User login
Promising HER2+/HR– breast cancer survival with de-escalated therapy
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
FROM ASCO 2021
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
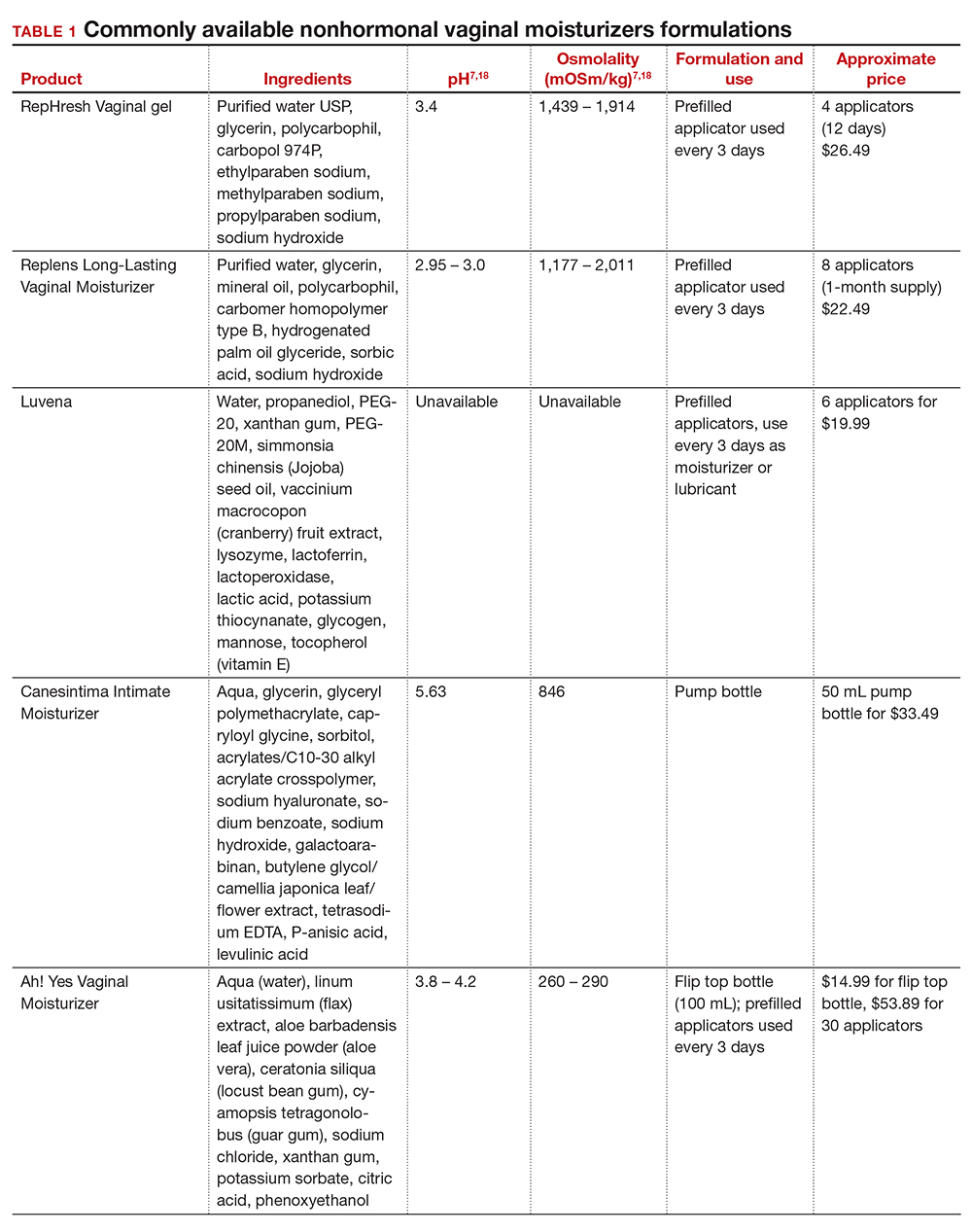
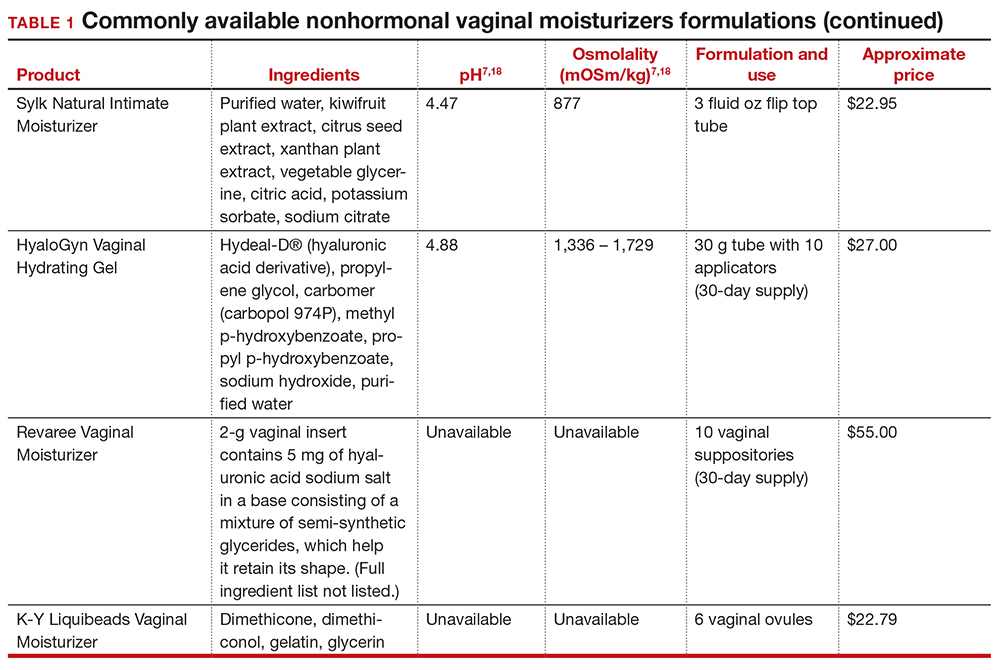
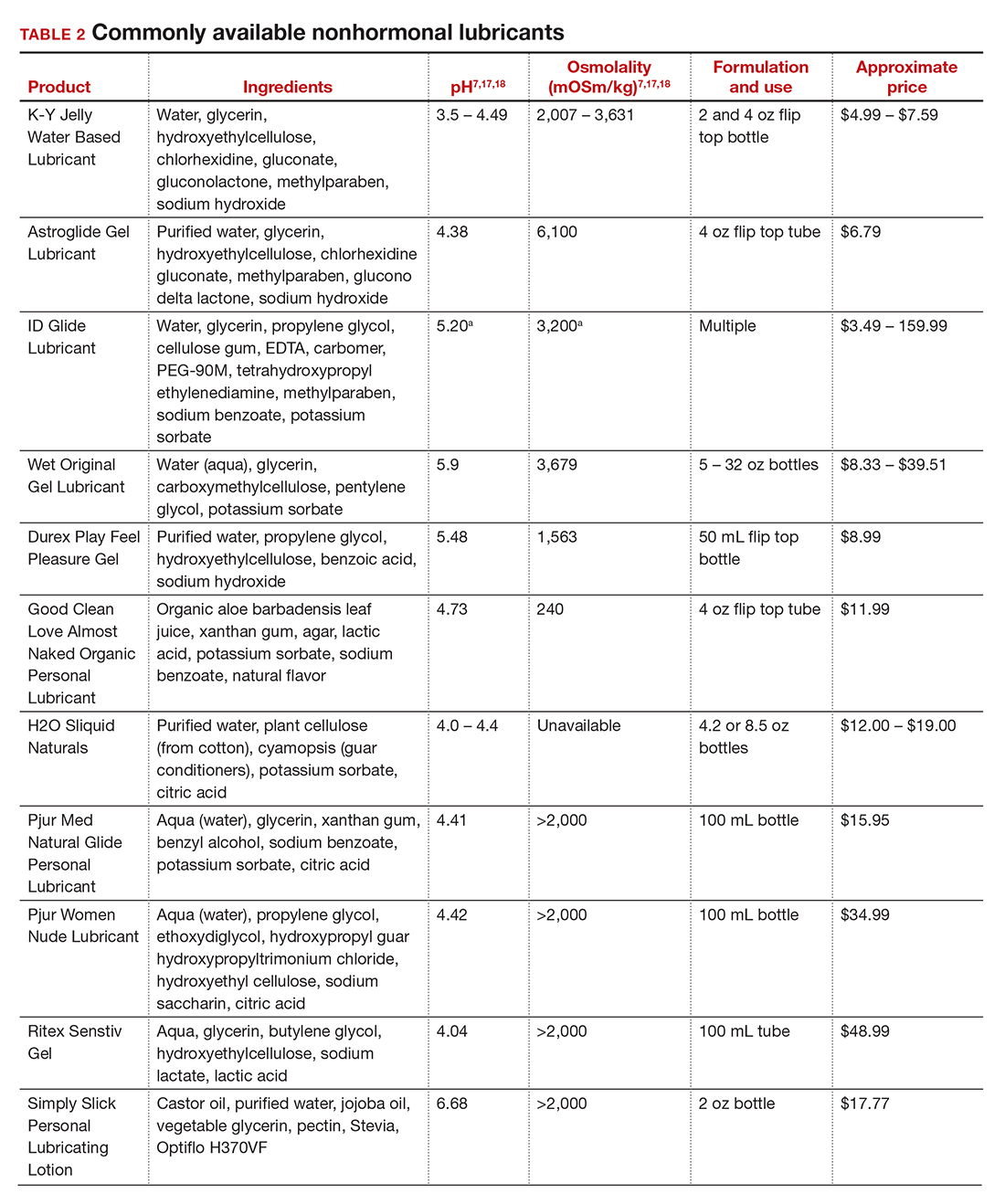
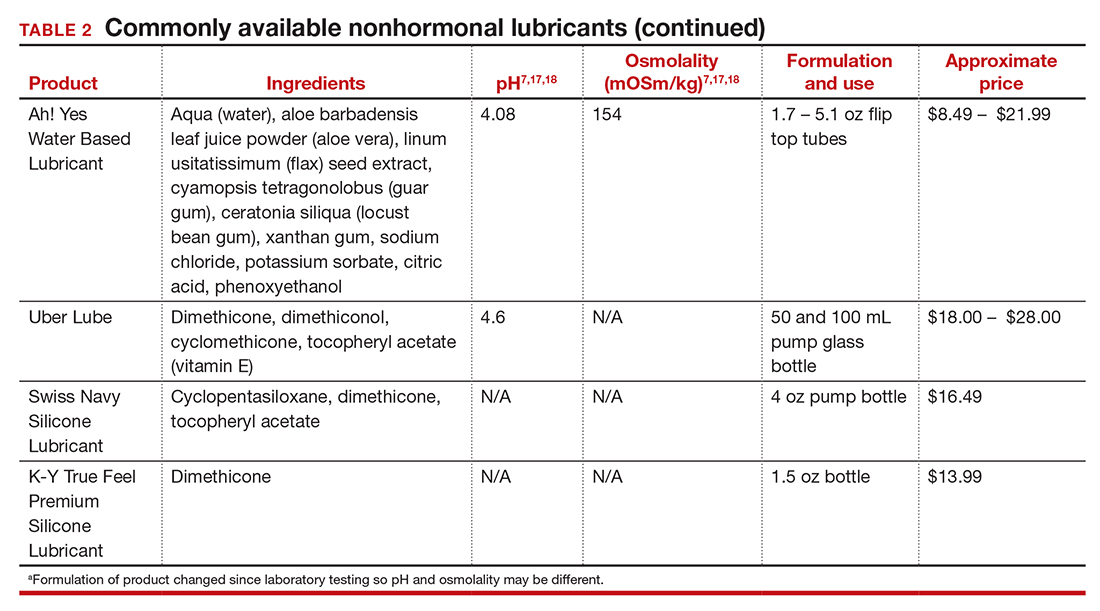
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Many comatose TBI patients recover consciousness during rehab
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
FROM JAMA NEUROLOGY
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
CONCERT: Better QoL but not survival with cabazitaxel in metastatic HER2– breast cancer
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
For patients with HER2-negative metastatic breast cancer, first line chemotherapy with cabazitaxel (Jevtana) every 3 weeks offers efficacy comparable to that of once-weekly paclitaxel, but with lower risk for peripheral neuropathy and better patient-reported quality of life, investigators in the multicenter CONCERT trial found.
In an open-label clinical trial of 158 patients from 14 hospitals in the United Kingdom, there was no difference in the primary endpoint of progression-free survival (PFS) or a secondary overall survival endpoint between patients randomly assigned to initial chemotherapy with cabazitaxel every 3 weeks or weekly paclitaxel, reported Amit Bahl, MD, of University Hospital Bristol, England, and colleagues.
“Cabazitaxel is safe and well tolerated for metastatic breast cancer and requires fewer hospital visits than weekly paclitaxel, which is very important for patients and health care providers, but more so in the current situation,” he said in an oral abstract session at the American Society of Clinical Oncology annual meeting (Abstract 1008).
Cabazitaxel is currently approved in the United States and Europe in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. It is not currently approved for the treatment of metastatic breast cancer, but has been explored for this indication in clinical trials.
“In the metastatic setting, where patients continue on treatment pretty much indefinitely until disease progression or unacceptable toxicity, the use of an every-3-week regimen could be attractive, because it means less visits for the patients, and it appears that this drug has lower toxicity in terms of peripheral neuropathy,” said breast cancer specialist Aditya Bardia, MD, MPH, who was not involved in the study.
Dr. Bardia, of Mass General Cancer Center in Boston, commented on the study in an interview.
Although paclitaxel is commonly used as first-line chemotherapy for HER2-negative metastatic breast cancer, it is associated with only modest response rates, ranging from 21.5% to 53.7% and carries significant risk of peripheral neuropathy, Dr. Bahl and colleagues noted.
“There is an unmet need for an alternative first-line cytotoxic chemotherapy agent, and cabazitaxel is a taxoid agent which has showed promising results in phase 2 trial of metastatic breast cancer patients in the second-line setting, even those with taxane resistance,” he said.
Open-label trial
To see whether cabazitaxel could meet those requirements, the investigators conducted a phase 2 randomized trial in which patients with HER2-negative metastatic breast cancer not previously treated with cytotoxic chemotherapy were assigned, 79 in each arm, to receive cabazitaxel 25 mg/m2 every 3 weeks, or paclitaxel 80 mg/m2 weekly.
The median patient age was 56 years in the cabazitaxel group and 61 years in the paclitaxel group. Roughly two-thirds of patients in each arm had Eastern Cooperative Oncology Group performance status 0, and the remainder had ECOG performance status 1.
In each arm, the median time on treatment was 15 weeks, but treatment delays and dose reductions were more common among patients on paclitaxel than cabazitaxel (61% vs. 39%, and 37% vs. 24%, respectively).
There were 149 PFS events at the time of the analysis. The median PFS with cabazitaxel was 6.7 months vs. 5.8 months with paclitaxel. This difference was not statistically significant. Median overall survival was 20.6 months in the cabazitaxel arm, vs. 18.2 months 20.0 months, respectively.
Similarly, there were no significant differences in either the overall response rates (42% vs. 37%), or time to response.
There were no complete responses with cabazitaxel vs. two (2.5%) with paclitaxel. The respective partial response rates were 41.8% vs. 34.2%.
In a subgroup analysis of PFS, there were no significant between-arm differences, except for an improved PFS in patients 65 and older with cabazitaxel (hazard ratio 0.45, 95% confidence interval, 0.25-0.80).
Quality of life favors cabazitaxel
Grade 3 or greater adverse events occurred in 42% of patients on cabazitaxel vs. 51% on paclitaxel. Diarrhea, febrile neutropenia, and nausea were the most common grade 3 or greater events in the cabazitaxel arm, whereas grade 3 or greater lung infection and peripheral neuropathy were more common with paclitaxel.
Sensory peripheral neuropathy of any grade occurred in 16% of patients assigned to cabazitaxel, compared with 54% assigned to paclitaxel. The respective rates of alopecia were 27% and 42%.
Over the course of treatment, the mean EuroQuol EQ-5D-5L single index utility score and visual analogue scale score were higher with cabazitaxel arm compared to paclitaxel, suggesting better patient quality of life with cabazitaxel.
In addition, throughout treatment patients in the cabazitaxel arm reported significantly better scores on The Functional Assessment of Cancer Therapy – Breast (FACT-B) breast cancer subscale, Dr. Bahl said.
Second-line may be better
ASCO invited discussant Marleen Kok, MD, PhD, from the Netherlands Cancer Institute in Amsterdam, pointed out that in the phase 2 GENEVIEVE trial comparing the efficacy and safety of cabazitaxel versus weekly paclitaxel as neoadjuvant treatment in patients with triple negative or luminal B/HER2 normal breast cancer the pathologic complete response rate with cabazitaxel was 1.2%, compared with 11% with paclitaxel.
“This GENEVIEVE trial, together with the CONCERT trial, suggests that there is not a big role for cabazitaxel to be used upfront before other taxanes,” she said.
However, in a phase 2 study of cabazitaxel as second-line therapy in patients with HER2-negative metastatic breast cancer who had previously been treated with taxanes, the overall response rate was 23%, “which is still of interest and importance for our patients,” she added.
Dr. Kok did not address quality of life differences between the regimens, however.
In a side note, Dr. Bardia said that “if there were an oral form of paclitaxel, that would certainly be very welcome, in that an oral drug is more convenient for patients, and would require fewer visits to the hospital.”
The CONCERT trial was funded by an investigator-sponsored study grant from Sanofi. Dr. Bahl disclosed honoraria and institutional research funding from Sanofi/Aventis and others, and travel expenses from Bayer and Roche. Dr. Kok disclosed a consulting or advisory role for Bristol Myers Squibb/Medarex, and institutional research funding from that company and others. Dr. Bardia disclosed a consulting or advisory role and research funding to his institution from multiple companies.
FROM ASCO 2021
Genomic signature predicts safety of omitting RT in early breast cancer
A novel 16-gene panel based on the biology of locoregional recurrence in early invasive breast cancer can both identify patients with a low risk of recurrence if they were to skip postsurgery radiation therapy, and predict which patients would be unlikely to benefit from adjuvant radiation, investigators reported.
Among 354 patients with stage I or II invasive estrogen receptor–positive (ER+), HER2-negative breast cancers who did not receive adjuvant systemic therapy, the genomic signature, dubbed POLAR (Profile for the Omission of Local Adjuvant Radiation) was prognostic for locoregional recurrence in patients who did not undergo radiation therapy, reported Martin Sjöström, MD, PhD, of the University of California, San Francisco, and colleagues.
They reported their findings in a poster presented during the American Society of Clinical Oncology (ASCO) annual meeting (Abstract 512).
“This is to our knowledge the first radiation-omission signature that is both prognostic and predictive: prognostic for outcomes in the absence of radiation, and predictive of benefits,” coauthor Corey Speers MD, PhD, of the University of Michigan, Ann Arbor, said in an interview.
The investigators conducted a retrospective analysis of data on patients enrolled in the SweBCG 91 RT trial, in which 1,187 patients with T1-2N0M0 breast cancer underwent a standardized radical sector resection and were then randomly assigned to either postoperative radiotherapy or no further treatment.
As the investigators reported in a long-term follow-up study presented in 2010 at the San Antonio Breast Cancer Symposium, the addition of postoperative radiation did not significantly affect overall survival, but was associated with a significant improvement in recurrence-free survival.
In the study presented at ASCO 2021, Dr. Sjöström and colleagues in Sweden, the United States, and Canada sought to determine whether they could identify a genomic signature that would identify women at very low risk for recurrence who could safely be spared from radiotherapy.
They focused on those patients in the study with ER+, HER2-negative tumors who did not receive adjuvant systemic therapy. The patients were divided into a training cohort of 243, and a validation cohort of 354 patients.
The investigators performed transcriptome-wide profiling of tumors, and identified both biological gene sets and individuals genes associated with locoregional recurrence among patients in the training set who did not receive radiotherapy.
The final POLAR genomic signature, containing 16 genes, was locked prior to testing in the validation cohort.
In a multivariable Cox model adjusting for age, grade, tumor size and luminal A vs. luminal B subtype, the POLAR gene set was prognostic for locoregional recurrence, with a hazard ratio (HR) of 1.7 (P < .001).
The 10-year locoregional recurrence rate for patients in the POLAR low-risk category who had not received radiation was 7%, and there was no significant benefit for POLAR low-risk patients who did receive radiation (HR 1.1, P = ns).
In contrast, patients classified as POLAR high risk who received radiotherapy had significantly lower risk for locoregional recurrence than high-risk patients who did not receive radiotherapy (HR 0.43, P = .0053).
Dr. Speers said that the POLAR signature appears to be unique in its ability to discriminate radiation-omission risk.
“At least looking in this cohort in the Swedish trial, none of other previously derived signatures – Mammaprint, ProSigna, Oncotype – were prognostic or predictive of locoregional recurrence events with radiation,” he said.
The investigators are currently exploring the POLAR signature in other clinical trials in which patients were randomized to receive radiation or no radiation.
‘A true victory’
Invited discussant Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, pointed out that in randomized clinical trials, approximately 60% of patients treated for early breast cancer did not experience recurrence in the absence of radiation, and that radiation prevented recurrence in about 30%, while about 10% experienced recurrence despite receiving radiation.
He said that the study by Dr. Sjöström and colleagues asks the question “can we use molecular factors to help identify patients who will not recur with lumpectomy alone without radiation therapy?”
The 60% of patients who will not experience recurrence in the absence of radiation can be categorized into two subpopulations: those with no residual malignant clonogens – the population included in this study by Dr. Sjöström and colleagues – and those with residual clonogens in the breast or elsewhere in the body that are sensitive to adjuvant endocrine therapy.
He said that the 7% 10-year risk of recurrence among patients in the POLAR low-risk group, who had neither radiation nor endocrine therapy, “is an exceptional outcome, which should be applauded, and I would point out that this risk of local recurrence of only 7% is at least in the same ballpark as the risk of recurrence that we accept every day when we treat early stage breast cancer patients with mastectomy alone, so this is a true victory.
“When we reflect on these provocative results from Dr. Sjöström and colleagues, it prompts in mind the question, could there be a group of patients with early breast cancer for whom a true ‘one-and-done’ strategy could be effective and safe?” Dr. Smith said.
Getting there will require a multidisciplinary, multimodality approach, involving imaging features of the primary tumor, clinical and pathologic features, and molecular information such as that provided by the POLAR genomic signature, he said.
The study was supported by PFS Genomics. Dr. Sjöström reported institutional funding from PFS Genomics. Dr. Speers disclosed stock and other ownership interests in the company, and he has applied for a patent on methods and genomic classifiers for prognosis of breast cancer and predicting benefit from adjuvant radiotherapy. Dr. Smith reported an equity interest in Oncora Medical, and an uncompensated relationship with the American Society for Radiation Oncology.
A novel 16-gene panel based on the biology of locoregional recurrence in early invasive breast cancer can both identify patients with a low risk of recurrence if they were to skip postsurgery radiation therapy, and predict which patients would be unlikely to benefit from adjuvant radiation, investigators reported.
Among 354 patients with stage I or II invasive estrogen receptor–positive (ER+), HER2-negative breast cancers who did not receive adjuvant systemic therapy, the genomic signature, dubbed POLAR (Profile for the Omission of Local Adjuvant Radiation) was prognostic for locoregional recurrence in patients who did not undergo radiation therapy, reported Martin Sjöström, MD, PhD, of the University of California, San Francisco, and colleagues.
They reported their findings in a poster presented during the American Society of Clinical Oncology (ASCO) annual meeting (Abstract 512).
“This is to our knowledge the first radiation-omission signature that is both prognostic and predictive: prognostic for outcomes in the absence of radiation, and predictive of benefits,” coauthor Corey Speers MD, PhD, of the University of Michigan, Ann Arbor, said in an interview.
The investigators conducted a retrospective analysis of data on patients enrolled in the SweBCG 91 RT trial, in which 1,187 patients with T1-2N0M0 breast cancer underwent a standardized radical sector resection and were then randomly assigned to either postoperative radiotherapy or no further treatment.
As the investigators reported in a long-term follow-up study presented in 2010 at the San Antonio Breast Cancer Symposium, the addition of postoperative radiation did not significantly affect overall survival, but was associated with a significant improvement in recurrence-free survival.
In the study presented at ASCO 2021, Dr. Sjöström and colleagues in Sweden, the United States, and Canada sought to determine whether they could identify a genomic signature that would identify women at very low risk for recurrence who could safely be spared from radiotherapy.
They focused on those patients in the study with ER+, HER2-negative tumors who did not receive adjuvant systemic therapy. The patients were divided into a training cohort of 243, and a validation cohort of 354 patients.
The investigators performed transcriptome-wide profiling of tumors, and identified both biological gene sets and individuals genes associated with locoregional recurrence among patients in the training set who did not receive radiotherapy.
The final POLAR genomic signature, containing 16 genes, was locked prior to testing in the validation cohort.
In a multivariable Cox model adjusting for age, grade, tumor size and luminal A vs. luminal B subtype, the POLAR gene set was prognostic for locoregional recurrence, with a hazard ratio (HR) of 1.7 (P < .001).
The 10-year locoregional recurrence rate for patients in the POLAR low-risk category who had not received radiation was 7%, and there was no significant benefit for POLAR low-risk patients who did receive radiation (HR 1.1, P = ns).
In contrast, patients classified as POLAR high risk who received radiotherapy had significantly lower risk for locoregional recurrence than high-risk patients who did not receive radiotherapy (HR 0.43, P = .0053).
Dr. Speers said that the POLAR signature appears to be unique in its ability to discriminate radiation-omission risk.
“At least looking in this cohort in the Swedish trial, none of other previously derived signatures – Mammaprint, ProSigna, Oncotype – were prognostic or predictive of locoregional recurrence events with radiation,” he said.
The investigators are currently exploring the POLAR signature in other clinical trials in which patients were randomized to receive radiation or no radiation.
‘A true victory’
Invited discussant Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, pointed out that in randomized clinical trials, approximately 60% of patients treated for early breast cancer did not experience recurrence in the absence of radiation, and that radiation prevented recurrence in about 30%, while about 10% experienced recurrence despite receiving radiation.
He said that the study by Dr. Sjöström and colleagues asks the question “can we use molecular factors to help identify patients who will not recur with lumpectomy alone without radiation therapy?”
The 60% of patients who will not experience recurrence in the absence of radiation can be categorized into two subpopulations: those with no residual malignant clonogens – the population included in this study by Dr. Sjöström and colleagues – and those with residual clonogens in the breast or elsewhere in the body that are sensitive to adjuvant endocrine therapy.
He said that the 7% 10-year risk of recurrence among patients in the POLAR low-risk group, who had neither radiation nor endocrine therapy, “is an exceptional outcome, which should be applauded, and I would point out that this risk of local recurrence of only 7% is at least in the same ballpark as the risk of recurrence that we accept every day when we treat early stage breast cancer patients with mastectomy alone, so this is a true victory.
“When we reflect on these provocative results from Dr. Sjöström and colleagues, it prompts in mind the question, could there be a group of patients with early breast cancer for whom a true ‘one-and-done’ strategy could be effective and safe?” Dr. Smith said.
Getting there will require a multidisciplinary, multimodality approach, involving imaging features of the primary tumor, clinical and pathologic features, and molecular information such as that provided by the POLAR genomic signature, he said.
The study was supported by PFS Genomics. Dr. Sjöström reported institutional funding from PFS Genomics. Dr. Speers disclosed stock and other ownership interests in the company, and he has applied for a patent on methods and genomic classifiers for prognosis of breast cancer and predicting benefit from adjuvant radiotherapy. Dr. Smith reported an equity interest in Oncora Medical, and an uncompensated relationship with the American Society for Radiation Oncology.
A novel 16-gene panel based on the biology of locoregional recurrence in early invasive breast cancer can both identify patients with a low risk of recurrence if they were to skip postsurgery radiation therapy, and predict which patients would be unlikely to benefit from adjuvant radiation, investigators reported.
Among 354 patients with stage I or II invasive estrogen receptor–positive (ER+), HER2-negative breast cancers who did not receive adjuvant systemic therapy, the genomic signature, dubbed POLAR (Profile for the Omission of Local Adjuvant Radiation) was prognostic for locoregional recurrence in patients who did not undergo radiation therapy, reported Martin Sjöström, MD, PhD, of the University of California, San Francisco, and colleagues.
They reported their findings in a poster presented during the American Society of Clinical Oncology (ASCO) annual meeting (Abstract 512).
“This is to our knowledge the first radiation-omission signature that is both prognostic and predictive: prognostic for outcomes in the absence of radiation, and predictive of benefits,” coauthor Corey Speers MD, PhD, of the University of Michigan, Ann Arbor, said in an interview.
The investigators conducted a retrospective analysis of data on patients enrolled in the SweBCG 91 RT trial, in which 1,187 patients with T1-2N0M0 breast cancer underwent a standardized radical sector resection and were then randomly assigned to either postoperative radiotherapy or no further treatment.
As the investigators reported in a long-term follow-up study presented in 2010 at the San Antonio Breast Cancer Symposium, the addition of postoperative radiation did not significantly affect overall survival, but was associated with a significant improvement in recurrence-free survival.
In the study presented at ASCO 2021, Dr. Sjöström and colleagues in Sweden, the United States, and Canada sought to determine whether they could identify a genomic signature that would identify women at very low risk for recurrence who could safely be spared from radiotherapy.
They focused on those patients in the study with ER+, HER2-negative tumors who did not receive adjuvant systemic therapy. The patients were divided into a training cohort of 243, and a validation cohort of 354 patients.
The investigators performed transcriptome-wide profiling of tumors, and identified both biological gene sets and individuals genes associated with locoregional recurrence among patients in the training set who did not receive radiotherapy.
The final POLAR genomic signature, containing 16 genes, was locked prior to testing in the validation cohort.
In a multivariable Cox model adjusting for age, grade, tumor size and luminal A vs. luminal B subtype, the POLAR gene set was prognostic for locoregional recurrence, with a hazard ratio (HR) of 1.7 (P < .001).
The 10-year locoregional recurrence rate for patients in the POLAR low-risk category who had not received radiation was 7%, and there was no significant benefit for POLAR low-risk patients who did receive radiation (HR 1.1, P = ns).
In contrast, patients classified as POLAR high risk who received radiotherapy had significantly lower risk for locoregional recurrence than high-risk patients who did not receive radiotherapy (HR 0.43, P = .0053).
Dr. Speers said that the POLAR signature appears to be unique in its ability to discriminate radiation-omission risk.
“At least looking in this cohort in the Swedish trial, none of other previously derived signatures – Mammaprint, ProSigna, Oncotype – were prognostic or predictive of locoregional recurrence events with radiation,” he said.
The investigators are currently exploring the POLAR signature in other clinical trials in which patients were randomized to receive radiation or no radiation.
‘A true victory’
Invited discussant Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, pointed out that in randomized clinical trials, approximately 60% of patients treated for early breast cancer did not experience recurrence in the absence of radiation, and that radiation prevented recurrence in about 30%, while about 10% experienced recurrence despite receiving radiation.
He said that the study by Dr. Sjöström and colleagues asks the question “can we use molecular factors to help identify patients who will not recur with lumpectomy alone without radiation therapy?”
The 60% of patients who will not experience recurrence in the absence of radiation can be categorized into two subpopulations: those with no residual malignant clonogens – the population included in this study by Dr. Sjöström and colleagues – and those with residual clonogens in the breast or elsewhere in the body that are sensitive to adjuvant endocrine therapy.
He said that the 7% 10-year risk of recurrence among patients in the POLAR low-risk group, who had neither radiation nor endocrine therapy, “is an exceptional outcome, which should be applauded, and I would point out that this risk of local recurrence of only 7% is at least in the same ballpark as the risk of recurrence that we accept every day when we treat early stage breast cancer patients with mastectomy alone, so this is a true victory.
“When we reflect on these provocative results from Dr. Sjöström and colleagues, it prompts in mind the question, could there be a group of patients with early breast cancer for whom a true ‘one-and-done’ strategy could be effective and safe?” Dr. Smith said.
Getting there will require a multidisciplinary, multimodality approach, involving imaging features of the primary tumor, clinical and pathologic features, and molecular information such as that provided by the POLAR genomic signature, he said.
The study was supported by PFS Genomics. Dr. Sjöström reported institutional funding from PFS Genomics. Dr. Speers disclosed stock and other ownership interests in the company, and he has applied for a patent on methods and genomic classifiers for prognosis of breast cancer and predicting benefit from adjuvant radiotherapy. Dr. Smith reported an equity interest in Oncora Medical, and an uncompensated relationship with the American Society for Radiation Oncology.
FROM ASCO 2021
ACE inhibitor prevents trastuzumab-associated LVEF decline after anthracyclines in BC treatment
For women with HER2-positive early breast cancer treated with an anthracycline-based regimen followed by trastuzumab (Herceptin), concurrent therapy with the angiotensin-converting enzyme (ACE) inhibitor lisinopril can help to prevent a decline in left-ventricular ejection fraction (LVEF) below 50%, investigators in a community-based study found.
Among 424 women with HER2-positive early-stage breast cancer, the rate of LVEF decline to below 50% was 21.4% among patients randomized to receive an anthracycline-based regimen, compared with 4.1% for patients who were treated with a non–anthracycline-based regimen.
Among patients in the anthracycline arm, treatment with lisinopril, but not carvedilol or placebo, was associated with significant protection against LVEF decline, reported Pamela N. Munster, MD, of the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“The findings of this study suggested that the drop in left ventricular ejection fraction to below 50% or to below normal was much larger than previously reported in the anthracycline group,” she said in a video presentation during a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 509).
Although HER2 inhibition is a highly effective treatment strategy for patients with HER2-positive tumors, trastuzumab-associated decline in LVEF and clinical heart failure can result in treatment interruption or discontinuation of therapy, the authors noted.
They conducted the prospective randomized trial to see whether trastuzumab-associated cardiotoxicity could be mitigated by concurrent use of either an ACE inhibitor or beta-blocker.
They enrolled women with early HER2-positive breast cancer from 127 community-based oncology centers.
The patients were randomly assigned to receive either an anthracycline- or non–anthracycline-containing regimen, followed by a year of trastuzumab, and were then randomized again to receive either lisinopril, carvedilol, or placebo concurrently with trastuzumab.
The patients were assessed for cardiotoxicity every 12 weeks with a multiple-gated acquisition (MUGA) scan and echocardiogram. Cardiotoxicity was defined as an absolute decrease in LVEF of 10% or more, or at least 5% decrease in LVEF below 50% at baseline.
Small LVEF declines in all
The investigators observed that all patients in the study experienced a small but not clinically relevant decrease in LVEF during trastuzumab therapy, and that this decline was not significantly ameliorated by any of the interventions.
However, as noted before, among patients assigned to anthracycline-based regimens the rate of decline to LVEF below 50% was 21.4%, compared with 4.1 for patients assigned to non–anthracycline regimens.
LVEF declines of at least 10% occurred in 5.5% of patients who received anthracyclines, and in 14.4% of those who received a non-anthracycline regimen.
Among patients who received anthracycline, only 10.8% randomized to receive lisinopril prophylaxis experienced a decline in LVEF, compared with 30.5% assigned to placebo (P = .045).
“In contrast, while carvedilol showed a numerical prevention of LVEF below 50% to 22.8%, this was not statistically significant,” Dr. Munster said.
Decreases in LVEF of 10% or greater within the normal LVEF range were similar in both chemotherapy arms and were not affected by either lisinopril or carvedilol.
“Lisinopril was well tolerated in this group, even in patients without hypertension,” she said.
“In women treated in a community-based environment who could benefit from anthracyclines or where anthracyclines are indicated, one should anticipate the decrease in left ventricular ejection fraction to below normal to be larger than is reported by other groups. However, this could be prevented by lisinopril,” she concluded.
Low numbers overall
Invited discussant Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, noted that the overall number of cardiotoxicity events and the rate of important decreases in LVEF were comparatively low.
She also pointed out that the findings are in line with another recent analysis of the same cohort by the same authors, although in the earlier analysis the investigators found that the effect on cardiotoxicity-free survival was good with both agents compared with placebo, but slightly better with carvedilol (hazard ratio 0.49, P = .009) than with lisinopril (HR 0.53, P = .015).
“Lisinopril has the most important effect regarding preventing decrease of LVEF below 50%. When we look at the curves of LVEF drops above 10%, carvedilol seems to have a slightly bigger effect,” she said.
The trial was cosponsored by the SunCoast Community Clinical Oncology Programs Research Base at University of South Florida, Tampa, and by the National Cancer Institute. Dr. Munster disclosed leadership positions, stock ownership, and consulting or advisory roles with several companies. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
For women with HER2-positive early breast cancer treated with an anthracycline-based regimen followed by trastuzumab (Herceptin), concurrent therapy with the angiotensin-converting enzyme (ACE) inhibitor lisinopril can help to prevent a decline in left-ventricular ejection fraction (LVEF) below 50%, investigators in a community-based study found.
Among 424 women with HER2-positive early-stage breast cancer, the rate of LVEF decline to below 50% was 21.4% among patients randomized to receive an anthracycline-based regimen, compared with 4.1% for patients who were treated with a non–anthracycline-based regimen.
Among patients in the anthracycline arm, treatment with lisinopril, but not carvedilol or placebo, was associated with significant protection against LVEF decline, reported Pamela N. Munster, MD, of the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“The findings of this study suggested that the drop in left ventricular ejection fraction to below 50% or to below normal was much larger than previously reported in the anthracycline group,” she said in a video presentation during a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 509).
Although HER2 inhibition is a highly effective treatment strategy for patients with HER2-positive tumors, trastuzumab-associated decline in LVEF and clinical heart failure can result in treatment interruption or discontinuation of therapy, the authors noted.
They conducted the prospective randomized trial to see whether trastuzumab-associated cardiotoxicity could be mitigated by concurrent use of either an ACE inhibitor or beta-blocker.
They enrolled women with early HER2-positive breast cancer from 127 community-based oncology centers.
The patients were randomly assigned to receive either an anthracycline- or non–anthracycline-containing regimen, followed by a year of trastuzumab, and were then randomized again to receive either lisinopril, carvedilol, or placebo concurrently with trastuzumab.
The patients were assessed for cardiotoxicity every 12 weeks with a multiple-gated acquisition (MUGA) scan and echocardiogram. Cardiotoxicity was defined as an absolute decrease in LVEF of 10% or more, or at least 5% decrease in LVEF below 50% at baseline.
Small LVEF declines in all
The investigators observed that all patients in the study experienced a small but not clinically relevant decrease in LVEF during trastuzumab therapy, and that this decline was not significantly ameliorated by any of the interventions.
However, as noted before, among patients assigned to anthracycline-based regimens the rate of decline to LVEF below 50% was 21.4%, compared with 4.1 for patients assigned to non–anthracycline regimens.
LVEF declines of at least 10% occurred in 5.5% of patients who received anthracyclines, and in 14.4% of those who received a non-anthracycline regimen.
Among patients who received anthracycline, only 10.8% randomized to receive lisinopril prophylaxis experienced a decline in LVEF, compared with 30.5% assigned to placebo (P = .045).
“In contrast, while carvedilol showed a numerical prevention of LVEF below 50% to 22.8%, this was not statistically significant,” Dr. Munster said.
Decreases in LVEF of 10% or greater within the normal LVEF range were similar in both chemotherapy arms and were not affected by either lisinopril or carvedilol.
“Lisinopril was well tolerated in this group, even in patients without hypertension,” she said.
“In women treated in a community-based environment who could benefit from anthracyclines or where anthracyclines are indicated, one should anticipate the decrease in left ventricular ejection fraction to below normal to be larger than is reported by other groups. However, this could be prevented by lisinopril,” she concluded.
Low numbers overall
Invited discussant Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, noted that the overall number of cardiotoxicity events and the rate of important decreases in LVEF were comparatively low.
She also pointed out that the findings are in line with another recent analysis of the same cohort by the same authors, although in the earlier analysis the investigators found that the effect on cardiotoxicity-free survival was good with both agents compared with placebo, but slightly better with carvedilol (hazard ratio 0.49, P = .009) than with lisinopril (HR 0.53, P = .015).
“Lisinopril has the most important effect regarding preventing decrease of LVEF below 50%. When we look at the curves of LVEF drops above 10%, carvedilol seems to have a slightly bigger effect,” she said.
The trial was cosponsored by the SunCoast Community Clinical Oncology Programs Research Base at University of South Florida, Tampa, and by the National Cancer Institute. Dr. Munster disclosed leadership positions, stock ownership, and consulting or advisory roles with several companies. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
For women with HER2-positive early breast cancer treated with an anthracycline-based regimen followed by trastuzumab (Herceptin), concurrent therapy with the angiotensin-converting enzyme (ACE) inhibitor lisinopril can help to prevent a decline in left-ventricular ejection fraction (LVEF) below 50%, investigators in a community-based study found.
Among 424 women with HER2-positive early-stage breast cancer, the rate of LVEF decline to below 50% was 21.4% among patients randomized to receive an anthracycline-based regimen, compared with 4.1% for patients who were treated with a non–anthracycline-based regimen.
Among patients in the anthracycline arm, treatment with lisinopril, but not carvedilol or placebo, was associated with significant protection against LVEF decline, reported Pamela N. Munster, MD, of the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“The findings of this study suggested that the drop in left ventricular ejection fraction to below 50% or to below normal was much larger than previously reported in the anthracycline group,” she said in a video presentation during a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 509).
Although HER2 inhibition is a highly effective treatment strategy for patients with HER2-positive tumors, trastuzumab-associated decline in LVEF and clinical heart failure can result in treatment interruption or discontinuation of therapy, the authors noted.
They conducted the prospective randomized trial to see whether trastuzumab-associated cardiotoxicity could be mitigated by concurrent use of either an ACE inhibitor or beta-blocker.
They enrolled women with early HER2-positive breast cancer from 127 community-based oncology centers.
The patients were randomly assigned to receive either an anthracycline- or non–anthracycline-containing regimen, followed by a year of trastuzumab, and were then randomized again to receive either lisinopril, carvedilol, or placebo concurrently with trastuzumab.
The patients were assessed for cardiotoxicity every 12 weeks with a multiple-gated acquisition (MUGA) scan and echocardiogram. Cardiotoxicity was defined as an absolute decrease in LVEF of 10% or more, or at least 5% decrease in LVEF below 50% at baseline.
Small LVEF declines in all
The investigators observed that all patients in the study experienced a small but not clinically relevant decrease in LVEF during trastuzumab therapy, and that this decline was not significantly ameliorated by any of the interventions.
However, as noted before, among patients assigned to anthracycline-based regimens the rate of decline to LVEF below 50% was 21.4%, compared with 4.1 for patients assigned to non–anthracycline regimens.
LVEF declines of at least 10% occurred in 5.5% of patients who received anthracyclines, and in 14.4% of those who received a non-anthracycline regimen.
Among patients who received anthracycline, only 10.8% randomized to receive lisinopril prophylaxis experienced a decline in LVEF, compared with 30.5% assigned to placebo (P = .045).
“In contrast, while carvedilol showed a numerical prevention of LVEF below 50% to 22.8%, this was not statistically significant,” Dr. Munster said.
Decreases in LVEF of 10% or greater within the normal LVEF range were similar in both chemotherapy arms and were not affected by either lisinopril or carvedilol.
“Lisinopril was well tolerated in this group, even in patients without hypertension,” she said.
“In women treated in a community-based environment who could benefit from anthracyclines or where anthracyclines are indicated, one should anticipate the decrease in left ventricular ejection fraction to below normal to be larger than is reported by other groups. However, this could be prevented by lisinopril,” she concluded.
Low numbers overall
Invited discussant Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, noted that the overall number of cardiotoxicity events and the rate of important decreases in LVEF were comparatively low.
She also pointed out that the findings are in line with another recent analysis of the same cohort by the same authors, although in the earlier analysis the investigators found that the effect on cardiotoxicity-free survival was good with both agents compared with placebo, but slightly better with carvedilol (hazard ratio 0.49, P = .009) than with lisinopril (HR 0.53, P = .015).
“Lisinopril has the most important effect regarding preventing decrease of LVEF below 50%. When we look at the curves of LVEF drops above 10%, carvedilol seems to have a slightly bigger effect,” she said.
The trial was cosponsored by the SunCoast Community Clinical Oncology Programs Research Base at University of South Florida, Tampa, and by the National Cancer Institute. Dr. Munster disclosed leadership positions, stock ownership, and consulting or advisory roles with several companies. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
FROM ASCO 2021
Pregnancy effect on chemotherapy does not affect maternal breast cancer outcomes
Reassuring news for women who receive a diagnosis of breast cancer during pregnancy: Pregnancy-induced alterations in the pharmacokinetics of chemotherapy do not appear to compromise outcomes for the mother.
That’s according to investigators who reviewed registry data on 662 pregnant women and 2,081 nonpregnant women with a diagnosis of breast cancer. After a median follow-up of 66 months, there were no significant differences in either disease-free survival (DFS) or overall survival (OS), and women who received more than 60% of their chemotherapy doses during pregnancy had survival comparable to that of nonpregnant women, reported Frédéric Amant, MD, PhD, of University Hospitals Leuven (Belgium).
“These results support initiation of chemotherapy for breast cancer during pregnancy where indicated for oncological reasons,” they reported in a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 515).
Although in general a diagnosis of breast cancer during pregnancy does not appear to affect the mother’s prognosis when standard therapy is used, “caution is warranted as gestational changes in pharmacokinetics with respect to the distribution, metabolism, and excretion of drugs may lead to reduced chemotherapy concentration in pregnant patients,” the authors wrote.
To get a better picture of the prognosis for women diagnosed with breast cancer during pregnancy, the investigators created a cohort of patients from two multicenter registries: the International Network of Cancer, Infertility, and Pregnancy and the German Breast Group. Both registries collect data retrospectively and prospectively,
They used propensity scoring to smooth out differences in the baseline characteristics of pregnant women and nonpregnant controls.
The median age at diagnosis was 34 year for pregnant women, and 38 years for controls. Pregnant women were more likely than were controls to have stage II disease (60.1% vs. 56, 1%, P = .035), grade 3 tumors (74% vs. 62.2%, P < .001), hormone receptor–negative breast tumors (48.4% vs. 30%), and triple-negative breast cancer (38.9% vs. 26.9%, P < .001).
In Cox proportional hazard regression analysis controlling for age, stage, grade, hormone receptor status, HER2 status and histology, there were no significant differences between pregnant women and controls in either DFS (hazard ratio [HR] 1.02, P = .83) or OS (HR 1.08, P = .57).
As noted before, a subgroup analysis of 339 women who received more than 60% of their assigned chemotherapy doses during pregnancy also showed that survival was not significantly different from that of nonpregnant women (HR for DFS 0,71, P = .13; HR for OS 0.85, P = .39).
Termination does not benefit the mother
“Thanks to the important work of Dr. Amant in the INCIP [International Network on Cancer, Infertility, and Pregnancy] network and others around the world, we now have sufficient data to know that it’s safe to treat breast cancer during pregnancy, and that the prognosis of breast cancer during pregnancy is comparable to nonpregnant controls if we adjust for certain characteristics such as age and others,” said Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, the invited discussant.
“With this and other studies, we can come to the conclusion that pregnancy-induced alterations in the chemotherapy concentration due to altered pharmacokinetics does not seem to affect maternal prognosis, and therefore we should initiate treatment of breast cancer during wherever it’s indicated for oncological reasons, knowing that you can only use chemotherapy during the second or third trimester,” she said.
Dr. Cardoso emphasized that breast cancer during pregnancy is a rare situation requiring that treatment be given in a specialized center by an experienced multidisciplinary team, and that interrupting the pregnancy does not improve the mother’s prognosis.
“We have to spread the word to all health professionals who come across these women to stop advising them to immediately terminate pregnancy. For the children, the most important take-home message is avoid prematurely delivery,” she said.
Treatment for women with a diagnosis of breast cancer during pregnancy should be similar to that for nonpregnant women, with the exception of endocrine therapy and anti-HER2 agents, which should be withheld until after delivery, she added.
The study was supported by the European Research Council, Research Foundation Flanders, and Kom op tegen kanker (Stand Up to Cancer). Dr. Amant disclosed a consulting or advisory role for AstraZeneca and Clovis Oncology. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
Reassuring news for women who receive a diagnosis of breast cancer during pregnancy: Pregnancy-induced alterations in the pharmacokinetics of chemotherapy do not appear to compromise outcomes for the mother.
That’s according to investigators who reviewed registry data on 662 pregnant women and 2,081 nonpregnant women with a diagnosis of breast cancer. After a median follow-up of 66 months, there were no significant differences in either disease-free survival (DFS) or overall survival (OS), and women who received more than 60% of their chemotherapy doses during pregnancy had survival comparable to that of nonpregnant women, reported Frédéric Amant, MD, PhD, of University Hospitals Leuven (Belgium).
“These results support initiation of chemotherapy for breast cancer during pregnancy where indicated for oncological reasons,” they reported in a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 515).
Although in general a diagnosis of breast cancer during pregnancy does not appear to affect the mother’s prognosis when standard therapy is used, “caution is warranted as gestational changes in pharmacokinetics with respect to the distribution, metabolism, and excretion of drugs may lead to reduced chemotherapy concentration in pregnant patients,” the authors wrote.
To get a better picture of the prognosis for women diagnosed with breast cancer during pregnancy, the investigators created a cohort of patients from two multicenter registries: the International Network of Cancer, Infertility, and Pregnancy and the German Breast Group. Both registries collect data retrospectively and prospectively,
They used propensity scoring to smooth out differences in the baseline characteristics of pregnant women and nonpregnant controls.
The median age at diagnosis was 34 year for pregnant women, and 38 years for controls. Pregnant women were more likely than were controls to have stage II disease (60.1% vs. 56, 1%, P = .035), grade 3 tumors (74% vs. 62.2%, P < .001), hormone receptor–negative breast tumors (48.4% vs. 30%), and triple-negative breast cancer (38.9% vs. 26.9%, P < .001).
In Cox proportional hazard regression analysis controlling for age, stage, grade, hormone receptor status, HER2 status and histology, there were no significant differences between pregnant women and controls in either DFS (hazard ratio [HR] 1.02, P = .83) or OS (HR 1.08, P = .57).
As noted before, a subgroup analysis of 339 women who received more than 60% of their assigned chemotherapy doses during pregnancy also showed that survival was not significantly different from that of nonpregnant women (HR for DFS 0,71, P = .13; HR for OS 0.85, P = .39).
Termination does not benefit the mother
“Thanks to the important work of Dr. Amant in the INCIP [International Network on Cancer, Infertility, and Pregnancy] network and others around the world, we now have sufficient data to know that it’s safe to treat breast cancer during pregnancy, and that the prognosis of breast cancer during pregnancy is comparable to nonpregnant controls if we adjust for certain characteristics such as age and others,” said Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, the invited discussant.
“With this and other studies, we can come to the conclusion that pregnancy-induced alterations in the chemotherapy concentration due to altered pharmacokinetics does not seem to affect maternal prognosis, and therefore we should initiate treatment of breast cancer during wherever it’s indicated for oncological reasons, knowing that you can only use chemotherapy during the second or third trimester,” she said.
Dr. Cardoso emphasized that breast cancer during pregnancy is a rare situation requiring that treatment be given in a specialized center by an experienced multidisciplinary team, and that interrupting the pregnancy does not improve the mother’s prognosis.
“We have to spread the word to all health professionals who come across these women to stop advising them to immediately terminate pregnancy. For the children, the most important take-home message is avoid prematurely delivery,” she said.
Treatment for women with a diagnosis of breast cancer during pregnancy should be similar to that for nonpregnant women, with the exception of endocrine therapy and anti-HER2 agents, which should be withheld until after delivery, she added.
The study was supported by the European Research Council, Research Foundation Flanders, and Kom op tegen kanker (Stand Up to Cancer). Dr. Amant disclosed a consulting or advisory role for AstraZeneca and Clovis Oncology. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
Reassuring news for women who receive a diagnosis of breast cancer during pregnancy: Pregnancy-induced alterations in the pharmacokinetics of chemotherapy do not appear to compromise outcomes for the mother.
That’s according to investigators who reviewed registry data on 662 pregnant women and 2,081 nonpregnant women with a diagnosis of breast cancer. After a median follow-up of 66 months, there were no significant differences in either disease-free survival (DFS) or overall survival (OS), and women who received more than 60% of their chemotherapy doses during pregnancy had survival comparable to that of nonpregnant women, reported Frédéric Amant, MD, PhD, of University Hospitals Leuven (Belgium).
“These results support initiation of chemotherapy for breast cancer during pregnancy where indicated for oncological reasons,” they reported in a poster discussion session at the American Society of Clinical Oncology annual meeting. (Abstract 515).
Although in general a diagnosis of breast cancer during pregnancy does not appear to affect the mother’s prognosis when standard therapy is used, “caution is warranted as gestational changes in pharmacokinetics with respect to the distribution, metabolism, and excretion of drugs may lead to reduced chemotherapy concentration in pregnant patients,” the authors wrote.
To get a better picture of the prognosis for women diagnosed with breast cancer during pregnancy, the investigators created a cohort of patients from two multicenter registries: the International Network of Cancer, Infertility, and Pregnancy and the German Breast Group. Both registries collect data retrospectively and prospectively,
They used propensity scoring to smooth out differences in the baseline characteristics of pregnant women and nonpregnant controls.
The median age at diagnosis was 34 year for pregnant women, and 38 years for controls. Pregnant women were more likely than were controls to have stage II disease (60.1% vs. 56, 1%, P = .035), grade 3 tumors (74% vs. 62.2%, P < .001), hormone receptor–negative breast tumors (48.4% vs. 30%), and triple-negative breast cancer (38.9% vs. 26.9%, P < .001).
In Cox proportional hazard regression analysis controlling for age, stage, grade, hormone receptor status, HER2 status and histology, there were no significant differences between pregnant women and controls in either DFS (hazard ratio [HR] 1.02, P = .83) or OS (HR 1.08, P = .57).
As noted before, a subgroup analysis of 339 women who received more than 60% of their assigned chemotherapy doses during pregnancy also showed that survival was not significantly different from that of nonpregnant women (HR for DFS 0,71, P = .13; HR for OS 0.85, P = .39).
Termination does not benefit the mother
“Thanks to the important work of Dr. Amant in the INCIP [International Network on Cancer, Infertility, and Pregnancy] network and others around the world, we now have sufficient data to know that it’s safe to treat breast cancer during pregnancy, and that the prognosis of breast cancer during pregnancy is comparable to nonpregnant controls if we adjust for certain characteristics such as age and others,” said Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, the invited discussant.
“With this and other studies, we can come to the conclusion that pregnancy-induced alterations in the chemotherapy concentration due to altered pharmacokinetics does not seem to affect maternal prognosis, and therefore we should initiate treatment of breast cancer during wherever it’s indicated for oncological reasons, knowing that you can only use chemotherapy during the second or third trimester,” she said.
Dr. Cardoso emphasized that breast cancer during pregnancy is a rare situation requiring that treatment be given in a specialized center by an experienced multidisciplinary team, and that interrupting the pregnancy does not improve the mother’s prognosis.
“We have to spread the word to all health professionals who come across these women to stop advising them to immediately terminate pregnancy. For the children, the most important take-home message is avoid prematurely delivery,” she said.
Treatment for women with a diagnosis of breast cancer during pregnancy should be similar to that for nonpregnant women, with the exception of endocrine therapy and anti-HER2 agents, which should be withheld until after delivery, she added.
The study was supported by the European Research Council, Research Foundation Flanders, and Kom op tegen kanker (Stand Up to Cancer). Dr. Amant disclosed a consulting or advisory role for AstraZeneca and Clovis Oncology. Dr. Cardoso disclosed consulting or advisory roles and travel support from multiple companies.
FROM ASCO 2021
Deep brain stimulation is effective over the long haul
, new research indicates.
“Subthalamic nucleus stimulation is a well recognized treatment used for improving motor conditions and quality of life in people with Parkinson’s disease. Our study, for the first time, supports its efficacy in the very long term – 15 years after surgery and 25 years since the Parkinson’s disease diagnosis,” said Elena Moro, MD, PhD, Grenoble Alpes University, Grenoble, France.
“This information is relevant for physicians, patients, and their families when they need to decide about the surgical option to deal with Parkinson’s disease,” said Dr. Moro.
The study was published online June 2 in Neurology.
‘Don’t delay’
The findings are based on 51 patients with Parkinson’s disease who underwent treatment with bilateral STN-DBS for an average of 17 years (range, 15-24 years). Their average age at diagnosis was 40 years, and the average age at device implantation was 51 years.
The results demonstrate that STN-DBS continues to be effective for motor complications for longer than 15 years, reducing time spent with dyskinesia by 75% and time spent in the off-state by 58.7%. This is similar to the amount of improvement seen 1 year after surgery.
Doses of dopaminergic medications continued to be low at long-term follow-up; dosing was reduced by 50.6% compared with baseline.
There was also continued improvement in quality of life. Scores on the Parkinson’s Disease Quality of Life Questionnaire in the very long term were 13.8% better compared with baseline.
“Few and mostly manageable device-related adverse events were observed during the follow-up,” the authors reported in their article.
“Deep brain stimulation is already recommended when a patient’s conditions are not optimized by medical treatment. Patients with Parkinson’s disease without dementia and in good general health conditions are the best candidates for this surgery,” said Dr. Moro.
“Taking into account our results and the data available in the literature, DBS surgery should not be delayed when motor conditions and quality of life decline despite medical treatment, if patients meet the inclusion criteria,” she added.
A revolutionary treatment
The authors of an accompanying editorial say these results, which indicate better motor outcomes with less medication, “reinforce why STN-DBS has revolutionized treatment for advanced Parkinson’s disease.”
Kelvin Chou, MD, of the University of Michigan, Ann Arbor, and David Charles, MD, of Vanderbilt University, Nashville, Tenn., pointed out that longer disease duration is associated with an increase in the likelihood of cognitive impairment and psychosis, both of which are risk factors for nursing home placement, and they limit the ability to use dopaminergic medications.
Although many of the patients in this cohort experienced hallucinations and psychosis over the long follow-up period, “one can imagine that the number and severity would be higher without DBS therapy,” they wrote.
Key caveats, said Dr. Chou and Dr. Charles, are that the results are based on a highly selected cohort and that the patients were managed by experts in the field of movement disorders and DBS.
Additionally, the patients’ conditions were highly responsive to levodopa; there was a 75.3% baseline improvement in Unified Parkinson’s Disease Rating Scale motor scores from the off-state to the on-state. In general, most DBS centers consider a levodopa response of approximately 30% as an acceptable cutoff for moving forward with STN-DBS, they noted.
Despite these caveats and limitations, the results of the study are important with respect to counseling potential candidates for DBS, Dr. Chou and Dr. Charles said.
“A common question that patients have is, ‘How long do the benefits of DBS last?’ We can now reassure them that, at least for STN-DBS, improvement in motor complications lasts beyond 15 years and is often accompanied by improvement in quality of life. In other words, with STN-DBS, we can uncomplicate their motor complications for the long haul,” the editorial writers concluded.
The research had no targeted funding. Moro has received honoraria from Medtronic and Abbott for consulting and lecturing and an educational grant from Boston and Newronika. A complete list of disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
, new research indicates.
“Subthalamic nucleus stimulation is a well recognized treatment used for improving motor conditions and quality of life in people with Parkinson’s disease. Our study, for the first time, supports its efficacy in the very long term – 15 years after surgery and 25 years since the Parkinson’s disease diagnosis,” said Elena Moro, MD, PhD, Grenoble Alpes University, Grenoble, France.
“This information is relevant for physicians, patients, and their families when they need to decide about the surgical option to deal with Parkinson’s disease,” said Dr. Moro.
The study was published online June 2 in Neurology.
‘Don’t delay’
The findings are based on 51 patients with Parkinson’s disease who underwent treatment with bilateral STN-DBS for an average of 17 years (range, 15-24 years). Their average age at diagnosis was 40 years, and the average age at device implantation was 51 years.
The results demonstrate that STN-DBS continues to be effective for motor complications for longer than 15 years, reducing time spent with dyskinesia by 75% and time spent in the off-state by 58.7%. This is similar to the amount of improvement seen 1 year after surgery.
Doses of dopaminergic medications continued to be low at long-term follow-up; dosing was reduced by 50.6% compared with baseline.
There was also continued improvement in quality of life. Scores on the Parkinson’s Disease Quality of Life Questionnaire in the very long term were 13.8% better compared with baseline.
“Few and mostly manageable device-related adverse events were observed during the follow-up,” the authors reported in their article.
“Deep brain stimulation is already recommended when a patient’s conditions are not optimized by medical treatment. Patients with Parkinson’s disease without dementia and in good general health conditions are the best candidates for this surgery,” said Dr. Moro.
“Taking into account our results and the data available in the literature, DBS surgery should not be delayed when motor conditions and quality of life decline despite medical treatment, if patients meet the inclusion criteria,” she added.
A revolutionary treatment
The authors of an accompanying editorial say these results, which indicate better motor outcomes with less medication, “reinforce why STN-DBS has revolutionized treatment for advanced Parkinson’s disease.”
Kelvin Chou, MD, of the University of Michigan, Ann Arbor, and David Charles, MD, of Vanderbilt University, Nashville, Tenn., pointed out that longer disease duration is associated with an increase in the likelihood of cognitive impairment and psychosis, both of which are risk factors for nursing home placement, and they limit the ability to use dopaminergic medications.
Although many of the patients in this cohort experienced hallucinations and psychosis over the long follow-up period, “one can imagine that the number and severity would be higher without DBS therapy,” they wrote.
Key caveats, said Dr. Chou and Dr. Charles, are that the results are based on a highly selected cohort and that the patients were managed by experts in the field of movement disorders and DBS.
Additionally, the patients’ conditions were highly responsive to levodopa; there was a 75.3% baseline improvement in Unified Parkinson’s Disease Rating Scale motor scores from the off-state to the on-state. In general, most DBS centers consider a levodopa response of approximately 30% as an acceptable cutoff for moving forward with STN-DBS, they noted.
Despite these caveats and limitations, the results of the study are important with respect to counseling potential candidates for DBS, Dr. Chou and Dr. Charles said.
“A common question that patients have is, ‘How long do the benefits of DBS last?’ We can now reassure them that, at least for STN-DBS, improvement in motor complications lasts beyond 15 years and is often accompanied by improvement in quality of life. In other words, with STN-DBS, we can uncomplicate their motor complications for the long haul,” the editorial writers concluded.
The research had no targeted funding. Moro has received honoraria from Medtronic and Abbott for consulting and lecturing and an educational grant from Boston and Newronika. A complete list of disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
, new research indicates.
“Subthalamic nucleus stimulation is a well recognized treatment used for improving motor conditions and quality of life in people with Parkinson’s disease. Our study, for the first time, supports its efficacy in the very long term – 15 years after surgery and 25 years since the Parkinson’s disease diagnosis,” said Elena Moro, MD, PhD, Grenoble Alpes University, Grenoble, France.
“This information is relevant for physicians, patients, and their families when they need to decide about the surgical option to deal with Parkinson’s disease,” said Dr. Moro.
The study was published online June 2 in Neurology.
‘Don’t delay’
The findings are based on 51 patients with Parkinson’s disease who underwent treatment with bilateral STN-DBS for an average of 17 years (range, 15-24 years). Their average age at diagnosis was 40 years, and the average age at device implantation was 51 years.
The results demonstrate that STN-DBS continues to be effective for motor complications for longer than 15 years, reducing time spent with dyskinesia by 75% and time spent in the off-state by 58.7%. This is similar to the amount of improvement seen 1 year after surgery.
Doses of dopaminergic medications continued to be low at long-term follow-up; dosing was reduced by 50.6% compared with baseline.
There was also continued improvement in quality of life. Scores on the Parkinson’s Disease Quality of Life Questionnaire in the very long term were 13.8% better compared with baseline.
“Few and mostly manageable device-related adverse events were observed during the follow-up,” the authors reported in their article.
“Deep brain stimulation is already recommended when a patient’s conditions are not optimized by medical treatment. Patients with Parkinson’s disease without dementia and in good general health conditions are the best candidates for this surgery,” said Dr. Moro.
“Taking into account our results and the data available in the literature, DBS surgery should not be delayed when motor conditions and quality of life decline despite medical treatment, if patients meet the inclusion criteria,” she added.
A revolutionary treatment
The authors of an accompanying editorial say these results, which indicate better motor outcomes with less medication, “reinforce why STN-DBS has revolutionized treatment for advanced Parkinson’s disease.”
Kelvin Chou, MD, of the University of Michigan, Ann Arbor, and David Charles, MD, of Vanderbilt University, Nashville, Tenn., pointed out that longer disease duration is associated with an increase in the likelihood of cognitive impairment and psychosis, both of which are risk factors for nursing home placement, and they limit the ability to use dopaminergic medications.
Although many of the patients in this cohort experienced hallucinations and psychosis over the long follow-up period, “one can imagine that the number and severity would be higher without DBS therapy,” they wrote.
Key caveats, said Dr. Chou and Dr. Charles, are that the results are based on a highly selected cohort and that the patients were managed by experts in the field of movement disorders and DBS.
Additionally, the patients’ conditions were highly responsive to levodopa; there was a 75.3% baseline improvement in Unified Parkinson’s Disease Rating Scale motor scores from the off-state to the on-state. In general, most DBS centers consider a levodopa response of approximately 30% as an acceptable cutoff for moving forward with STN-DBS, they noted.
Despite these caveats and limitations, the results of the study are important with respect to counseling potential candidates for DBS, Dr. Chou and Dr. Charles said.
“A common question that patients have is, ‘How long do the benefits of DBS last?’ We can now reassure them that, at least for STN-DBS, improvement in motor complications lasts beyond 15 years and is often accompanied by improvement in quality of life. In other words, with STN-DBS, we can uncomplicate their motor complications for the long haul,” the editorial writers concluded.
The research had no targeted funding. Moro has received honoraria from Medtronic and Abbott for consulting and lecturing and an educational grant from Boston and Newronika. A complete list of disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
From Neurology
ASCO 2021: Breast cancer sessions not to miss
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.

Hello. It’s Dr. Kathy Miller from Indiana University.
I have to admit that time has snuck up on me this year. It is already time for the American Society of Clinical Oncology Annual Meeting.
I found it hard to keep track of time this year with the pandemic. Many of the things that help mark the passage of time haven’t happened, have happened at different times of the year than is typical, or have happened in different ways that just haven’t had the same impact in my brain.
Just recently, I was taking a look through the breast cancer program at ASCO and there is a special clinical science symposium that I want to make sure you know about and tune into. It’s the sort of session that might not otherwise reach you.
This has been a year of incredible turmoil and critical thinking about issues of race, ethnicity, justice, and how we can make sure that the medical care we’re providing is inclusive and equitable. How we can make sure we are giving the best outcome to all of our patients.
This special clinical science symposium this year includes several presentations that will delve into how genetically determined ancestry and socially determined race might impact the outcome of our patients. This is a tangled web that is difficult to unpack and separate, but there are clear distinctions here: The genes we inherit do affect how we metabolize drugs, what side effects we might have from drugs, and what drugs might be the best choices for us.
Our socially determined race affects how the world interacts with us. Those biases, be they conscious or unconscious, can affect where we live, where we go to school, how people treat us, what opportunities we have, and how the medical system treats us. They’re related, but they’re not the same. Tune into that clinical science symposium to begin thinking about those differences and how we can make sure we give our patients the best care.
There are other high-profile presentations that you’re going to want to see as well, looking at how we can optimize therapy in patients with HER2-positive disease and beginning to think about who might not need chemotherapy to have an excellent outcome in early-stage disease.
Also, we will be thinking about those patients with triple-negative disease who have residual disease after neoadjuvant chemotherapy. We were all caught off guard with the results of the CREATE-X trial, quite frankly, several years ago.
This year we will hear the results of a postneoadjuvant trial coordinated by the Eastern Cooperative Oncology Group comparing platinum therapy with capecitabine. Tune in to think more about whether capecitabine really should be the standard of care in this population.
As always, I’m interested in your thoughts before or after ASCO. What stood out for you this year in breast cancer? Drop us a comment and let us know about these sessions and what else you found worthwhile.
Dr. Miller is associate director of clinical research and codirector of the breast cancer program at the Melvin and Bren Simon Cancer Center at Indiana University, Indianapolis. Her career has combined both laboratory and clinical research in breast cancer.
A version of this article first appeared on Medscape.com.




