User login
Severity of side effects key to choosing between lithium and quetiapine in bipolar I and II
HOLLYWOOD, FLA. – Because there is no clinically significant difference in bipolar spectrum symptom relief offered by either lithium or quetiapine, which mood stabilizer clinicians choose to prescribe should largely depend on what side effects patients can tolerate.
That’s the conclusion of a panel of experts who presented data from the Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) clinical trial at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
By randomly assigning the study’s 482 participating outpatient adults to either lithium or quetiapine, while allowing clinicians to prescribe adjunctive treatment as necessary; and by using a broader-than-usual set of inclusion criteria, the investigators hoped to provide clinicians with "real world" data on how the respective mood stabilizers perform.
"To my knowledge, this is the first randomized comparative effectiveness study of regular, expert care that looks at lithium versus quetiapine strategies of treatment, both with adjunctive personalized treatment when necessary," study coauthor Dr. Andrew A. Nierenberg told the audience in a well-attended session.
"We tortured the data to get a P value to pop out," said Dr. Nierenberg, who is the director of the Bipolar Clinic and Research Program at Massachusetts General Hospital in Boston. "The bottom line was that the treatment strategies, based on either lithium or quetiapine, were very similar. I think the clinical implication is that the choice of treatment really depends on someone’s tolerability. This may resurrect lithium as something [clinicians] should at least consider, because its use has gone down dramatically in the last 20 years or so."
Side effects such as metabolic or sleep disturbances were slightly less frequent in the lithium group, although the difference was not statistically significant; rates of suicidality and discontinuation of medication due to side effects were also similar across the two groups, according to Dr. David E. Kemp of the psychiatry department at Case Western Reserve University, Cleveland, and a study coauthor who also presented data during the session. Changes from baseline in mood stabilization were also virtually the same across the groups.
‘Rules of engagement’
The study enrolled adults aged 18-68 years who were at least mildly ill with either bipolar disorder I (68%) or II, with a Clinical Global Impression (CGI) score of at least 3, although the average CGI score was 4.5 (standard deviation of plus or minus 0.9). Of the 482 enrolled, 364 completed the study.
The so-called rules of engagement for clinicians from the 11 participating centers were that "they do everything they could to get their patients well," with the caveat that the lithium patient group not receive any antipsychotic, and the quetiapine patient group not receive lithium or any other antipsychotic.
"But they could get anything else that they needed," Dr. Nierenberg said.
At the time of enrollment, subjects were, in the estimation of their treating physician, experiencing symptoms that warranted a change in treatment, and that either lithium or quetiapine would be viable therapeutic options. Patients did not need to be lithium- or quetiapine-naive, but they could not have been treated with either drug in the previous 30 days. If they were already taking a second-generation antipsychotic, participants had to be willing to discontinue that medication and to be randomly assigned to either of the study drugs.
This strict adherence to one mood stabilizer, combined with adjunctive personalized treatments such as antidepressants or benzodiazepines, prescribed at the discretion of the treating clinician, ensured that the study tested the strategy of using either lithium or quetiapine as the base of treatment, Dr. Nierenberg said.
‘Similar’ results
Clinicians also were instructed to use the maximum tolerated dose of the respective mood stabilizer. For the 240 patients in the lithium group, the mean maximum tolerated dose was 1,007.5 mg, with the median dose being a "perfectly reasonable 900 mg," Dr. Nierenberg said. Blood lithium levels were taken at weeks 2, 16, and 24 and also were all found to be "reasonable," with most patients getting to at least 0.6 mEq/L by week 24.
For the 242 members of the quetiapine group, the maximum tolerated dose was 344.9 mg, with a median tolerated dose of 300 mg, which Dr. Nierenberg said was "what you would expect."
The overall result was that regardless of which mood stabilizer was prescribed along with adjunctive personalized treatment, the coprimary outcomes of the benefits and harms ratio from treatment effects over time (the CGI efficacy index), and the number of necessary clinical adjustments (changes in medication excluding titrations according to scale), were virtually parallel: For each primary outcome, the P value for the estimated change in baseline was less than .0001 for both lithium plus adjunctive treatment, and quetiapine plus adjunctive treatment.
Further, Dr. Nierenberg reported the "unexpected" result that at 6 months, about a quarter of each group was observed to be doing well without any adjunctive personalized treatments (23.8% of the lithium group; 27.3% of the quetiapine group; P = .14). "We thought that everybody would be on multiple things," Dr. Nierenberg said.
There was also no real difference in the time to discontinuation. "We were actually surprised that at 6 months, about three-quarters of the patients were still on the study drug, which was pretty good considering that this was a very ill population," Dr. Nierenberg said.
Using a CGI bipolar severity scale rating of up to 2, maintained for at least 8 weeks, the investigators found that overall, 20% were considered doing "really well"; it was also about 20% for the lithium plus adjunctive therapy group and the quetiapine and adjunctive therapy group (P = .14 for all three).
Because the Agency for Healthcare Research and Quality–funded study mandated that researchers end their investigation at precisely 36 months, Dr. Nierenberg said the study sites "randomized the 482 patients to 6 months of treatment within 36 months from start to finish," but he added that the investigators would have preferred to conduct a full 2-year study of participants.
"The good news was that most of the patients actually did improve substantially; the bad news is that while maybe a quarter did really well by the end of 6 months, we don’t know whether if we were to extend the study out further, we would see a difference between the two groups, whether they would continue to improve, or whether they would relapse," he said.
Adverse effects, predictors of response
Because study participants were followed for only 6 months, the long-term adverse effects of many years of exposure to either mood stabilizer, such as renal impairment with lithium or type II diabetes with quetiapine, were not considered, Dr. Kemp said. The adverse-effect profile for lithium generally includes a narrow therapeutic index; nausea, vomiting, and diarrhea; renal impairment; and hypothyroidism. Although Dr. Kemp said that the long-term adverse effects of quetiapine are understudied, known short-term side effects include the potential for sedation or somnolence, and weight gain.
Changes in baseline on the Frequency and Intensity of Side Effects Ratings for the quetiapine plus adjunctive personalized treatment arm were slightly more adverse than for the lithium and additional treatment arm. For the lithium plus adjunctive treatment group, the mean frequency of side effects was –1.41 (–1.78, –1.04 SD); the mean intensity of side effects was –1.48 (–1.80, –1.15 SD); and the mean level of impairment was –1.13 (–1.43, –0.82 SD). For the quetiapine and adjunctive treatment group, the mean frequency measure was –1.08 (–1.45, –0.72 SD); the intensity was –1.12 (–1.44, –0.79 SD); and the impairment was –0.77 (–1.07, –0.47 SD). For all scores in both groups, P was less than .001.
The changes from baseline on the Bipolar Index Severity Scale (BISS) overall for the quetiapine plus adjunctive treatment group was –28.56 (–10.91, –26.21 SD; P less than .0001). For the lithium plus adjunctive treatment group, it was –27.61 (–29.99, –25.24 SD; P less than .0001). The overall difference between the two groups was 0.94 (–2.10, 3.99 SD; P = .54).
Scores on the Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool also slightly favored lithium: –3.74 (–4.29, –3.19 SD) vs. –3.6 (–4.15, –3.07 SD) for quetiapine (P less than .0001 for all).
To the investigators’ surprise, among those with comorbid anxiety, the lithium plus adjunctive treatment group had fewer necessary clinical adjustments per month than did the quetiapine plus adjunctive treatment group, according to Dr. Nierenberg: –0.83 vs. 1.11 (P = .02).
"That seemed counterintuitive, and this difference was only with anxiety, not with any other comorbid psychiatric conditions," Dr. Nierenberg said. He hypothesized that it was possible benzodiazepines were used more frequently and easily with lithium than with quetiapine, but said future analyses would give a clearer answer. "We have a detailed database of every other medication used: when it was started, when it was stopped, and the reason why everything was done."
Not having current anxiety disorder was predictive of a better outcome (odds ratio, 1.81; P = .02), as was employment (OR, 1.67; P = .04). Those with bipolar II disorder responded better to treatment than those with bipolar disorder I, having an OR of response to treatment of nearly 1.8 P = .03). "That was a surprise to us, too," Dr. Nierenberg said.
Just over a quarter (27%) of the study population had metabolic syndrome, according to Dr. Kemp, who said this was not found to influence treatment outcomes.
Nearly half of the overall population was obese (48%), and slightly less than half (44%) had adipose. Prospective analysis indicated that patients with either obesity or adiposity tended to show less improvement in their CGI scores in a statistically significant way, regardless of which mood stabilizer they were on.
"Part of that might be due to overlapping pathophysiology between bipolar disorder and obesity," Dr. Kemp said. "Much of that is centered around inflammation in the central nervous system, as well as alterations in adipokines receptor levels and mitochondrial dysfunction."
"A key issue is to take care of obesity," said panel discussant Dr. Mauricio Tohen, chairman of psychiatry at the University of New Mexico in Albuquerque. "Regardless of the treatment, when there is obesity, the outcome will not be as good."
Efficacy vs. ‘generalizability’
Adjunctive personalized treatment was measured to help minimize the lack of assay sensitivity and internal validity typically inherent in comparative effectiveness trials, while also achieving more broadly applicable results.
"The right study depends on the right question," Dr. Tohen said. "If the question is whether a particular treatment has efficacy and is safe, then of course, we need an efficacy study. The problem with efficacy studies is that they limit the generalizability."
Instead, Dr. Tohen, who disclosed he spent more than a decade evaluating efficacy studies for both Eli Lily and AstraZeneca (makers of quetiapine), praised the metric of the adjunctive personalized treatments, and said a study should answer the question, "Of all the patients in my clinic, who will do better on which treatment? With efficacy studies you cannot answer that question because of the exclusion criteria that need to be taken into account."
In addition, Dr. Tohen said the metric likely would increase in importance under the Affordable Care Act, where efficacy is not the only consideration. "For example, if a patient relapses earlier, it might not be reimbursed, so asking what other outcomes we need to measure in [these kinds of] comparative effectiveness studies is very important."
In the CHOICE study, which had no placebo group, participating physicians were asked to track each patient’s dosage changes, missed doses, new medications added, discontinued medications, and the specific reasons for any of these changes. Changes that were made because of lack of effectiveness or intolerance were measured; however, planned dosage titrations according to normal scales were not considered necessary clinical adjustments but simply the regular course of treatment.
This heterogeneity of adjunctive treatment was seen by the investigators as a way to reflect "real world" practice, although they also noted it could be seen as a limitation to the study.
Another way the study was designed to reflect real-world practice was the inclusion of a broader-than-usual group of participants, and asking them for their feedback at the end of the study period.
"Before we put together these data, we held a stakeholder summit," Dr. Nierenberg said. "We invited patients and their advocates to help us interpret the study. They felt strongly that this was a reasonably positive study, because they thought that people really did get better. You could report these data toward the negative or the positive, but we reported toward the positive because that is what the stakeholders asked us to do." Dr. Tohen said that this kind of feedback in a comparative effectiveness study was another way these kinds of studies could help guide practice in the era of the Affordable Care Act.
As for who was actually admitted to the study, "exclusions were minimal," said Dr. Edward S. Friedman of the psychiatry department at the University of Pittsburgh. Those with a history of drug or alcohol dependence in the previous 30 days, a demonstrated intolerance to either study drug, or severe cardiovascular or renal disease were excluded, as were those with unstable thyroid disease, and pregnant or breastfeeding women.
Overall, the group was multiethnic, although nearly three-quarters were white and 20% were black. Roughly a third of the entire study population was employed, a third unemployed, and the rest were students, retirees, or those on disability.
The overall BISS score at enrollment averaged 56.1 (SD plus or minus 18.8). The average BISS depression score was 37.6 (SD plus or minus 14.0), and the average BISS mania score was 18.5 (SD plus or minus 12.1).
There were many comorbid psychiatric conditions, ranging from panic disorder to agoraphobia, although substance use was highest (61.4%). Current anxiety disorder also was prevalent at 58%.
"The CHOICE sample was similar in age and gender to previous efficacy studies," Dr. Friedman said. "It was more representative of the U.S. population than efficacy studies such as STEP-BD or LITMUS."
The 283 women (59% of the study) were more likely to report having spent a greater percentage of time depressed in the previous year, although the percentage of time spent in manic or hypomanic states was equal across both genders.
"This study was composed of sick individuals who had been sick for a considerable period of time," Dr. Friedman said.
For example, in the CHOICE study, 47% had been hospitalized for their bipolar disorder previously, vs. 43% in the LITMUS study. CHOICE participants had an attempted suicide rate of 36%, which fell between the 41% in the LITMUS trial and the 33.3% in an efficacy study conducted by Dr. Joseph R. Calabrese and his colleagues (J. Clin. Psychiatry 1999;60:79-88).
The average age for the first depressive episode for CHOICE participants was 16.4 years; the first manic episode tended to be at 20 years; and the first mood episode tended to be around 15.5 years. These data were similar to those in the LITMUS study, but differed from data in several efficacy studies such as the one by Dr. Calabrese, where the average age was 31.3 years.
"You might anticipate that in these specialty clinics we would have very few medication-naive patients, and yet we did," Dr. Friedman said during a postpanel audience participation session. "Looking for patients with a low threshold, we brought in patients who were very sick. They were also older, with lots of previous episodes. Maybe we missed younger patients who didn’t have as many previous episodes."
"These are the patients you would see in your practice," Dr. Nierenberg said before asking the audience what they think should be studied next in a comparative effectiveness trial. "We could look at the use of different antipsychotics, the combinations we use, with or without lamotrigine," he said.
"Most of the decisions that are made in medicine are made without evidence. Most of the things that are done are done without evidence. You have combinations that were never studied before or never even used before, and it’s across all medicine." Because medicine needs to be learning more, he said the audience needed to participate. "What are the questions that need to be asked?"
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Because there is no clinically significant difference in bipolar spectrum symptom relief offered by either lithium or quetiapine, which mood stabilizer clinicians choose to prescribe should largely depend on what side effects patients can tolerate.
That’s the conclusion of a panel of experts who presented data from the Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) clinical trial at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
By randomly assigning the study’s 482 participating outpatient adults to either lithium or quetiapine, while allowing clinicians to prescribe adjunctive treatment as necessary; and by using a broader-than-usual set of inclusion criteria, the investigators hoped to provide clinicians with "real world" data on how the respective mood stabilizers perform.
"To my knowledge, this is the first randomized comparative effectiveness study of regular, expert care that looks at lithium versus quetiapine strategies of treatment, both with adjunctive personalized treatment when necessary," study coauthor Dr. Andrew A. Nierenberg told the audience in a well-attended session.
"We tortured the data to get a P value to pop out," said Dr. Nierenberg, who is the director of the Bipolar Clinic and Research Program at Massachusetts General Hospital in Boston. "The bottom line was that the treatment strategies, based on either lithium or quetiapine, were very similar. I think the clinical implication is that the choice of treatment really depends on someone’s tolerability. This may resurrect lithium as something [clinicians] should at least consider, because its use has gone down dramatically in the last 20 years or so."
Side effects such as metabolic or sleep disturbances were slightly less frequent in the lithium group, although the difference was not statistically significant; rates of suicidality and discontinuation of medication due to side effects were also similar across the two groups, according to Dr. David E. Kemp of the psychiatry department at Case Western Reserve University, Cleveland, and a study coauthor who also presented data during the session. Changes from baseline in mood stabilization were also virtually the same across the groups.
‘Rules of engagement’
The study enrolled adults aged 18-68 years who were at least mildly ill with either bipolar disorder I (68%) or II, with a Clinical Global Impression (CGI) score of at least 3, although the average CGI score was 4.5 (standard deviation of plus or minus 0.9). Of the 482 enrolled, 364 completed the study.
The so-called rules of engagement for clinicians from the 11 participating centers were that "they do everything they could to get their patients well," with the caveat that the lithium patient group not receive any antipsychotic, and the quetiapine patient group not receive lithium or any other antipsychotic.
"But they could get anything else that they needed," Dr. Nierenberg said.
At the time of enrollment, subjects were, in the estimation of their treating physician, experiencing symptoms that warranted a change in treatment, and that either lithium or quetiapine would be viable therapeutic options. Patients did not need to be lithium- or quetiapine-naive, but they could not have been treated with either drug in the previous 30 days. If they were already taking a second-generation antipsychotic, participants had to be willing to discontinue that medication and to be randomly assigned to either of the study drugs.
This strict adherence to one mood stabilizer, combined with adjunctive personalized treatments such as antidepressants or benzodiazepines, prescribed at the discretion of the treating clinician, ensured that the study tested the strategy of using either lithium or quetiapine as the base of treatment, Dr. Nierenberg said.
‘Similar’ results
Clinicians also were instructed to use the maximum tolerated dose of the respective mood stabilizer. For the 240 patients in the lithium group, the mean maximum tolerated dose was 1,007.5 mg, with the median dose being a "perfectly reasonable 900 mg," Dr. Nierenberg said. Blood lithium levels were taken at weeks 2, 16, and 24 and also were all found to be "reasonable," with most patients getting to at least 0.6 mEq/L by week 24.
For the 242 members of the quetiapine group, the maximum tolerated dose was 344.9 mg, with a median tolerated dose of 300 mg, which Dr. Nierenberg said was "what you would expect."
The overall result was that regardless of which mood stabilizer was prescribed along with adjunctive personalized treatment, the coprimary outcomes of the benefits and harms ratio from treatment effects over time (the CGI efficacy index), and the number of necessary clinical adjustments (changes in medication excluding titrations according to scale), were virtually parallel: For each primary outcome, the P value for the estimated change in baseline was less than .0001 for both lithium plus adjunctive treatment, and quetiapine plus adjunctive treatment.
Further, Dr. Nierenberg reported the "unexpected" result that at 6 months, about a quarter of each group was observed to be doing well without any adjunctive personalized treatments (23.8% of the lithium group; 27.3% of the quetiapine group; P = .14). "We thought that everybody would be on multiple things," Dr. Nierenberg said.
There was also no real difference in the time to discontinuation. "We were actually surprised that at 6 months, about three-quarters of the patients were still on the study drug, which was pretty good considering that this was a very ill population," Dr. Nierenberg said.
Using a CGI bipolar severity scale rating of up to 2, maintained for at least 8 weeks, the investigators found that overall, 20% were considered doing "really well"; it was also about 20% for the lithium plus adjunctive therapy group and the quetiapine and adjunctive therapy group (P = .14 for all three).
Because the Agency for Healthcare Research and Quality–funded study mandated that researchers end their investigation at precisely 36 months, Dr. Nierenberg said the study sites "randomized the 482 patients to 6 months of treatment within 36 months from start to finish," but he added that the investigators would have preferred to conduct a full 2-year study of participants.
"The good news was that most of the patients actually did improve substantially; the bad news is that while maybe a quarter did really well by the end of 6 months, we don’t know whether if we were to extend the study out further, we would see a difference between the two groups, whether they would continue to improve, or whether they would relapse," he said.
Adverse effects, predictors of response
Because study participants were followed for only 6 months, the long-term adverse effects of many years of exposure to either mood stabilizer, such as renal impairment with lithium or type II diabetes with quetiapine, were not considered, Dr. Kemp said. The adverse-effect profile for lithium generally includes a narrow therapeutic index; nausea, vomiting, and diarrhea; renal impairment; and hypothyroidism. Although Dr. Kemp said that the long-term adverse effects of quetiapine are understudied, known short-term side effects include the potential for sedation or somnolence, and weight gain.
Changes in baseline on the Frequency and Intensity of Side Effects Ratings for the quetiapine plus adjunctive personalized treatment arm were slightly more adverse than for the lithium and additional treatment arm. For the lithium plus adjunctive treatment group, the mean frequency of side effects was –1.41 (–1.78, –1.04 SD); the mean intensity of side effects was –1.48 (–1.80, –1.15 SD); and the mean level of impairment was –1.13 (–1.43, –0.82 SD). For the quetiapine and adjunctive treatment group, the mean frequency measure was –1.08 (–1.45, –0.72 SD); the intensity was –1.12 (–1.44, –0.79 SD); and the impairment was –0.77 (–1.07, –0.47 SD). For all scores in both groups, P was less than .001.
The changes from baseline on the Bipolar Index Severity Scale (BISS) overall for the quetiapine plus adjunctive treatment group was –28.56 (–10.91, –26.21 SD; P less than .0001). For the lithium plus adjunctive treatment group, it was –27.61 (–29.99, –25.24 SD; P less than .0001). The overall difference between the two groups was 0.94 (–2.10, 3.99 SD; P = .54).
Scores on the Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool also slightly favored lithium: –3.74 (–4.29, –3.19 SD) vs. –3.6 (–4.15, –3.07 SD) for quetiapine (P less than .0001 for all).
To the investigators’ surprise, among those with comorbid anxiety, the lithium plus adjunctive treatment group had fewer necessary clinical adjustments per month than did the quetiapine plus adjunctive treatment group, according to Dr. Nierenberg: –0.83 vs. 1.11 (P = .02).
"That seemed counterintuitive, and this difference was only with anxiety, not with any other comorbid psychiatric conditions," Dr. Nierenberg said. He hypothesized that it was possible benzodiazepines were used more frequently and easily with lithium than with quetiapine, but said future analyses would give a clearer answer. "We have a detailed database of every other medication used: when it was started, when it was stopped, and the reason why everything was done."
Not having current anxiety disorder was predictive of a better outcome (odds ratio, 1.81; P = .02), as was employment (OR, 1.67; P = .04). Those with bipolar II disorder responded better to treatment than those with bipolar disorder I, having an OR of response to treatment of nearly 1.8 P = .03). "That was a surprise to us, too," Dr. Nierenberg said.
Just over a quarter (27%) of the study population had metabolic syndrome, according to Dr. Kemp, who said this was not found to influence treatment outcomes.
Nearly half of the overall population was obese (48%), and slightly less than half (44%) had adipose. Prospective analysis indicated that patients with either obesity or adiposity tended to show less improvement in their CGI scores in a statistically significant way, regardless of which mood stabilizer they were on.
"Part of that might be due to overlapping pathophysiology between bipolar disorder and obesity," Dr. Kemp said. "Much of that is centered around inflammation in the central nervous system, as well as alterations in adipokines receptor levels and mitochondrial dysfunction."
"A key issue is to take care of obesity," said panel discussant Dr. Mauricio Tohen, chairman of psychiatry at the University of New Mexico in Albuquerque. "Regardless of the treatment, when there is obesity, the outcome will not be as good."
Efficacy vs. ‘generalizability’
Adjunctive personalized treatment was measured to help minimize the lack of assay sensitivity and internal validity typically inherent in comparative effectiveness trials, while also achieving more broadly applicable results.
"The right study depends on the right question," Dr. Tohen said. "If the question is whether a particular treatment has efficacy and is safe, then of course, we need an efficacy study. The problem with efficacy studies is that they limit the generalizability."
Instead, Dr. Tohen, who disclosed he spent more than a decade evaluating efficacy studies for both Eli Lily and AstraZeneca (makers of quetiapine), praised the metric of the adjunctive personalized treatments, and said a study should answer the question, "Of all the patients in my clinic, who will do better on which treatment? With efficacy studies you cannot answer that question because of the exclusion criteria that need to be taken into account."
In addition, Dr. Tohen said the metric likely would increase in importance under the Affordable Care Act, where efficacy is not the only consideration. "For example, if a patient relapses earlier, it might not be reimbursed, so asking what other outcomes we need to measure in [these kinds of] comparative effectiveness studies is very important."
In the CHOICE study, which had no placebo group, participating physicians were asked to track each patient’s dosage changes, missed doses, new medications added, discontinued medications, and the specific reasons for any of these changes. Changes that were made because of lack of effectiveness or intolerance were measured; however, planned dosage titrations according to normal scales were not considered necessary clinical adjustments but simply the regular course of treatment.
This heterogeneity of adjunctive treatment was seen by the investigators as a way to reflect "real world" practice, although they also noted it could be seen as a limitation to the study.
Another way the study was designed to reflect real-world practice was the inclusion of a broader-than-usual group of participants, and asking them for their feedback at the end of the study period.
"Before we put together these data, we held a stakeholder summit," Dr. Nierenberg said. "We invited patients and their advocates to help us interpret the study. They felt strongly that this was a reasonably positive study, because they thought that people really did get better. You could report these data toward the negative or the positive, but we reported toward the positive because that is what the stakeholders asked us to do." Dr. Tohen said that this kind of feedback in a comparative effectiveness study was another way these kinds of studies could help guide practice in the era of the Affordable Care Act.
As for who was actually admitted to the study, "exclusions were minimal," said Dr. Edward S. Friedman of the psychiatry department at the University of Pittsburgh. Those with a history of drug or alcohol dependence in the previous 30 days, a demonstrated intolerance to either study drug, or severe cardiovascular or renal disease were excluded, as were those with unstable thyroid disease, and pregnant or breastfeeding women.
Overall, the group was multiethnic, although nearly three-quarters were white and 20% were black. Roughly a third of the entire study population was employed, a third unemployed, and the rest were students, retirees, or those on disability.
The overall BISS score at enrollment averaged 56.1 (SD plus or minus 18.8). The average BISS depression score was 37.6 (SD plus or minus 14.0), and the average BISS mania score was 18.5 (SD plus or minus 12.1).
There were many comorbid psychiatric conditions, ranging from panic disorder to agoraphobia, although substance use was highest (61.4%). Current anxiety disorder also was prevalent at 58%.
"The CHOICE sample was similar in age and gender to previous efficacy studies," Dr. Friedman said. "It was more representative of the U.S. population than efficacy studies such as STEP-BD or LITMUS."
The 283 women (59% of the study) were more likely to report having spent a greater percentage of time depressed in the previous year, although the percentage of time spent in manic or hypomanic states was equal across both genders.
"This study was composed of sick individuals who had been sick for a considerable period of time," Dr. Friedman said.
For example, in the CHOICE study, 47% had been hospitalized for their bipolar disorder previously, vs. 43% in the LITMUS study. CHOICE participants had an attempted suicide rate of 36%, which fell between the 41% in the LITMUS trial and the 33.3% in an efficacy study conducted by Dr. Joseph R. Calabrese and his colleagues (J. Clin. Psychiatry 1999;60:79-88).
The average age for the first depressive episode for CHOICE participants was 16.4 years; the first manic episode tended to be at 20 years; and the first mood episode tended to be around 15.5 years. These data were similar to those in the LITMUS study, but differed from data in several efficacy studies such as the one by Dr. Calabrese, where the average age was 31.3 years.
"You might anticipate that in these specialty clinics we would have very few medication-naive patients, and yet we did," Dr. Friedman said during a postpanel audience participation session. "Looking for patients with a low threshold, we brought in patients who were very sick. They were also older, with lots of previous episodes. Maybe we missed younger patients who didn’t have as many previous episodes."
"These are the patients you would see in your practice," Dr. Nierenberg said before asking the audience what they think should be studied next in a comparative effectiveness trial. "We could look at the use of different antipsychotics, the combinations we use, with or without lamotrigine," he said.
"Most of the decisions that are made in medicine are made without evidence. Most of the things that are done are done without evidence. You have combinations that were never studied before or never even used before, and it’s across all medicine." Because medicine needs to be learning more, he said the audience needed to participate. "What are the questions that need to be asked?"
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Because there is no clinically significant difference in bipolar spectrum symptom relief offered by either lithium or quetiapine, which mood stabilizer clinicians choose to prescribe should largely depend on what side effects patients can tolerate.
That’s the conclusion of a panel of experts who presented data from the Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) clinical trial at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
By randomly assigning the study’s 482 participating outpatient adults to either lithium or quetiapine, while allowing clinicians to prescribe adjunctive treatment as necessary; and by using a broader-than-usual set of inclusion criteria, the investigators hoped to provide clinicians with "real world" data on how the respective mood stabilizers perform.
"To my knowledge, this is the first randomized comparative effectiveness study of regular, expert care that looks at lithium versus quetiapine strategies of treatment, both with adjunctive personalized treatment when necessary," study coauthor Dr. Andrew A. Nierenberg told the audience in a well-attended session.
"We tortured the data to get a P value to pop out," said Dr. Nierenberg, who is the director of the Bipolar Clinic and Research Program at Massachusetts General Hospital in Boston. "The bottom line was that the treatment strategies, based on either lithium or quetiapine, were very similar. I think the clinical implication is that the choice of treatment really depends on someone’s tolerability. This may resurrect lithium as something [clinicians] should at least consider, because its use has gone down dramatically in the last 20 years or so."
Side effects such as metabolic or sleep disturbances were slightly less frequent in the lithium group, although the difference was not statistically significant; rates of suicidality and discontinuation of medication due to side effects were also similar across the two groups, according to Dr. David E. Kemp of the psychiatry department at Case Western Reserve University, Cleveland, and a study coauthor who also presented data during the session. Changes from baseline in mood stabilization were also virtually the same across the groups.
‘Rules of engagement’
The study enrolled adults aged 18-68 years who were at least mildly ill with either bipolar disorder I (68%) or II, with a Clinical Global Impression (CGI) score of at least 3, although the average CGI score was 4.5 (standard deviation of plus or minus 0.9). Of the 482 enrolled, 364 completed the study.
The so-called rules of engagement for clinicians from the 11 participating centers were that "they do everything they could to get their patients well," with the caveat that the lithium patient group not receive any antipsychotic, and the quetiapine patient group not receive lithium or any other antipsychotic.
"But they could get anything else that they needed," Dr. Nierenberg said.
At the time of enrollment, subjects were, in the estimation of their treating physician, experiencing symptoms that warranted a change in treatment, and that either lithium or quetiapine would be viable therapeutic options. Patients did not need to be lithium- or quetiapine-naive, but they could not have been treated with either drug in the previous 30 days. If they were already taking a second-generation antipsychotic, participants had to be willing to discontinue that medication and to be randomly assigned to either of the study drugs.
This strict adherence to one mood stabilizer, combined with adjunctive personalized treatments such as antidepressants or benzodiazepines, prescribed at the discretion of the treating clinician, ensured that the study tested the strategy of using either lithium or quetiapine as the base of treatment, Dr. Nierenberg said.
‘Similar’ results
Clinicians also were instructed to use the maximum tolerated dose of the respective mood stabilizer. For the 240 patients in the lithium group, the mean maximum tolerated dose was 1,007.5 mg, with the median dose being a "perfectly reasonable 900 mg," Dr. Nierenberg said. Blood lithium levels were taken at weeks 2, 16, and 24 and also were all found to be "reasonable," with most patients getting to at least 0.6 mEq/L by week 24.
For the 242 members of the quetiapine group, the maximum tolerated dose was 344.9 mg, with a median tolerated dose of 300 mg, which Dr. Nierenberg said was "what you would expect."
The overall result was that regardless of which mood stabilizer was prescribed along with adjunctive personalized treatment, the coprimary outcomes of the benefits and harms ratio from treatment effects over time (the CGI efficacy index), and the number of necessary clinical adjustments (changes in medication excluding titrations according to scale), were virtually parallel: For each primary outcome, the P value for the estimated change in baseline was less than .0001 for both lithium plus adjunctive treatment, and quetiapine plus adjunctive treatment.
Further, Dr. Nierenberg reported the "unexpected" result that at 6 months, about a quarter of each group was observed to be doing well without any adjunctive personalized treatments (23.8% of the lithium group; 27.3% of the quetiapine group; P = .14). "We thought that everybody would be on multiple things," Dr. Nierenberg said.
There was also no real difference in the time to discontinuation. "We were actually surprised that at 6 months, about three-quarters of the patients were still on the study drug, which was pretty good considering that this was a very ill population," Dr. Nierenberg said.
Using a CGI bipolar severity scale rating of up to 2, maintained for at least 8 weeks, the investigators found that overall, 20% were considered doing "really well"; it was also about 20% for the lithium plus adjunctive therapy group and the quetiapine and adjunctive therapy group (P = .14 for all three).
Because the Agency for Healthcare Research and Quality–funded study mandated that researchers end their investigation at precisely 36 months, Dr. Nierenberg said the study sites "randomized the 482 patients to 6 months of treatment within 36 months from start to finish," but he added that the investigators would have preferred to conduct a full 2-year study of participants.
"The good news was that most of the patients actually did improve substantially; the bad news is that while maybe a quarter did really well by the end of 6 months, we don’t know whether if we were to extend the study out further, we would see a difference between the two groups, whether they would continue to improve, or whether they would relapse," he said.
Adverse effects, predictors of response
Because study participants were followed for only 6 months, the long-term adverse effects of many years of exposure to either mood stabilizer, such as renal impairment with lithium or type II diabetes with quetiapine, were not considered, Dr. Kemp said. The adverse-effect profile for lithium generally includes a narrow therapeutic index; nausea, vomiting, and diarrhea; renal impairment; and hypothyroidism. Although Dr. Kemp said that the long-term adverse effects of quetiapine are understudied, known short-term side effects include the potential for sedation or somnolence, and weight gain.
Changes in baseline on the Frequency and Intensity of Side Effects Ratings for the quetiapine plus adjunctive personalized treatment arm were slightly more adverse than for the lithium and additional treatment arm. For the lithium plus adjunctive treatment group, the mean frequency of side effects was –1.41 (–1.78, –1.04 SD); the mean intensity of side effects was –1.48 (–1.80, –1.15 SD); and the mean level of impairment was –1.13 (–1.43, –0.82 SD). For the quetiapine and adjunctive treatment group, the mean frequency measure was –1.08 (–1.45, –0.72 SD); the intensity was –1.12 (–1.44, –0.79 SD); and the impairment was –0.77 (–1.07, –0.47 SD). For all scores in both groups, P was less than .001.
The changes from baseline on the Bipolar Index Severity Scale (BISS) overall for the quetiapine plus adjunctive treatment group was –28.56 (–10.91, –26.21 SD; P less than .0001). For the lithium plus adjunctive treatment group, it was –27.61 (–29.99, –25.24 SD; P less than .0001). The overall difference between the two groups was 0.94 (–2.10, 3.99 SD; P = .54).
Scores on the Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool also slightly favored lithium: –3.74 (–4.29, –3.19 SD) vs. –3.6 (–4.15, –3.07 SD) for quetiapine (P less than .0001 for all).
To the investigators’ surprise, among those with comorbid anxiety, the lithium plus adjunctive treatment group had fewer necessary clinical adjustments per month than did the quetiapine plus adjunctive treatment group, according to Dr. Nierenberg: –0.83 vs. 1.11 (P = .02).
"That seemed counterintuitive, and this difference was only with anxiety, not with any other comorbid psychiatric conditions," Dr. Nierenberg said. He hypothesized that it was possible benzodiazepines were used more frequently and easily with lithium than with quetiapine, but said future analyses would give a clearer answer. "We have a detailed database of every other medication used: when it was started, when it was stopped, and the reason why everything was done."
Not having current anxiety disorder was predictive of a better outcome (odds ratio, 1.81; P = .02), as was employment (OR, 1.67; P = .04). Those with bipolar II disorder responded better to treatment than those with bipolar disorder I, having an OR of response to treatment of nearly 1.8 P = .03). "That was a surprise to us, too," Dr. Nierenberg said.
Just over a quarter (27%) of the study population had metabolic syndrome, according to Dr. Kemp, who said this was not found to influence treatment outcomes.
Nearly half of the overall population was obese (48%), and slightly less than half (44%) had adipose. Prospective analysis indicated that patients with either obesity or adiposity tended to show less improvement in their CGI scores in a statistically significant way, regardless of which mood stabilizer they were on.
"Part of that might be due to overlapping pathophysiology between bipolar disorder and obesity," Dr. Kemp said. "Much of that is centered around inflammation in the central nervous system, as well as alterations in adipokines receptor levels and mitochondrial dysfunction."
"A key issue is to take care of obesity," said panel discussant Dr. Mauricio Tohen, chairman of psychiatry at the University of New Mexico in Albuquerque. "Regardless of the treatment, when there is obesity, the outcome will not be as good."
Efficacy vs. ‘generalizability’
Adjunctive personalized treatment was measured to help minimize the lack of assay sensitivity and internal validity typically inherent in comparative effectiveness trials, while also achieving more broadly applicable results.
"The right study depends on the right question," Dr. Tohen said. "If the question is whether a particular treatment has efficacy and is safe, then of course, we need an efficacy study. The problem with efficacy studies is that they limit the generalizability."
Instead, Dr. Tohen, who disclosed he spent more than a decade evaluating efficacy studies for both Eli Lily and AstraZeneca (makers of quetiapine), praised the metric of the adjunctive personalized treatments, and said a study should answer the question, "Of all the patients in my clinic, who will do better on which treatment? With efficacy studies you cannot answer that question because of the exclusion criteria that need to be taken into account."
In addition, Dr. Tohen said the metric likely would increase in importance under the Affordable Care Act, where efficacy is not the only consideration. "For example, if a patient relapses earlier, it might not be reimbursed, so asking what other outcomes we need to measure in [these kinds of] comparative effectiveness studies is very important."
In the CHOICE study, which had no placebo group, participating physicians were asked to track each patient’s dosage changes, missed doses, new medications added, discontinued medications, and the specific reasons for any of these changes. Changes that were made because of lack of effectiveness or intolerance were measured; however, planned dosage titrations according to normal scales were not considered necessary clinical adjustments but simply the regular course of treatment.
This heterogeneity of adjunctive treatment was seen by the investigators as a way to reflect "real world" practice, although they also noted it could be seen as a limitation to the study.
Another way the study was designed to reflect real-world practice was the inclusion of a broader-than-usual group of participants, and asking them for their feedback at the end of the study period.
"Before we put together these data, we held a stakeholder summit," Dr. Nierenberg said. "We invited patients and their advocates to help us interpret the study. They felt strongly that this was a reasonably positive study, because they thought that people really did get better. You could report these data toward the negative or the positive, but we reported toward the positive because that is what the stakeholders asked us to do." Dr. Tohen said that this kind of feedback in a comparative effectiveness study was another way these kinds of studies could help guide practice in the era of the Affordable Care Act.
As for who was actually admitted to the study, "exclusions were minimal," said Dr. Edward S. Friedman of the psychiatry department at the University of Pittsburgh. Those with a history of drug or alcohol dependence in the previous 30 days, a demonstrated intolerance to either study drug, or severe cardiovascular or renal disease were excluded, as were those with unstable thyroid disease, and pregnant or breastfeeding women.
Overall, the group was multiethnic, although nearly three-quarters were white and 20% were black. Roughly a third of the entire study population was employed, a third unemployed, and the rest were students, retirees, or those on disability.
The overall BISS score at enrollment averaged 56.1 (SD plus or minus 18.8). The average BISS depression score was 37.6 (SD plus or minus 14.0), and the average BISS mania score was 18.5 (SD plus or minus 12.1).
There were many comorbid psychiatric conditions, ranging from panic disorder to agoraphobia, although substance use was highest (61.4%). Current anxiety disorder also was prevalent at 58%.
"The CHOICE sample was similar in age and gender to previous efficacy studies," Dr. Friedman said. "It was more representative of the U.S. population than efficacy studies such as STEP-BD or LITMUS."
The 283 women (59% of the study) were more likely to report having spent a greater percentage of time depressed in the previous year, although the percentage of time spent in manic or hypomanic states was equal across both genders.
"This study was composed of sick individuals who had been sick for a considerable period of time," Dr. Friedman said.
For example, in the CHOICE study, 47% had been hospitalized for their bipolar disorder previously, vs. 43% in the LITMUS study. CHOICE participants had an attempted suicide rate of 36%, which fell between the 41% in the LITMUS trial and the 33.3% in an efficacy study conducted by Dr. Joseph R. Calabrese and his colleagues (J. Clin. Psychiatry 1999;60:79-88).
The average age for the first depressive episode for CHOICE participants was 16.4 years; the first manic episode tended to be at 20 years; and the first mood episode tended to be around 15.5 years. These data were similar to those in the LITMUS study, but differed from data in several efficacy studies such as the one by Dr. Calabrese, where the average age was 31.3 years.
"You might anticipate that in these specialty clinics we would have very few medication-naive patients, and yet we did," Dr. Friedman said during a postpanel audience participation session. "Looking for patients with a low threshold, we brought in patients who were very sick. They were also older, with lots of previous episodes. Maybe we missed younger patients who didn’t have as many previous episodes."
"These are the patients you would see in your practice," Dr. Nierenberg said before asking the audience what they think should be studied next in a comparative effectiveness trial. "We could look at the use of different antipsychotics, the combinations we use, with or without lamotrigine," he said.
"Most of the decisions that are made in medicine are made without evidence. Most of the things that are done are done without evidence. You have combinations that were never studied before or never even used before, and it’s across all medicine." Because medicine needs to be learning more, he said the audience needed to participate. "What are the questions that need to be asked?"
On Twitter @whitneymcknight
AT THE ASCP ANNUAL MEETING
Key clinical point: Determining whether to use lithium or quetiapine in bipolar I and II comes down to side effects.
Major finding: No clinically significant difference was found for bipolar I and bipolar II symptom improvements in patients given lithium or quetiapine (P less than .0001).
Data source: Multicenter, randomized comparative effectiveness study of 482 outpatients on the bipolar spectrum, followed for 6 months as they were given either lithium plus adjunctive therapy, quetiapine plus adjunctive therapy, or monotherapy of either mood stabilizer.
Disclosures: The Agency for Healthcare Research and Quality funded this study in its entirety.
Gold and nickel lead list of eyelid irritants
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Chances are good that if your patient presents with eyelid dermatitis, allergic contact with gold or nickel is the culprit.
"Gold is not thought to be easily released from jewelry, which would be the typical exposure with eyelids, unless it comes into contact with sweat, friction, or abrasives," Dr. Amber Reck Atwater told attendees of a session on facial dermatoses at the American Academy of Dermatology summer meeting.
However, when gold comes into contact with titanium dioxide, a common active ingredient in many cosmetics such as eye shadow, patients are at risk for eyelid irritation.
"If I am wearing a gold ring, and I put this on my eyelids using my finger, the gold will be more easily released, and I will be more likely to get a reaction on my lids," said Dr. Atwater, of the department of dermatology at Duke University, Durham, N.C.
Nickel is another leading cause of eyelid dermatitis, Dr. Atwater said. She warned of the metal’s pernicious tendency to hide in personal care products such as eyelash curlers that do not list it as an active or inactive ingredient.
A simple and relatively inexpensive dimethylglyoxime test, which Dr. Atwater said can be purchased on the consumer market, can help identify items that may contain nickel. Rub a drop of dimethylglyoxime onto an item, such as a house key, with a cotton swab. If the key turns bright pink, then you know it has nickel in it.
"So you can imagine that if I am holding my keys in my hands, I could be transferring nickel from the keys to my eyelids," said Dr. Atwater.
Other potential sources of nickel include faucets, sunglasses with metal frames, barbells, and other weight-lifting equipment.
Other common causes of eyelid dermatitis are products that contain fragrance, including balsam of Peru, neomycin (typically found in antibacterial eye drops), formaldehyde and bronopol (preservatives that are found in certain cosmetics), skin care products, and topicals.
Allergic contact dermatitis in the eyelid can present in an upper, lower, unilateral, or bilateral fashion on the eyelids alone, but typically presents in combination with dermatitis on other areas of the face, or even other areas of the body, according to Dr. Atwater.
"You should be highly suspicious that it’s contact dermatitis when you see eyelid dermatitis with other parts of the body involved," she said.
When the dermatitis presents in the eyelids alone, other factors such as seborrheic dermatitis or aspecific xerotic dermatitis could be the cause.
"Still, a good 30%-50% of our patients will have allergic contact dermatitis when we see them with eyelid dermatitis alone," Dr. Atwater said.
The eyelids are particularly susceptible to irritation in part because the skin is extremely thin – 0.55 mm – compared with other facial areas where the average skin thickness is about 2 mm, Dr. Atwater explained.
"And it’s really easy to transfer substances from our hands to our eyes. People rub their eyes and their faces a lot throughout the day," she noted.
Dr. Atwater had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
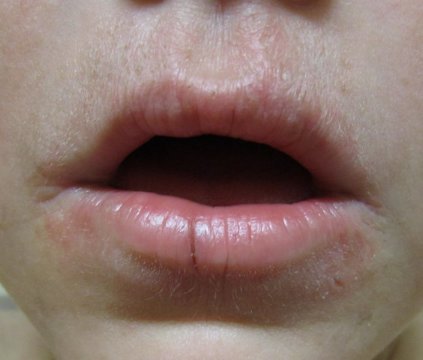
Consider a zero therapy approach to periorificial dermatitis
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – For periorificial dermatitis, don’t do a thing. That was the first-line therapy suggested at this year’s American Academy of Dermatology summer meeting.
"Sometimes you have to be brave to tell a patient you’re sending them home with nothing," said Dr. Sarah Wolfe of Duke University in Durham, N.C. "But ‘zero’ therapy is important. It’s simply counseling your patient to recognize the disease and avoid anything that could be exacerbating or precipitating it."
In theory, this advice includes abstinence from using any cosmetics for about 2 months, but "I am not sure whom that will work for," said Dr. Wolfe.
Periorificial dermatitis can affect anyone, but typically occurs in women aged 20-45 years across all ethnicities; it generally presents as erythematous pustules up to 2 mm in size near the nose and mouth, and patients report a burning sensation.
In children, the condition occurs equally in both genders, and tends to peak by age 5 years. Some children develop a distinctive form of the disease – childhood granulomatous periorificial dermatitis – that features persistent, inflammatory papules and pustules that appear symmetrically around the mouth. Although this form tends to be harder to treat and lasts longer, reassure patients that the condition will heal without scarring, Dr. Wolfe said.
Role of steroids
The minimalist approach to treating periorificial dermatitis is particularly important if the condition is related to steroid use, Dr. Wolfe said. Although the prevailing wisdom has been that corticosteroids caused periorificial dermatitis, enough evidence exists to show that corticosteroids likely exacerbate, rather than cause the problem, she explained.
Sometimes patients will use topical steroids (often medications prescribed for other family members or for other ailments) to help calm the burning, unaware of steroids’ potential to make their symptoms worse, she added.
If steroids are implicated, prepare your patient for the possibility of a steroid rebound that lasts a couple of weeks once they stop using the topicals.
"You have to either counsel them through it, or consider using a lower-potency steroid such as a hydrocortisone 2.5% or 1% to get them through that period," Dr. Wolfe said.
Inhaled steroids also have been associated with periorificial dermatitis, occurring where the inhalant mask touches the skin and lips. Intranasal steroids, particularly mometasone furoate and fluticasone furoate, have similarly been associated with the condition in cases of pustules starting near the nose and then spreading to the mouth and chin (Allergy 2001;56:944-8), (Cutan. Ocul. Toxicol. 2012;31:160-3).
Systemic steroids such as prednisone have been associated with the development of periorificial dermatitis upon tapering them, although this association has not been well studied. Because of the risk for the unintentional transfer of topical steroids to the face from other parts of the body being treated, counsel patients to be scrupulous about handwashing after applying their medications, Dr. Wolf emphasized.
Similarly, unintentional corticosteroid use can occur with the use of over the counter skin-lightening products marketed as "herbal" or "all natural," she said.
Oral, topical options
If you and your patient prefer to actively treat the condition, Dr. Wolfe recommended using the most evidence-based therapy available: Oral tetracycline 250-500 mg twice per day, a therapy which showed clearance in 4 to 8 weeks when compared to placebo (G. Ital. Dermatol. Venereol. 2010 Aug;145:433-44).
She did not recommend using this therapy in children younger than 8 years, because of the drug’s association with dental abnormalities. Other antibiotics such as doxycycline, minocycline, and azithromycin (in children) have not been well studied, Dr. Wolfe said, although she noted anecdotal reports of pediatric dermatologists prescribing azithromycin if the patient’s presentation is "slightly disfiguring."
Although erythromycin may be easier to use when controlling costs, both it and pimecrolimus are viable options for topical therapy, Dr. Wolfe said. Pimecrolimus 1% cream has been shown to reduce disease severity in 2 weeks, while erythromycin 2% solution, in ointment or gel form twice daily, brought clearance in 3-7 weeks in a randomized, placebo-controlled trial. She emphasized the importance of discussing all topical exposures with patients, regardless of the treatment option chosen.
Dr. Wolfe had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
VIDEO: Stress and inflammatory skin diseases – Does the science prove a link?
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
CHICAGO – The data are still being developed, but evidence of a direct link between stress and inflammatory skin conditions continues to mount.
The research is especially compelling for psoriasis; experimental data suggest that stress triggers the nerves to release elevated levels of neuropeptides and neurotransmitters, which in turn affect the nervous system.
Dr. Richard Granstein, chairman of the dermatology department at Weill Cornell Medical College in New York, gave an exclusive interview to Frontline Medical News in which he described studies of how calming the nerves clearly interrupts psoriatic and other inflammatory skin conditions. Dr. Granstein discussed the implications of this new, and still controversial, line of research, and what this means for clinicians: Should they prescribe stress management programs to their patients with these skin conditions?
Dr. Granstein disclosed he has financial relationships with Velius and Clinique.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
AUDIO: Unusual approaches to unusual tumors
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
CHICAGO – Doctors who don’t abide by accepted norms can sometimes achieve better outcomes when treating patients who have unusual and tenacious tumors. This was the theme of a session covering cases of confounding tumors at the American Academy of Dermatology summer meeting.
In an interview after the session, panel moderator and case presenter Dr. John A. Carucci, chief of Mohs and dermatologic surgery at New York (N.Y.) University, shared his thoughts on when certain kinds of imaging are more appropriate than others, even if it’s not the "usual way."
Dr. Carucci also addressed the use of postsurgical negative pressure wound therapy, radiation therapy in conjunction with Mohs surgery, and potentially controversial topics such as the use of a protein kinase inhibitor as a neoadjuvant therapy.
And what new information on staging squamous cell carcinomas has Dr. Carucci and his colleagues excited? Listen and find out. Dr. Carucci said that he had no financial conflicts to disclose.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
AUDIO: An interview with Dr. Thomas Insel – Part I
HOLLYWOOD, FLA. – Imagine if you’d gone to medical school with the intention of becoming a psychiatrist, only to be told that part of your core curriculum would include a course on biomaterials engineering or nanoscience.
If all goes according to the current plan of President Barack Obama’s BRAIN Initiative (Brain Research through Advancing Innovative Neurotechnologies), announced in April 2013, these disciplines, and many other so-called nontraditional medical sciences, will be foundational to understanding the human mind and brain.
It’s a development welcomed by the director of the National Institute of Mental Health, Dr. Thomas Insel, who says that neuropsychiatry lacks the necessary tools to identify, treat, and prevent mental and neurologic illnesses.
We spoke with Dr. Insel after the plenary session of this year’s American Society of Clinical Psychopharmacology annual meeting, formerly known as the New Clinical Drug Evaluation Unit meeting. We discussed how the BRAIN Initiative could change practice for psychiatrists and other mental health professionals. This is part I of that conversation.
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Imagine if you’d gone to medical school with the intention of becoming a psychiatrist, only to be told that part of your core curriculum would include a course on biomaterials engineering or nanoscience.
If all goes according to the current plan of President Barack Obama’s BRAIN Initiative (Brain Research through Advancing Innovative Neurotechnologies), announced in April 2013, these disciplines, and many other so-called nontraditional medical sciences, will be foundational to understanding the human mind and brain.
It’s a development welcomed by the director of the National Institute of Mental Health, Dr. Thomas Insel, who says that neuropsychiatry lacks the necessary tools to identify, treat, and prevent mental and neurologic illnesses.
We spoke with Dr. Insel after the plenary session of this year’s American Society of Clinical Psychopharmacology annual meeting, formerly known as the New Clinical Drug Evaluation Unit meeting. We discussed how the BRAIN Initiative could change practice for psychiatrists and other mental health professionals. This is part I of that conversation.
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Imagine if you’d gone to medical school with the intention of becoming a psychiatrist, only to be told that part of your core curriculum would include a course on biomaterials engineering or nanoscience.
If all goes according to the current plan of President Barack Obama’s BRAIN Initiative (Brain Research through Advancing Innovative Neurotechnologies), announced in April 2013, these disciplines, and many other so-called nontraditional medical sciences, will be foundational to understanding the human mind and brain.
It’s a development welcomed by the director of the National Institute of Mental Health, Dr. Thomas Insel, who says that neuropsychiatry lacks the necessary tools to identify, treat, and prevent mental and neurologic illnesses.
We spoke with Dr. Insel after the plenary session of this year’s American Society of Clinical Psychopharmacology annual meeting, formerly known as the New Clinical Drug Evaluation Unit meeting. We discussed how the BRAIN Initiative could change practice for psychiatrists and other mental health professionals. This is part I of that conversation.
On Twitter @whitneymcknight
VIDEO: Biogels boost targeted delivery of growth factor in severe wounds
DANA POINT, CALIF.– Materials science meets medicine. That’s the new frontier for many specialties, thanks to advances in the understanding of native tissue dynamics and how bioengineered materials will perform in vivo.
Sarah Heilshorn, Ph.D., of the department of materials science and engineering and the department of bioengineering at Stanford (Calif.) University, was one of several presenters at this year’s Summit in Aesthetic Medicine, with a focus on the use of synthetic materials for reconstructive surgery and treatment for acute and traumatic tissue injury.
In this video, Dr. Heilshorn explains how materials science and bioengineering are promoting angiogenesis to help patients who have suffered spinal injuries, chronic wounds, and conditions involving severe tissue injury at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications. She describes how revascularization, bone regeneration, and tissue growth are all possible through the use of highly specific and targeted biogels injected into the body to improve delivery of growth factors.
Dr. Heilshorn said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
DANA POINT, CALIF.– Materials science meets medicine. That’s the new frontier for many specialties, thanks to advances in the understanding of native tissue dynamics and how bioengineered materials will perform in vivo.
Sarah Heilshorn, Ph.D., of the department of materials science and engineering and the department of bioengineering at Stanford (Calif.) University, was one of several presenters at this year’s Summit in Aesthetic Medicine, with a focus on the use of synthetic materials for reconstructive surgery and treatment for acute and traumatic tissue injury.
In this video, Dr. Heilshorn explains how materials science and bioengineering are promoting angiogenesis to help patients who have suffered spinal injuries, chronic wounds, and conditions involving severe tissue injury at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications. She describes how revascularization, bone regeneration, and tissue growth are all possible through the use of highly specific and targeted biogels injected into the body to improve delivery of growth factors.
Dr. Heilshorn said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
DANA POINT, CALIF.– Materials science meets medicine. That’s the new frontier for many specialties, thanks to advances in the understanding of native tissue dynamics and how bioengineered materials will perform in vivo.
Sarah Heilshorn, Ph.D., of the department of materials science and engineering and the department of bioengineering at Stanford (Calif.) University, was one of several presenters at this year’s Summit in Aesthetic Medicine, with a focus on the use of synthetic materials for reconstructive surgery and treatment for acute and traumatic tissue injury.
In this video, Dr. Heilshorn explains how materials science and bioengineering are promoting angiogenesis to help patients who have suffered spinal injuries, chronic wounds, and conditions involving severe tissue injury at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications. She describes how revascularization, bone regeneration, and tissue growth are all possible through the use of highly specific and targeted biogels injected into the body to improve delivery of growth factors.
Dr. Heilshorn said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE 2014
VIDEO: Lasers plus angiogenesis inhibitors equal potential for treating port wine stains
DANA POINT, CALIF. – Although lasers are standard in the treatment of port wine stains, they don’t give full clearance or prevent recurrence, according to Dr. Kristen M. Kelly of the University of California, Irvine.
Using imaging, Dr. Kelly and her team observed that lasers provided patients with "very nice" destruction of blood vessels, but in as little as a week, revascularization would begin. The investigators sought a way to interfere with angiogenesis in order to extend clearance of the port wine stains.
In this video, recorded during the Summit in Aesthetic Medicine 2014, Dr. Kelly says that she doesn’t think "we have the optimal treatment yet." However, a proof of concept study she and others conducted that combined laser treatment with Food and Drug Administration–approved agents, such as imiquimod and rapamycin, showed "a slight improvement over lasers alone."
Dr. Kelly added that the current research is only a "first step," and that other agents will prove equally or more effective as antiangiogenesis gains recognition as a viable treatment for a range of cutaneous conditions.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Kelly said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
DANA POINT, CALIF. – Although lasers are standard in the treatment of port wine stains, they don’t give full clearance or prevent recurrence, according to Dr. Kristen M. Kelly of the University of California, Irvine.
Using imaging, Dr. Kelly and her team observed that lasers provided patients with "very nice" destruction of blood vessels, but in as little as a week, revascularization would begin. The investigators sought a way to interfere with angiogenesis in order to extend clearance of the port wine stains.
In this video, recorded during the Summit in Aesthetic Medicine 2014, Dr. Kelly says that she doesn’t think "we have the optimal treatment yet." However, a proof of concept study she and others conducted that combined laser treatment with Food and Drug Administration–approved agents, such as imiquimod and rapamycin, showed "a slight improvement over lasers alone."
Dr. Kelly added that the current research is only a "first step," and that other agents will prove equally or more effective as antiangiogenesis gains recognition as a viable treatment for a range of cutaneous conditions.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Kelly said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
DANA POINT, CALIF. – Although lasers are standard in the treatment of port wine stains, they don’t give full clearance or prevent recurrence, according to Dr. Kristen M. Kelly of the University of California, Irvine.
Using imaging, Dr. Kelly and her team observed that lasers provided patients with "very nice" destruction of blood vessels, but in as little as a week, revascularization would begin. The investigators sought a way to interfere with angiogenesis in order to extend clearance of the port wine stains.
In this video, recorded during the Summit in Aesthetic Medicine 2014, Dr. Kelly says that she doesn’t think "we have the optimal treatment yet." However, a proof of concept study she and others conducted that combined laser treatment with Food and Drug Administration–approved agents, such as imiquimod and rapamycin, showed "a slight improvement over lasers alone."
Dr. Kelly added that the current research is only a "first step," and that other agents will prove equally or more effective as antiangiogenesis gains recognition as a viable treatment for a range of cutaneous conditions.
The Summit in Aesthetic Medicine is held by the Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Kelly said she had no relevant financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM SUMMIT IN AESTHETIC MEDICINE
ADHD drug works by stimulating brain’s motivation-reward system
HOLLYWOOD, FLA. – Activity in the regions of the brain associated with motivation and reward correlated with clinical improvements in attention-deficit/hyperactivity disorder symptoms in adults given lisdexamfetamine dimesylate, the results of an imaging study have shown.
"What we saw is that Vyvanse (lisdexamfetamine dimesylate) increases activity in the caudate and anterior cingulate, which then seems to show that the medication increases sensitivity to reward," Stephanie Duhoux, Ph.D., said in an interview about her poster presentation at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
"You could say that when they are taking this medication, these patients don’t need as much reward in order to get them to engage in an activity," said Dr. Duhoux of Icahn School of Medicine at Mount Sinai, New York, who was a new investigator award winner at this year’s ASCP meeting.
Building upon recent findings by Dr. Nora D. Volkow, director of the National Institute on Drug Abuse and her colleagues (Mol. Psychiatry 2011;16:1147-54) indicating that a dopaminergic dysfunction in the brain’s motivation-reward system might contribute to ADHD, Dr. Duhoux and her colleagues used imaging to study changes in the brains of 20 adults with ADHD after being treated with lisdexamfetamine dimesylate. Study participants, 11 of whom were men, ranged in age from 19 to 52 years. Lisdexamfetamine dimesylate, a class II stimulant, was approved in 2012 by the Food and Drug Administration for adult ADHD.
In the randomized, placebo-controlled, crossover study, each participant received the active drug for at least 4 weeks in an escalating stepped dose titration (30, 50, 70 mg). The participants were scanned twice; once after taking the placebo and also after at least 2 weeks of taking their best tolerated dose of the active drug.
The scans were done while the subject performed a passive-avoidance learning task where participants learned to associate whether their response to a specific image corresponded with a high chance of either winning or losing money. The participants were asked to respond when they thought they were going to win and avoid responding when they thought they were going to lose. The scans were intended to capture the effect of the drug on components of the motivation/reward circuitry and its impact on decision-making processes.
Each participant’s blood oxygen level–dependent (BOLD) signals were modeled according to the moment they chose or refused to respond to an image, and when they received the resulting feedback as to whether they’d won or lost money. Decision-related activity was modulated by the expected value that the participant had for the image, and the feedback processing was modulated by the difference between the result they had expected from their choice and the result that actually occurred (the prediction error).
For the scans, whole-brain analyses were used (P less than .005); the extent threshold was defined via Monte Carlo simulation. A small-volume correction was performed on the ventral striatum with a P value FWE (family-wise error) corrected of less than .05.
To examine the relationship between each participant’s regional BOLD signals during the imaging and their respective clinician ratings on the ADHD Rating Scale for adults, Dr. Duhoux and her colleagues used regression analyses and found that when compared to placebo, when the participant chose not to respond to an image, the stimulant increased the modulation of BOLD responses by the expected value of the image in the dorsolateral prefrontal cortex.
The drug also was associated with increased BOLD response in the ventral striatum when a reward was received. This response was modulated by the prediction error in the ventromedial prefrontal cortex. Increased activation in the ventral striatum and ventromedial prefrontal cortex with reward was positively correlated with greater improvement in ADHD Rating Scale scores.
These results suggest that "the medication helps increase the value of the stimulus and the person’s perception of the stimulus and also reinforces the ability to avoid responding to stimuli considered to be less rewarding," Dr. Duhoux said. "Practically speaking, this is associated with clinical improvements and makes patients more sensitive to an activity, so they engage more easily."
Dr. Duhoux said she had no relevant disclosures. This study was funded by Shire, maker of Vyvanse.
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Activity in the regions of the brain associated with motivation and reward correlated with clinical improvements in attention-deficit/hyperactivity disorder symptoms in adults given lisdexamfetamine dimesylate, the results of an imaging study have shown.
"What we saw is that Vyvanse (lisdexamfetamine dimesylate) increases activity in the caudate and anterior cingulate, which then seems to show that the medication increases sensitivity to reward," Stephanie Duhoux, Ph.D., said in an interview about her poster presentation at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
"You could say that when they are taking this medication, these patients don’t need as much reward in order to get them to engage in an activity," said Dr. Duhoux of Icahn School of Medicine at Mount Sinai, New York, who was a new investigator award winner at this year’s ASCP meeting.
Building upon recent findings by Dr. Nora D. Volkow, director of the National Institute on Drug Abuse and her colleagues (Mol. Psychiatry 2011;16:1147-54) indicating that a dopaminergic dysfunction in the brain’s motivation-reward system might contribute to ADHD, Dr. Duhoux and her colleagues used imaging to study changes in the brains of 20 adults with ADHD after being treated with lisdexamfetamine dimesylate. Study participants, 11 of whom were men, ranged in age from 19 to 52 years. Lisdexamfetamine dimesylate, a class II stimulant, was approved in 2012 by the Food and Drug Administration for adult ADHD.
In the randomized, placebo-controlled, crossover study, each participant received the active drug for at least 4 weeks in an escalating stepped dose titration (30, 50, 70 mg). The participants were scanned twice; once after taking the placebo and also after at least 2 weeks of taking their best tolerated dose of the active drug.
The scans were done while the subject performed a passive-avoidance learning task where participants learned to associate whether their response to a specific image corresponded with a high chance of either winning or losing money. The participants were asked to respond when they thought they were going to win and avoid responding when they thought they were going to lose. The scans were intended to capture the effect of the drug on components of the motivation/reward circuitry and its impact on decision-making processes.
Each participant’s blood oxygen level–dependent (BOLD) signals were modeled according to the moment they chose or refused to respond to an image, and when they received the resulting feedback as to whether they’d won or lost money. Decision-related activity was modulated by the expected value that the participant had for the image, and the feedback processing was modulated by the difference between the result they had expected from their choice and the result that actually occurred (the prediction error).
For the scans, whole-brain analyses were used (P less than .005); the extent threshold was defined via Monte Carlo simulation. A small-volume correction was performed on the ventral striatum with a P value FWE (family-wise error) corrected of less than .05.
To examine the relationship between each participant’s regional BOLD signals during the imaging and their respective clinician ratings on the ADHD Rating Scale for adults, Dr. Duhoux and her colleagues used regression analyses and found that when compared to placebo, when the participant chose not to respond to an image, the stimulant increased the modulation of BOLD responses by the expected value of the image in the dorsolateral prefrontal cortex.
The drug also was associated with increased BOLD response in the ventral striatum when a reward was received. This response was modulated by the prediction error in the ventromedial prefrontal cortex. Increased activation in the ventral striatum and ventromedial prefrontal cortex with reward was positively correlated with greater improvement in ADHD Rating Scale scores.
These results suggest that "the medication helps increase the value of the stimulus and the person’s perception of the stimulus and also reinforces the ability to avoid responding to stimuli considered to be less rewarding," Dr. Duhoux said. "Practically speaking, this is associated with clinical improvements and makes patients more sensitive to an activity, so they engage more easily."
Dr. Duhoux said she had no relevant disclosures. This study was funded by Shire, maker of Vyvanse.
On Twitter @whitneymcknight
HOLLYWOOD, FLA. – Activity in the regions of the brain associated with motivation and reward correlated with clinical improvements in attention-deficit/hyperactivity disorder symptoms in adults given lisdexamfetamine dimesylate, the results of an imaging study have shown.
"What we saw is that Vyvanse (lisdexamfetamine dimesylate) increases activity in the caudate and anterior cingulate, which then seems to show that the medication increases sensitivity to reward," Stephanie Duhoux, Ph.D., said in an interview about her poster presentation at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
"You could say that when they are taking this medication, these patients don’t need as much reward in order to get them to engage in an activity," said Dr. Duhoux of Icahn School of Medicine at Mount Sinai, New York, who was a new investigator award winner at this year’s ASCP meeting.
Building upon recent findings by Dr. Nora D. Volkow, director of the National Institute on Drug Abuse and her colleagues (Mol. Psychiatry 2011;16:1147-54) indicating that a dopaminergic dysfunction in the brain’s motivation-reward system might contribute to ADHD, Dr. Duhoux and her colleagues used imaging to study changes in the brains of 20 adults with ADHD after being treated with lisdexamfetamine dimesylate. Study participants, 11 of whom were men, ranged in age from 19 to 52 years. Lisdexamfetamine dimesylate, a class II stimulant, was approved in 2012 by the Food and Drug Administration for adult ADHD.
In the randomized, placebo-controlled, crossover study, each participant received the active drug for at least 4 weeks in an escalating stepped dose titration (30, 50, 70 mg). The participants were scanned twice; once after taking the placebo and also after at least 2 weeks of taking their best tolerated dose of the active drug.
The scans were done while the subject performed a passive-avoidance learning task where participants learned to associate whether their response to a specific image corresponded with a high chance of either winning or losing money. The participants were asked to respond when they thought they were going to win and avoid responding when they thought they were going to lose. The scans were intended to capture the effect of the drug on components of the motivation/reward circuitry and its impact on decision-making processes.
Each participant’s blood oxygen level–dependent (BOLD) signals were modeled according to the moment they chose or refused to respond to an image, and when they received the resulting feedback as to whether they’d won or lost money. Decision-related activity was modulated by the expected value that the participant had for the image, and the feedback processing was modulated by the difference between the result they had expected from their choice and the result that actually occurred (the prediction error).
For the scans, whole-brain analyses were used (P less than .005); the extent threshold was defined via Monte Carlo simulation. A small-volume correction was performed on the ventral striatum with a P value FWE (family-wise error) corrected of less than .05.
To examine the relationship between each participant’s regional BOLD signals during the imaging and their respective clinician ratings on the ADHD Rating Scale for adults, Dr. Duhoux and her colleagues used regression analyses and found that when compared to placebo, when the participant chose not to respond to an image, the stimulant increased the modulation of BOLD responses by the expected value of the image in the dorsolateral prefrontal cortex.
The drug also was associated with increased BOLD response in the ventral striatum when a reward was received. This response was modulated by the prediction error in the ventromedial prefrontal cortex. Increased activation in the ventral striatum and ventromedial prefrontal cortex with reward was positively correlated with greater improvement in ADHD Rating Scale scores.
These results suggest that "the medication helps increase the value of the stimulus and the person’s perception of the stimulus and also reinforces the ability to avoid responding to stimuli considered to be less rewarding," Dr. Duhoux said. "Practically speaking, this is associated with clinical improvements and makes patients more sensitive to an activity, so they engage more easily."
Dr. Duhoux said she had no relevant disclosures. This study was funded by Shire, maker of Vyvanse.
On Twitter @whitneymcknight
AT THE ASCP ANNUAL MEETING
Key clinical point: Patients with ADHD who are taking lisdexamfetamine dimesylate "don’t need as much reward in order to get them to engage in an activity."
Major finding: Clinical improvement in ADHD symptoms correlated with lisdexamfetamine dimesylate–induced activity in brain regions key to the motivation-reward system.
Data source: Randomized, placebo-controlled crossover study of 20 adults with ADHD given either placebo or lisdexamfetamine dimesylate.
Disclosures: Dr. Duhoux said she had no relevant disclosures. This study was funded by Shire, maker of Vyvanse.
VIDEO: Lasers – The scar treatment tools you already have
DANA POINT, CALIF. – When you think of lasers, do you consider them as the standard of care for treatment of a range of scars, from dog bites to burns? If not, Dr. Jill Waibel, director of the Miami Dermatology and Laser Institute, thinks you should.
"The sooner you get the lasers on the scars, the better," she emphasizes in this video, recorded during Summit in Aesthetic Medicine 2014. "They [lasers] are both preventative and therapeutic."
Dermatologists can combine lasers and medications that they already have in their practices to great effect to help make scars nearly invisible, Dr. Waibel explained at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Waibel disclosed that she has financial relationships with Alma, Syneron/Candela, Sciton, Lutronics, and Lumenis.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
DANA POINT, CALIF. – When you think of lasers, do you consider them as the standard of care for treatment of a range of scars, from dog bites to burns? If not, Dr. Jill Waibel, director of the Miami Dermatology and Laser Institute, thinks you should.
"The sooner you get the lasers on the scars, the better," she emphasizes in this video, recorded during Summit in Aesthetic Medicine 2014. "They [lasers] are both preventative and therapeutic."
Dermatologists can combine lasers and medications that they already have in their practices to great effect to help make scars nearly invisible, Dr. Waibel explained at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Waibel disclosed that she has financial relationships with Alma, Syneron/Candela, Sciton, Lutronics, and Lumenis.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
DANA POINT, CALIF. – When you think of lasers, do you consider them as the standard of care for treatment of a range of scars, from dog bites to burns? If not, Dr. Jill Waibel, director of the Miami Dermatology and Laser Institute, thinks you should.
"The sooner you get the lasers on the scars, the better," she emphasizes in this video, recorded during Summit in Aesthetic Medicine 2014. "They [lasers] are both preventative and therapeutic."
Dermatologists can combine lasers and medications that they already have in their practices to great effect to help make scars nearly invisible, Dr. Waibel explained at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Waibel disclosed that she has financial relationships with Alma, Syneron/Candela, Sciton, Lutronics, and Lumenis.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE