User login
Duration and Cessation of Treatment
The appropriate duration of antimicrobial therapy for serious infections such as hospital‐ or healthcare‐associated pneumonia, complicated intra‐abdominal infection, and bacteremia has not been well studied. To the extent that guidelines for treatment duration exist, they are largely based on observational studies, clinical experience, and consensus, rather than data from well‐designed clinical studiesalthough such studies and data are beginning to emerge, more so in some areas (pneumonia) than others (intra‐abdominal infections and catheter‐related bacteremia). Additional studies supporting treatment durations for these and other important infections are encouraged, given the widely recognized relationships between antimicrobial use and development of antimicrobial resistance, and between antimicrobial resistance and increased morbidity, mortality, and healthcare costs.13 Duration is a component of antimicrobial exposure, and together with optimal dosing, has been linked with antimicrobial resistance and other adverse or unintended consequences of antimicrobial therapy. The general idea is to eradicate (kill) the pathogen as soon as possible, and then stop therapy, since dead bugs don't mutate.
An overwhelming body of work has established a link between antimicrobial use and emergence of antimicrobial‐resistant bacteria. This relationship holds for most, if not all, antimicrobial,47 but appears to be particularly strong for broader‐spectrum agents like fluoroquinolones,814 extended‐spectrum cephalosporins,1518 and carbapenems.4, 1822 Using an antimicrobial from a particular drug class typically promotes development of resistance to all members of the class, but can also lead to more broad‐based resistance including other drug classes, depending on the mechanisms of resistance. Emergence of resistance is expected to be especially high when a suboptimal antimicrobial regimen is administered for a prolonged time or duration,7, 23 as these conditions optimize pressure for selection of preexistent resistant strains or development of new ones.
Optimal efficacy and safety of antimicrobial therapy depends, first, on avoiding antimicrobials when they are not indicated, and second, when they are used, focusing on the 4 Ds of optimal antimicrobial therapy: right Drug, right Dose, De‐escalation to pathogen‐directed therapy, and right Duration of therapy.24 Corresponding articles in this supplement have focused on the first 3 Ds: Dr Syndman on selection of the right drug and dose, and Dr Kaye on de‐escalation of initial empiric therapy, when circumstances warrant it. The current article examines the rationale for reducing the duration of antimicrobial therapy (when possible), and current evidence or guidelines supporting the use of shorter courses of antimicrobial therapy for such infections as pneumonia (community‐, hospital‐, or healthcare‐acquired/associated), complicated intra‐abdominal infection, and bacteremia or sepsis. Key points will be illustrated through 3 case studies dealing with each of these general infection categories.
ADJUSTING DURATION TO OPTIMIZE ANTIMICROBIAL THERAPY
The ultimate goals of short‐course antimicrobial therapy are to rapidly eradicate pathogenic microorganisms and reduce selective pressure for emergence of resistance. The primary potential advantages of shorter duration antimicrobial therapy include lower cost, less toxicity, better adherence, reduced antimicrobial resistance, and reduced disruption of endogenous flora and risk of superinfections, such as Clostridium difficile‐associated disease.23 Other potential benefits of shorter antimicrobial durations include a shorter length of hospital stay and (perhaps) earlier removal of an intravenous catheter, which would be expected to reduce risk of iatrogenic complications and facilitate early mobility and earlier return to full health. Effective short‐course antimicrobial therapy also appears to better meet patient expectations of therapy than longer courses.25
Rapid or early eradication of pathogens depends not only on selecting an agent or combination of agents with activity against the causative pathogen, but also administering the agent in a manner that enables it to achieve its pharmacodynamic (PD) target for pathogen eradication in a rapid fashion.23, 26 The PD parameter that best predicts efficacy will vary for different antimicrobial classes, but the general idea is to use a dose, dosing schedule, and route of administration that rapidly achieves adequate tissue penetration and drug concentration at the infection site for a sufficient length of time for maximum efficacy. In brief, the general concept for short‐course antimicrobial therapy is to hit hard and fast then leave as soon as possible.23
The World Health Organization (WHO) 2000 report on overcoming antimicrobial resistance also recognizes that ideal antimicrobial usage includes using the correct drug, administered by the best route, in the right amount, at optimal intervals, for the appropriate period, after an accurate diagnosis.27 Administering antimicrobials for the wrong period of time (ie, duration) increases risk of resistance. In essence, the WHO report is another call to treat aggressively with shorter courses to help reduce antimicrobial resistance, and to avoid antimicrobial therapy when it is not warranted.
However, while there is general agreement about the utility of using as short an antimicrobial course as is consistent with efficacy, there has been a general dearth of information about exactly what the optimal duration is for particular agents (or drug classes) used to treat particular infections. This is especially the case for most infections occurring in critically ill patients in the hospital setting. Appropriate duration of therapy has been established for some infections, notably group A streptococcus pharyngitis, urinary tract infections, and some sexually transmitted diseases,2831 but treatment duration has not been firmly established for most serious infections. Furthermore, clinicians are often reluctant to shorten the duration of antimicrobial therapy in patients with serious infections for fear of incompletely eradicating the pathogen, thereby leading to relapses and significant morbidity or mortality.
Nevertheless, several studies have now been published that point to the effectiveness of shorter‐course antimicrobial therapy for community‐acquired pneumonia (CAP)3235 and hospital‐acquired pneumonia (HAP) or ventilator‐associated pneumonia (VAP),3645 and a more limited number pointing to the effectiveness of shorter‐course therapy for intra‐abdominal infections38, 46, 47 or bacteremia.4851 In addition, clinical practice guidelines recommend shorter‐course antimicrobial therapy for most patients with CAP,52 uncomplicated healthcare‐associated pneumonia (HCAP) or HAP/VAP,53 and complicated intra‐abdominal infections54and clinical practice guidelines for the management of intravascular catheter‐related infection, including bacteremia, specify a standard duration of therapy and conditions under which a shorter (or longer) course may be considered.55 Shorter‐course therapy can be best implemented based on clinical parameters (eg, resolution of fever, reduction of leukocytosis) along with clinical judgment of the well‐informed clinician with guidance from evidenced‐based guidelines.
The remainder of this section will examine some of the preclinical and clinical evidence supporting shorter‐course therapy for CAP. Subsequent sections of the article utilize 3 case studies to discuss current guidelines and supportive evidence for use of shorter‐course antimicrobial therapy in patients with HCAP or HAP/VAP, complicated intra‐abdominal infections, and bacteremia. The discussion of CAP is intended as an introduction that lays down some general concepts concerning shorter‐duration therapy before delving into the serious hospital‐ or healthcare‐related infections outlined above. Because there is more clinical research on duration of treatment for patients with HAP/VAP than for complicated intra‐abdominal infections or bacteremia, the section on HCAP/HAP/VAP is much longer and detailed than the ones for complicated intra‐abdominal infections or bacteremia.
CAP is defined as pneumonia developing in individuals who are not residents in a nursing home or extended‐care facility, and who have not recently been hospitalized or had significant exposure to the healthcare setting. Pneumonia developing after 48 hours of hospital admission, and that was not incubating at the time of admission, is known as HAP,53, 56 and VAP is a subset of HAP, more precisely defined as HAP that arises after endotracheal intubation.53 HCAP includes patients characterized by residence in a nursing home or extended‐care facility or hospitalization for 2 days in the preceding 90 days or other significant exposure to the healthcare setting.53, 57, 58
DURATION OF THERAPY FOR CAP
A number of studies have reported similar efficacy with shortened versus longer durations of antimicrobial therapy for CAP.33, 5964 Consistent with this, 2 recent meta‐analyses of studies comparing shorter‐ versus longer‐course therapy for mild‐to‐moderate CAP (22 randomized controlled trials and >8000 patients between them) reported similar efficacy and safety with shorter‐course therapy.65, 66 In addition, other studies have reported an association between longer durations of antimicrobial therapy and development of resistance by community respiratory pathogens, especially when lower doses have been used.67, 68 These findings are consistent with the belief that prolonged treatment with a suboptimal antimicrobial regimen creates particularly fertile conditions for selection or development of antimicrobial‐resistant strains.65, 66
Data from preclinical studies provide a basis for understanding the effectiveness of shorter‐dosing regimens of adequate antimicrobial therapy for CAP or other forms of pneumonia. In particular, in vitro time‐kill studies6974 and animal models of infection7577 have demonstrated that Streptococcus pneumoniae can be rapidly eradicated without use of long‐term therapy when appropriate antimicrobials are used. Consistent with these preclinical data, various clinical studies have also shown that S pneumoniae and other respiratory pathogens are rapidly eradicated from lower respiratory tract secretions after initiation of appropriate antimicrobial treatment. For example, Montravers et al. reported that 94% of respiratory pathogens were eradicated from the lungs of 76 patients with VAP after just 3 days of antimicrobial therapy.78
Based on the available data, the 2007 Infectious Diseases Society of America (IDSA)/America Thoracic Society (ATS) guidelines for CAP management recommend a minimum of 5 days of antimicrobial treatment, while noting that most patients become clinically stable within 3‐7 days of treatment onset and rarely require longer durations.52 The guidelines further recommend that CAP patients should be afebrile for 4872 hours and should have no more than 1 CAP‐associated sign of clinical instability before discontinuation of therapy. Although the general movement is toward use of shorter‐duration treatment courses than the traditional 710 days or longer, the IDSA/ATS guidelines acknowledge that longer durations may be needed in certain situations.79
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
Case 1 is a 72‐year‐old woman admitted with findings consistent with HCAP who was initiated on an empiric therapy regimen of vancomycin and piperacillin‐tazobactam. Results from blood and sputum cultures obtained prior to treatment initiation came back on day 3, and were negative for pathogenic bacteria. White blood cell (WBC) counts were trending downward, and the patient appeared to be stabilizing. She still had an elevated WBC count, slight fever (temperature maximum of 101.4F for the past 24 hours), and lung crackles at the right lung base. Because Gram stain failed to identify Gram‐positive cocci clusters, and there was no culture evidence of methicillin‐resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa, vancomycin treatment was terminated and the patient was switched to single‐agent therapy with intravenous ceftriaxone, a nonpseudomonal third‐generation cephalosporin. On hospital day 5, there was continuing evidence of response to antimicrobial therapy. The patient reported feeling better and she was breathing comfortably. Her cough was much improved, sputum production was markedly decreased, and her fever had resolved. Now, on day 7, the patient is still afebrile, her WBC count is normal, and she has 96% oxygen saturation on room air.
The question before the clinician is whether to terminate or continue antimicrobial therapy, and if continued, with what regimen and for how long? In addition, if a decision is made to continue antimicrobial therapy, there is a possibility of switching from an intravenous to oral treatment regimen. An examination of the literature and current treatment guidelines for HCAP/HAP/VAP should enable a more informed decision, one that optimally benefits not only this patient, but all subsequent ones who might be exposed and infected with a resistant pathogen that develops when treatment is continued longer than necessary.
Using Clinical Parameters to Shorten Antimicrobial Therapy
A prospective study by Dennesen et al., published 10 years ago, was one of the first suggesting the possibility of shortened duration of antimicrobial therapy for VAP.80 At the time, duration of antimicrobial therapy for VAP typically ranged from 7 to 21 days, and was most commonly 14 to 21 days. In this study, Dennesen and coworkers examined symptom resolution in 27 patients diagnosed with VAP based on clinical, radiologic, and microbiological criteria, each of whom received appropriate antimicrobial therapy based on culture susceptibility data.80 Significant improvements were observed for all clinical parameters examined (highest temperature, leukocyte count, pressure of arterial oxygen to fractional inspired oxygen [PaO2/FIO2] ratio, semiquantitative culture result of endotracheal aspirate), usually first appearing within the first 6 days of antimicrobial therapy. Furthermore, analyses of specific pathogens showed that appropriate antimicrobial therapy rapidly eradicated endotracheal colonization with S pneumoniae, Haemophilus influenzae, and S aureus, but not of P aeruginosa or Enterobacteriaceae. Moreover, endotracheal colonization with resistant pathogens tended to occur when antimicrobial therapy was continued beyond the first week. Taken together, these results suggested that prolonged antimicrobials beyond 7 days usually did not benefit VAP patients, and in fact increased risk of superinfection with a resistant strain. However, it is important to make a distinction between VAP and, for example, skin or bloodstream infections involving S aureus. While improved signs and symptoms generally indicate clinical cure for VAP, this reasoning should not be applied to S aureus bacteremia.
The findings from Dennesen et al. are generally consistent with those from Montravers et al., which showed that 94% of respiratory pathogens were eradicated from the lungs of VAP patients 3 days after initiation of antimicrobial therapy.78 They are also consistent with the findings from a 2005 study by Vidaur et al., which demonstrated resolution of fever (38C), PaO2/FIO2 (>250 mmHg), and WBC/leukocyte count (10,000) in 73%, 75%, and 53% of VAP patients, respectively, without acute respiratory distress syndrome (ARDS; n = 75) after 3 days of appropriate antimicrobial therapy.81 However, Vidaur et al. reported that fever took roughly twice as long to resolve in VAP patients with ARDS (n = 20) versus without ARDS, and that hypoxia resolution was less useful when evaluating treatment response in ARDS patients. As with the Dennesen et al. study,80 the results from Vidaur et al. suggest that measures of core body temperature and oxygenation can be useful guides for clinicians in determining whether to shorten the duration of antimicrobial therapy for patients with VAP, HAP, or HCAP.81
Along the same lines, the clinical pulmonary infection score (CPIS) has established itself as a means for the early termination (shortening) of initial empiric antimicrobial therapy in particular VAP patients. The CPIS is derived by scoring 57 clinical indices relevant for the diagnosis of VAP, as illustrated in Table 1.82 A score of >6 is considered suggestive of pneumonia, while one 6 implies low likelihood of pneumonia. A 2000 study by Singh et al. randomized 81 consecutive patients with pulmonary infiltrates and a CPIS 6 to receive either standard antimicrobial therapy (at discretion of the clinician) or ciprofloxacin monotherapy, with the intention of reevaluating patients at day 3.45 For patients in the ciprofloxacin (experimental) group, antimicrobial therapy was terminated at day 3 if the CPIS remained 6. As a result, only 28% of patients in the experimental group had antimicrobial therapy continued beyond day 3, compared with 90% of patients in the standard therapy group (P = 0.0001). More importantly, there were no significant differences in mortality between patients in the 2 treatment groups, despite a significantly shorter treatment duration for those in the experimental group (3.0 vs 9.8 days, P = 0.0001). In addition, mean length of intensive care unit (ICU) stay was significantly shorter (9.4 vs 14.7 days, P= 0.04) and mean antimicrobial cost was significantly lower ($259 vs $640, P = 0.0001) for patients in the experimental versus standard therapy group.
| Points | |
|---|---|
| |
| Temperature C | |
| 36.5 and 38.4 | 0 |
| 38.5 and 38.9 | 1 |
| 39 or 36.0 | 2 |
| Tracheal secretions | |
| Absence of secretions | 0 |
| Presence of non‐purulent secretions | 1 |
| Presence of purulent secretions | 2 |
| Pulmonary radiography (chest X‐ray) | |
| No infiltrate | 0 |
| Diffused (or patchy) infiltrate | 1 |
| Localized infiltrate | 2 |
| WBCs, leukocytes/mm3 | |
| 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 |
| +Band forms 500 | 2 |
| Oxygenation: PaO2/FIO2 mmHg | |
| >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 |
| Culture of tracheal aspirate (semiquantitative: 012 or 3+) | |
| Pathogenic bacteria cultured 1+ or no growth | 0 |
| Pathogenic bacteria cultured >1+ + same pathogenic bacteria seen on the gram stain >1+ | 1 2 |
| Progression of pulmonary infiltrate | |
| No radiographic progression | 0 |
| Radiographic progression (ARDS excluded) | 2 |
Furthermore, a significantly greater proportion of patients in the standard versus experimental therapy group exhibited evidence of antimicrobial resistance or superinfections (38% vs 14%, P = 0.017). The 2005 clinical practice guidelines for HAP, VAP, or HCAP state, A modified CPIS of 6 or less for 3 days, proposed by Singh and coworkers, is an objective criterion to select patients at low risk for early discontinuation of empiric treatment of HAP.53 While the Singh et al. study provides the rationale for shorter‐course therapy in ICU patients with pulmonary infiltrates who have low likelihood of pneumonia (CPIS 6), this criterion may or may not pertain to HAP/VAP more strictly, and still requires validation in patients with more severe forms of VAP. Incidentally, although the CPIS was designed to define VAP, and there are no data validating its use for other types of pneumonia, the clinical experience by this author indicates that it can be helpful in evaluating HCAP and non‐VAP HAP as well.
Clinical Trial to Support Shortened Duration of HCAP/HAP/VAP Therapy
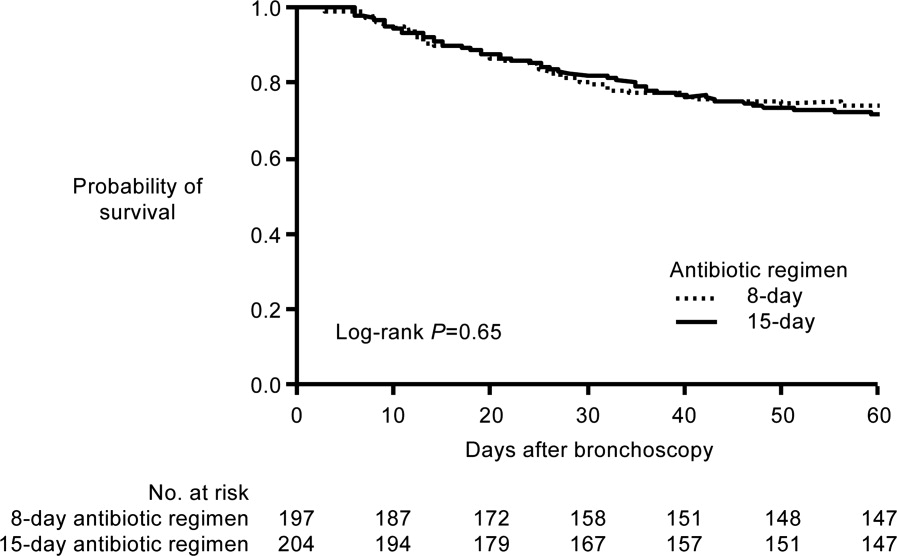
A French study published in JAMA in 2003 provides more direct support that approximately 1 week of antimicrobial therapy produces effectiveness comparable to more traditional 23‐week therapy for most patients with VAP.37 In this prospective, multicenter, randomized, double‐blind (until day 8) clinical trial, 401 patients with microbiologically proven VAP were randomly assigned to receive either 8 days (n = 197) or 15 days (n = 204) of initial empiric antimicrobial therapy selected by the treating physician. No significant differences were observed between the 8‐day and 15‐day treatment groups for the 2 primary efficacy endpoints of death from any cause (18.8% vs 17.2%) and microbiologically documented pulmonary infection recurrence (28.9% vs 26.0%). There were also no differences between the groups for number of mechanical ventilation‐free days (8.7 vs 9.1 days), number of organ‐failure‐free days (8.7 vs 8.9 days), length of ICU stay (30.0 vs 27.5 days), unfavorable outcome (death, pulmonary infection recurrence, or prescription of a new antimicrobial) (46.2% vs 43.6%), mortality rate on day 60 (25.4% vs 27.9%), or in‐hospital mortality (32% vs 29.9%).
Conversely, patients in the 8‐day treatment group had significantly more antimicrobial‐free days (13.1 vs 8.7 days, P 0.001), and among patients who developed recurrent infections, multidrug‐resistant pathogens emerged more frequently in patients in the 15‐day versus 8‐day treatment group (62.0% vs 42.1%, P = 0.04). However, there was an apparent exception to the general comparable efficacy of the 8‐ and 15‐day treatment regimens for infections caused by nonfermenting Gram‐negative bacilli, including P aeruginosa. For primary infections caused by nonfermenting Gram‐negative bacilli, the 8‐day versus 15‐day regimen was associated with higher rates of pulmonary recurrence (40.6% vs 25.4%). Interestingly, the 8‐day regimen was not associated with more adverse outcomes here, just a higher recurrence rate. With respect to primary infections caused by MRSA, no differences were observed between the 2 treatment regimens for death for all causes (23.4% vs 30.2%) or pulmonary infection recurrence (33.3% vs 42.9%). Figure 1 presents the probability of survival data for the 8‐day and 15‐day treatment groups.
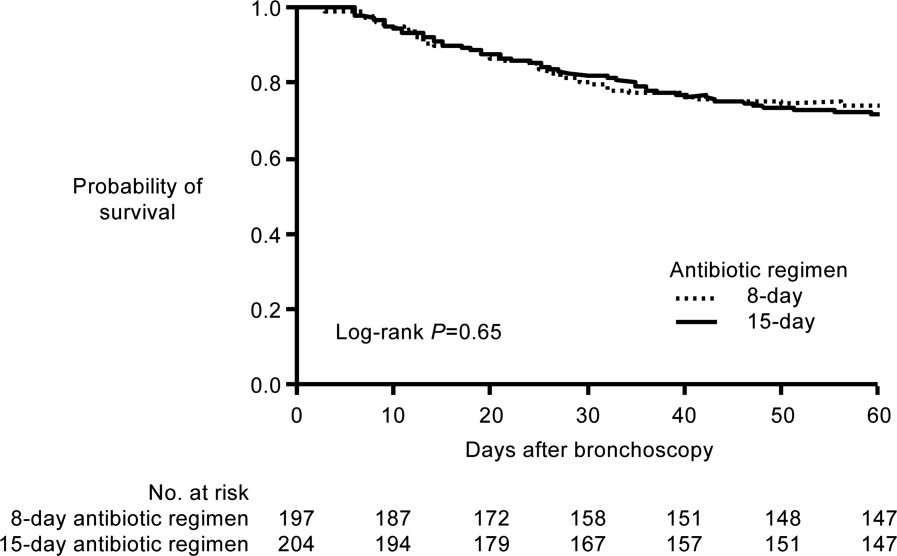
Hence, the data from the Chastre et al. study37 support use of an 8‐day (or shortened) regimen as standard antimicrobial therapy for most patients with VAP, with some possible exceptions. Additional studies provide further support for this general conclusion. For example, a prospective, randomized, controlled trial by Micek et al. evaluated the impact of using an antimicrobial discontinuation policy based on clinical criteria (discontinuation group; n = 150)versus the decision of treating physicians (conventional group, n = 140)to determine the duration of antimicrobial therapy for VAP, and observed a statistically shorter treatment duration in the discontinuation versus conventional management group (6.0 vs 8.0 days, P = 0.001), but no difference between the groups for hospital mortality (32.0% vs 37.1%), ICU length of stay (6.8 vs 7.0 days), or VAP recurrence (17.3% vs 19.3%).42 A prior study by the same group reported a shorter duration of antimicrobial therapy for VAP following implementation of an antimicrobial guideline (vs prior to implementation) (8.6 vs 14.8 days, P 0.001), and a lower rate of VAP recurrence among patients in the after period (7.7% vs 24.0%, P = 0.03). However, interpretation of the results was complicated by the fact that initial empiric therapy was more often appropriate during the after versus before guideline implementation period (94.2% vs 48.0%, P 0.001).40
A limited number of studies have focused further on shortened duration of therapy for patients with VAP caused by Gram‐negative bacteria, and particularly by nonfermenting Gram‐negative bacilli. A retrospective study by Hedrick et al. analyzed the relationship between antimicrobial duration and outcomes of 452 episodes of VAP in the ICU, 154 caused by nonfermenting Gram‐negative bacilli.39 In the study, 127 patients infected with a nonfermenting Gram‐negative bacillus received 9 days (mean 17.1 0.7 days) of antimicrobial therapy, while 27 received 3‐8 days (mean 6.4 0.3 days) of therapy. No significant differences were observed between the shorter‐ and longer‐duration groups for mortality (22% vs 14%, P = 0.38) or VAP recurrence (22% vs 34%, P = 0.27) for these patient populations. Table 2 provides the results for all 452 VAP episodes based on 8 days or 9 days of antimicrobial therapy.
| Patient Characteristic | 8 Days (n = 98) | 9 Days (n = 354) | P Value |
|---|---|---|---|
| |||
| Mean antimicrobial days | 6.2 | 16.8 | 0.0001 |
| Mean APACHE II | 18 | 20 | 0.0009 |
| % Trauma | 71 | 68 | 0.63 |
| Mean time to onset, days | 17.7 | 17.8 | 0.97 |
| Recurrence | 11% | 25% | 0.004 |
| Death | 13% | 11% | 0.59 |
| Nonfermenting Gram‐negative bacilli recurrence | 22% (n = 27) | 34% (n = 127) | 0.27 |
| Staphylococcus aureus recurrence | 20% (n = 10) | 38% (n = 47) | 0.47 |
The retrospective nature of the study limits the ability to more confidently interpret the results, but the data appear to be consistent with the conclusion that short‐duration therapy does not necessarily increase recurrence or worsen other outcomes in patients with VAP caused by nonfermenting Gram‐negative bacilli. The most common Gram‐negative bacilli associated with VAP in the study were P aeruginosa (18% of all infections), Enterobacter cloacae (11%), Acinetobacter spp (11%), Klebsiella pneumoniae (7%), Stenotrophomonas maltophilia (7%), Serratia spp (7%), H influenzae (6%), and Escherichia coli (4%). In addition, the study results suggest that short‐duration therapy is at least as effective as longer‐duration therapy for the overall VAP population, with potential benefits in terms of reduced antimicrobial use and lower rate of recurrence.
Another recent retrospective analysis examining an even shorter course of antimicrobial therapy (5 days) for patients with HAP associated with Gram‐negative bacteria reported a low overall recurrence rate (14%) and a critical care mortality rate (34.2%) in line with prior studies of short‐term therapy for VAP/HAP.44 However, the HAP relapse rate was significantly higher in patients with HAP caused by nonfermenting Gram‐negative bacilli versus other Gram‐negative species (17% vs 2%, P = 0.03).
A recent US pilot study explored the use of repeat bronchoalveolar lavage (BAL) to guide antimicrobial duration in 52 patients with VAP, and compared the results with a matched control group of 52 VAP patients treated before institution of the BAL pathway.43 Antimicrobial therapy in the pathway patients was discontinued if pathogen growth was 10,000 colony forming units/mL on the repeat BAL performed on day 4 of therapy. One objective was to determine whether a repeat BAL strategy, such as the one here, might be able to identify patients with VAP due to nonfermenting Gram‐negative bacilli or other microorganisms who could be safely and effectively treated with shorter‐duration therapy.
Results showed that the antimicrobial duration was significantly shorter for patients in the pathway group than the matched control group (9.8 vs 3.8 days, P 0.001), including the subset of patients with VAP associated with nonfermenting Gram‐negative bacilli (10.7 vs 14.4 days, P 0.001). No significant differences were observed between the overall treatment populations for VAP recurrence, mechanical ventilator‐free ICU days, ICU‐free hospital days, or mortality. Repeat BAL showed most VAP isolates in the study group (83%) responded to initial therapy with a mean duration of 8.8 days. Nonresponders without concomitant infections received significantly longer treatment than pure responding isolates (14.4 vs 7.3 days, P 0.001), and the most common nonresponding microorganisms were P aeruginosa (41% response rate) and S maltophilia (50% response rate), 2 nonfermenting Gram‐negative bacilli.
Most nonfermenting Gram‐negative bacilli‐associated VAP isolates in the study group did respond on repeat BAL (59%). These responders were treated for a mean duration of 8.2 days, and exhibited a similar recurrence rate versus that observed for the matched control group (12.0% vs 17.9%, P = 0.71). These pilot study results suggest that repeat BAL might be used to identify patients likely to benefit from short‐duration therapy, including patients infected with nonfermenting Gram‐negative bacilli. Further study on this is needed.
ATS/IDSA Guidelines for Duration of HCAP/HAP/VAP Therapy
Based largely on the studies by Dennesen et al.80 and Luna et al.83 indicating most VAP patients who respond to appropriate antimicrobial therapy do so within the first 6 days, and those by Chastre et al.37 and Singh et al.45 pointing to the efficacy and safety of shorter‐duration VAP therapy, the 2005 ATS/IDSA guidelines recommend the use of shorter‐duration antimicrobial therapy for most patients with HCAP or HAP/VAP.53 More specifically, the guidelines state, If patients receive an initially appropriate antimicrobial regimen, efforts should be made to shorten the duration of therapy from the traditional 14 to 21 days to periods as short as 7 days, provided that the etiologic pathogen is not P. aeruginosa, and that the patient has a good clinical response with resolution of clinical features of infection.53 Figure 2 presents an overview of the ATS/IDSA guidelines for HCAP/HAP/VAP management 48 to 72 hours after initiation of empiric antimicrobial therapy.53
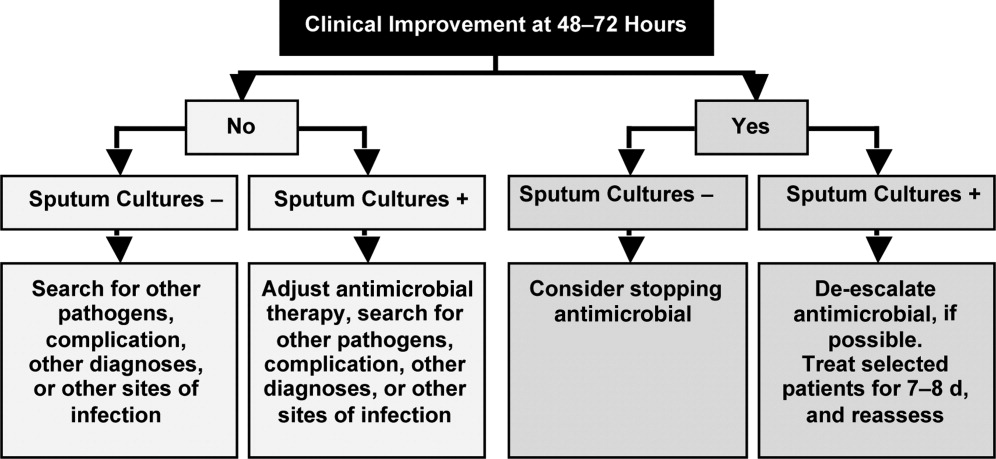
Note that the clinician should consider terminating antimicrobial therapy in patients with clinical improvement and negative cultures or other evidence suggestive of a noninfectious cause. CPIS can also be helpful when deciding whether to terminate initial empiric therapy in a patient with clinical improvement after 23 days of therapy and negative cultures. If cultures are positive, the clinician should consider whether antimicrobial de‐escalation is possible (as discussed by Dr Kaye in the corresponding supplement article), and aim to treat selected patients with an antimicrobial course lasting 78 days. After 78 days, patients should be reassessed for treatment termination or other appropriate actions.
The ATS/IDSA guidelines also provide recommendations for route of drug administration, and if and when to switch from an intravenous to oral agent. In particular, the guidelines state that all patients with HCAP, HAP, or VAP should initially receive therapy intravenously, but conversion to oral/enteral therapy may be possible in certain responding patients, ie, those with a good clinical response and a functioning intestinal tract.53 Fluoroquinolones and linezolid have oral formulations with bioavailability equivalent to the intravenous form, meaning the oral formulations are capable of achieving high levels at the site of infection. This may facilitate conversion to oral therapy in select patients. Early step‐down is safe and effective with fluoroquinolones.84, 85
Based on the information just reviewed, the antimicrobial can be terminated on day 7 for case 1. She is afebrile, and her WBC and oxygenation are normal. In fact, since her records show she was responding at day 5, consideration could have been given to switching from intravenous to oral therapy at that time, and perhaps even discharging her to the rehabilitation center.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with sepsis and diverticular abscess with walled‐off perforation. Upon hospital arrival, Interventional Radiology inserted a drain, and the patient was initiated on ciprofloxacin and metronidazole therapy. Day 3 examination showed improvement in WBC count and normal vital signs, but the patient still had a low‐grade fever (100.9F). Abdominal examination results were improved, but with some diffuse tenderness. Initial cultures of the abdominal abscess isolated Gram‐negative rods, and the patient was continued on ciprofloxacin/metronidazole. Further cultures on day 4 identified an extended‐spectrum ‐lactamase (ESBL)‐producing E coli organism as the causative pathogen. The patient was switched from ciprofloxacin/metronidazole to ertapenem. It is now hospital day 8, and the patient continues to show good response to treatment. She is afebrile and WBC count is normal. The abscess catheter is no longer draining. Her abdominal pain is improved, and she is complaining that she is hungry. A repeat computed tomography scan shows resolution of the abscess and no evidence of bowel perforation. Should antimicrobial therapy be continued in this patient, and if so, with what agent and for how long?
Guidelines from the Surgical Infection Society and IDSA state that antimicrobial therapy of established or complicated intra‐abdominal infection in adults should be limited to 47 days, unless it is difficult to achieve adequate source control.54 This is because extended antimicrobial exposure increases antimicrobial cost and risk of resistance, superinfection, C difficile‐associated colitis, or other untoward and unintended consequences of antimicrobial therapy, and there is no evidence that longer treatment durations improve outcomes.46, 47, 54, 86 Runyon et al. randomized 90 patients with spontaneous bacterial peritonitis or culture‐negative neutrocytic ascites to receive 5 days or 10 days of cefotaxime monotherapy, and reported similar rates of infection‐related mortality (0% vs 4.3%), hospitalization mortality (33% vs 43%), bacteriologic cure (93% vs 92%), and recurrence of ascitic fluid infection (12% vs 13%).46 Furthermore, shorter‐course therapy was associated with significantly lower antimicrobial administration and costs. Similarly, a recent prospective, randomized, double‐blind trial comparing 3 versus 5 days of ertapenem therapy in 111 patients with community‐acquired intra‐abdominal infection reported similar cure (93% vs 90%) and eradication rates (95% vs 94%).86 However, it should be noted that the mean duration of antimicrobial therapy in the longer‐duration group was still relatively short (5.7 days, range of 5‐10 days).
Studies also indicate there is a very low risk of infection recurrence or treatment failure when antimicrobial therapy is terminated in a patient diagnosed with a complicated intra‐abdominal infection who no longer shows signs of continuing infection.38, 87 Lennard et al. compared postoperative outcomes in 65 patients with or without leukocytosis and fever at the conclusion of antimicrobial therapy for intra‐abdominal sepsis, and reported development of intra‐abdominal infection in 7 of 21 (33%) with persistent leukocytosis.87 None of the 30 patients with normal WBC counts at the end of therapy developed an intra‐abdominal infection postoperatively. Furthermore, intra‐abdominal infection occurred postoperatively in 11 of 14 patients (79%) who responded to treatment but were still febrile at the time of antimicrobial discontinuation.
Similar results were obtained in a much larger, more recent study that retrospectively analyzed the relationship between duration of antimicrobial therapy and infectious complications for patients with intra‐abdominal infections.38 In the study, 929 patients with intra‐abdominal infections associated with either fever or leukocytosis were organized into 4 quartiles based on total duration of antimicrobial therapy (quartile 1: 07 days, n = 218; quartile 2: 812 days, n = 217; quartile 3: 1317 days, n = 246; and quartile 4: >17 days, n = 248) or antimicrobial duration after resolution of leukocytosis (quartile 1: 05 days, n = 130; quartile 2: 610 days, n = 127; quartile 3: 1115 days, n = 124; and quartile 4: >15 days, n = 118). Based on either total duration of antimicrobial therapy or duration after leukocytosis resolution, risk of recurrence was significantly higher for patients in quartiles 3 or 4 versus those in quartile 1, and there was no difference between quartiles 1 and 2.
Taken together, these results suggest that antimicrobial therapy for intra‐abdominal sepsis can be shortened in patients exhibiting a clinical response to treatment, if there are no signs of persistent leukocytosis or fever. Hence, clinicians should use the resolution of clinical signs of infection as a guide to determine when during the 47‐day window antimicrobial therapy should be terminated.54 In practical terms, this usually means treatment can be terminated when the patient is afebrile, has normal WBC counts, and is able to tolerate an oral diet.
Based on the clinical status of case 2 after 8 days of antimicrobial therapy (afebrile with normal WBC counts and requesting oral diet), the ertapenem regimen should be stopped. There is no reason to consider further outpatient antimicrobial therapy for this particular patient, but the Surgical Infection Society and IDSA guidelines discuss the type of patient who should be considered for oral or outpatient antimicrobial therapy. According to the guidelines, the patient convalescing from a complicated intra‐abdominal infection may receive oral antimicrobial therapy, but that therapy should only be included as a component within the brief treatment duration already mentioned, ie, in total, it should rarely exceed 7 days.54 Such therapy is rarely indicated for patients who are afebrile, with normal peripheral WBC/leukocyte counts, and with return of bowel function. These recommendations make it clear that no further antimicrobial therapy is warranted for case 2.
However, for appropriate patients who are recovering from a complicated intra‐abdominal infection and are able to tolerate an oral diet, an oral antimicrobial regimen selected on the basis of identified primary isolates may be used for completion of therapy.54 In the absence of cultures, an oral regimen that covers commonly isolated pathogens (eg, E coli, streptococci, and Bacteroides fragilis) should be considered. Common regimens include an oral cephalosporin or fluoroquinolone with metronidazole, or amoxicillin‐clavulanic acid, assuming susceptibility studies do not demonstrate resistance. Given the identification of an ESBL‐producing E coli for case 2a pathogen relatively resistant to oral antimicrobialan oral regimen probably would not have been viable for this patient even earlier in the treatment course. Lastly, a repeat computed tomography scan was used for the case here. It should be noted that there are currently no well‐established criteria for determining when repeat imaging is needed to confirm resolution of fluid collections. This should be a clinical decision. A general practice is that the catheter is left in place until there is minimal drainage (eg, 10 mL/day); catheter sinograms can also be helpful in determining the status of the abscess.
CASE 3: CENTRAL LINE‐ASSOCIATED BACTEREMIA
Case 3 is a 56‐year‐old man with status epilepticus, intubation, and ICU stay. He was initially treated with vancomycin and piperacillin‐tazobactam for a fever of 103.4F on day 5 of hospitalization. Blood cultures grew Gram‐positive cocci. The central venous catheter was removed, and the initial antimicrobial regimen was de‐escalated to vancomycin monotherapy, which was associated with continued improvement in fever and WBC count, and clinical stability on hospital day 7. At that time, further blood culture analyses isolated methicillin‐susceptible S aureus (MSSA), and the antimicrobial regimen was switched/de‐escalated from vancomycin to cefazolin. It is now hospital day 9 (day 3 of cefazolin) and the patient continues to respond and is afebrile. Repeat blood cultures show no bacterial growth, and a transesophageal echocardiograph (TEE) was performed and revealed normal heart valves. Should the antimicrobial therapy be continued for this patient, and if so, with what agent and for how long?
The IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal and 46 weeks of antimicrobial therapy for patients with S aureus catheter‐related bloodstream infection (CRBSI), unless the patient has exceptions allowing consideration of shorter‐duration therapy (minimum of 14 days, with day 1 being the first day of negative blood culture results).55 These exceptions include absence of diabetes; immunocompetence (no immunosuppression); removal of the infected catheter; no prosthetic intravascular device (eg, pacemaker or recently placed vascular graft); no evidence of endocarditis or suppurative thrombophlebitis on TEE and ultrasound, respectively; fever and bacteremia resolved within 72 hours after initiation of appropriate antimicrobial therapy; and no evidence of metastatic infection on physical examination and sign‐ or symptom‐directed diagnostic tests.
Short‐duration (10‐16 day) antimicrobial therapy has been reported to yield similarly low recurrence or relapse rates as longer courses of therapy in patients with uncomplicated catheter‐associated S aureus bacteremia.50, 51, 88, 89 A small 1989 study by Ehni and Reller prospectively followed 13 patients with S aureus CRBSI who had received short‐course therapy (17 days), and reported only 1 case of relapse with endocarditis (8% relapse rate).50 A subsequent study by Malanoski et al. retrospectively analyzed the data from 55 patients with S aureus CRBSI.51 Excluding the 8 patients with early complications, the authors observed similar rates of relapse in patients treated for 1015 days and those receiving longer courses of antimicrobial therapy (0% vs 4.7%). The clinical characteristics of the 2 treatment duration groups were similar, and delayed catheter removal was linked with persistence of bacteremia (P = 0.01).
A more recent multicenter, prospective observational study by Chang et al. examined recurrence and the impact of antimicrobial treatment in 505 consecutive patients with S aureus bacteremia, and determined that duration of antimicrobial therapy was not a factor associated with relapse.88 This was true both for patients with bacteremia resulting from endocarditis, bacteremia with no apparent source, or bacteremia due to a focus that could not be cured or removed (28 days therapy after defervescence, 28 days therapy, or 28 days therapy), or those with bacteremia resulting from a source amenable to definitive cure, such as an intravascular device that could be removed, an abscess that could be incised and drained, or an infected bone that could be resected (>14 days, 1014 days, or 10 days therapy). Similarly, a 2005 prospective study by Thomas and Morris determined there was no relationship between treatment duration (7 vs 8 days, 10 vs 10 days, or 14 vs 15 days; P = 0.62, 0.87, and 0.16, respectively) and rate of relapse for 276 patients with cannula‐associated S aureus bacteremia.89 Longer‐duration antimicrobial therapy is warranted in patients with CRBSI and an early complicated course, eg, fever and/or bacteremia persisting for >3 days after catheter removal.90
According to the IDSA guidelines, a TEE should be obtained for all patients with CRBSI involving S aureus who are being considered for a shorter duration of therapy, and the TEE should be performed at least 57 days after onset of bacteremia to minimize risk of false‐negative results.55 High rates of infective endocarditis are observed in patients with S aureus bacteremia,89, 9193 with higher rates in patients with MSSA versus MRSA bacteremia (43.4% vs 19.6%, P 0.009).91 TEE is essential to diagnose endocarditis and detect other complications of bacteremia.92, 93 This recommendation for use of TEE does not necessarily apply to all patients with CRBSI when S aureus is not involved.
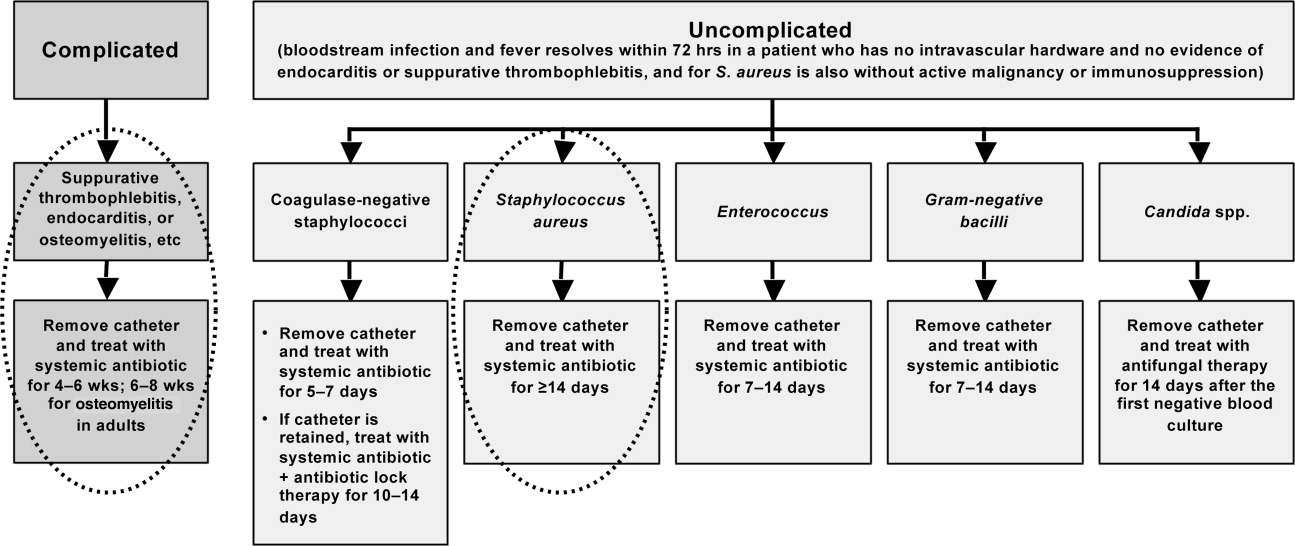
Figure 3 summarizes the general recommendations from the IDSA guidelines for the management of CRBSI in patients with a short‐term catheter.55 The figure illustrates the varied recommendations for treatment duration depending on whether the infection is complicated or uncomplicated, and based on the pathogenic microorganism. Returning to case 3, the patient meets the general criteria for shorter duration of antimicrobial therapy: he is not diabetic or immunosuppressed, his catheter has been removed, he does not have any prosthetic intravascular devices, his fever and bacteremia (based on blood cultures) resolved within 3 days of initiating cefazolin therapy, and there is no evidence of endocarditis or other complications of bacteremia. Hence, he is an excellent example of a patient with uncomplicated MSSA CRBSI who meets the criteria for consideration of shortened antimicrobial therapy. Based on the clinical practice guidelines, the patient should continue on intravenous cefazolin for a 14‐day course of therapy, at which time he can be re‐evaluated. A recent review of bloodstream infections caused by various pathogens similarly concluded that the minimum treatment duration for low‐risk patients with S aureus CRBSI is 14 days.48 As a final point, it is also important to note that there is no role for oral therapy in patients with CRBSI, so whether shortened or not, the chosen regimen should be administered intravenously.
CONCLUSIONS
Shortening the duration of appropriate and adequate antimicrobial therapy represents one strategy for reducing pressure for selection or development of resistant pathogenic microorganisms. Other potential benefits of shorter courses of antimicrobial therapy include reduced risk of antimicrobial‐associated infections (superinfection, C difficile‐associated diarrhea) and other antimicrobial‐related adverse events, improved compliance, and reduced antimicrobial costs. Clinicians are sometimes concerned that reducing antimicrobial courses for patients with serious infections, such as HCAP/HAP/VAP, complicated intra‐abdominal infection, and CRBSI, will lead to incomplete eradication of pathogenic microorganisms, leading to disease recurrence and increased morbidity and mortality. When managing patients with these serious infections, clinicians often turn to the literature and recommendations from professional organizations for guidance. Available data from randomized controlled and nonrandomized clinical trials indicate that shorter‐course therapy is effective and safe for patients with CAP, HCAP/HAP/VAP, complicated intra‐abdominal infections, and CRBSI. Based on these data, and consensus/expert opinion, clinical practice guidelines have been developed that recommend specific durations of antimicrobial therapy for each of these infections.
Although greater study of antimicrobial therapy duration is needed, the current and developing literature and current treatment guidelines should enable clinicians to recognize patients who would benefit from shortened courses of antimicrobial therapy. In doing so, they would help to lower antimicrobial costs and reduce the growing problem of antimicrobial resistance, with its wide‐ranging, negative consequences for current and future patients, and the clinicians who treat them.
- .The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs.Clin Infect Dis.2006;42(suppl 2):S82–S89.
- ,,.Clinical and economic burden of antimicrobial resistance.Expert Rev Anti Infect Ther.2008;6:751–763.
- .Economics of antibiotic resistance.Expert Rev Anti Infect Ther.2008;6:523–539.
- ,,,.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43:1379–1382.
- ,,,,.Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis.BMJ.2010;340:c2096.
- ,,,,,.Risk factors for invasive pneumococcal disease in children: a population‐based case‐control study in North America.Pediatrics.1999;103:E28.
- ,,.Antimicrobial resistance: consideration as an adverse drug event.Crit Care Med.2010;38:S155–S161.
- ,,,,,.Fluoroquinolone‐resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact.Am J Med.2006;119:526.e519–526.525.
- ,,, et al.Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates.Am J Med.2008;121:876–884.
- ,,,,,.Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes.Infect Control Hosp Epidemiol.2006;27:893–900.
- ,,,,.Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals.Clin Infect Dis.2005;41:435–440.
- ,,,.Evaluation of an intervention designed to decrease the rate of nosocomial methicillin‐resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use.Infect Control Hosp Epidemiol.2006;27:155–169.
- ,,,,,.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals.Infect Control Hosp Epidemiol.2007;28:1389–1395.
- ,,, et al.Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned.Arch Intern Med.2002;162:1515–1520.
- ,,,,.Nosocomial outbreak of Klebsiella infection resistant to late‐generation cephalosporins.Ann Intern Med.1993;119:353–358.
- ,,.The effect of an antimicrobial restriction program on Pseudomonas aeruginosa resistance to beta‐lactams in a large teaching hospital.Pharmacotherapy.2003;23:618–624.
- ,,,.Association between antibiotic usage and subsequent colonization or infection of extensive drug‐resistant Acinetobacter baumannii: a matched case‐control study in intensive care units.Diagn Microbiol Infect Dis.2008;62:298–305.
- ,,.Relationships between antimicrobial use and antimicrobial resistance in Gram‐negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan.Int J Antimicrob Agents.2005;26:463–472.
- ,,, et al.Risk factors for acquisition of imipenem‐resistant Acinetobacter baumannii: a case‐control study.Antimicrob Agents Chemother.2004;48:224–228.
- ,,,,.Consumption of imipenem correlates with beta‐lactam resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother.2002;46:2920–2925.
- ,,.Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem‐resistant Pseudomonas aeruginosa.Antimicrob Agents Chemother.2009;53:1983–1986.
- .Clinical efficacy of newer agents in short‐duration therapy for community‐acquired pneumonia.Clin Infect Dis.2004;39(suppl 3):S159–S164.
- ,.The role of carbapenems in the treatment of severe nosocomial respiratory tract infections.Expert Opin Pharmacother.2008;9:561–575.
- ,.Does short‐course antibiotic therapy better meet patient expectations?Int J Antimicrob Agents.2003;21:222–228.
- ,,,.Tackling empirical antibiotic therapy for ventilator‐associated pneumonia in your ICU: guidance for implementing the guidelines.Semin Respir Crit Care Med.2009;30:102–115.
- World Health Organization (WHO) report on infectious diseases 2000. Overcoming antimicrobial resistance. Chapter 5. Call to action: A massive effort to provide proper treatment. Available at: http://www.who.int/infectious‐disease‐report/2000/index.html. Accessed January 14,2011.
- ,,,,.Diagnosis and management of group A streptococcal pharyngitis: a practice guideline. Infectious Diseases Society of America.Clin Infect Dis.1997;25:574–583.
- ,,,,,.Shorter‐course antibiotic therapy (SCAT): principles, current data, and caveats. In: Owens RCJ, Lautenbach E, editors.Antimicrobial Resistance: Problem Pathogens and Clinical Countermeasures.New York, NY:Informa Healthcare;2008:337–370.
- ,,, et al.International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases.Clin Infect Dis.2011;52:e103–e120.
- ,.Sexually transmitted diseases treatment guidelines, 2006.MMWR Recomm Rep.2006;55:1–94.
- ,,,,.Novel, single‐dose microsphere formulation of azithromycin versus 7‐day levofloxacin therapy for treatment of mild to moderate community‐acquired pneumonia in adults.Antimicrob Agents Chemother.2005;49:4035–4041.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,.Randomized, multicentre study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of adults with mild to moderate community‐acquired pneumonia. Azithromycin Study Group.Eur J Clin Microbiol Infect Dis.1998;17:828–833.
- ,,,,,.A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia. The optimal duration of therapy.Chest.1996;110:965–971.
- ,,,.Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator‐associated pneumonia to decrease resistance in the intensive care unit.Clin Infect Dis.2006;43(suppl 2):S75–S81.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- ,,, et al.Can we define the ideal duration of antibiotic therapy?Surg Infect (Larchmt).2006;7:419–432.
- ,,,,,.Duration of antibiotic therapy for ventilator‐associated pneumonia caused by non‐fermentative gram‐negative bacilli.Surg Infect (Larchmt).2007;8:589–597.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,.Antibiotic utilization and outcomes for patients with clinically suspected ventilator‐associated pneumonia and negative quantitative BAL culture results.Chest.2005;128:2706–2713.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Repeat bronchoalveolar lavage to guide antibiotic duration for ventilator‐associated pneumonia.J Trauma.2007;63:1329–1337.
- ,,.Short course antibiotic therapy for Gram‐negative hospital‐acquired pneumonia in the critically ill.J Hosp Infect.2010;74:337–343.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,.Short‐course versus long‐course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients.Gastroenterology.1991;100:1737–1742.
- ,,,,.Complicated appendicitis: is there a minimum intravenous antibiotic requirement? A prospective randomized trial.Am Surg.2000;66:887–890.
- ,,.Short‐course therapy for bloodstream infections in immunocompetent adults.Int J Antimicrob Agents.2009;34(suppl 4):S47–S51.
- ,,,.Short‐course monotherapy strategy for treating bacteremia in the critically ill.Minerva Anestesiol.2006;72:841–857.
- ,.Short‐course therapy for catheter‐associated Staphylococcus aureus bacteremia.Arch Intern Med.1989;149:533–536.
- ,,,.Staphylococcus aureus catheter‐associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters.Arch Intern Med.1995;155:1161–1166.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44(suppl 2):S27–S72.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,.Hospital‐acquired pneumonia: pathophysiology, diagnosis, and treatment.Surg Clin North Am.2009;89:439–461, ix.
- ,,.Healthcare‐associated pneumonia in adults: management principles to improve outcomes.Infect Dis Clin North Am.2004;18:939–962.
- ,,.Healthcare‐associated infections. A useful concept?Curr Opin Crit Care.2009;15:419–424.
- ,,,,,.Efficacy of 750‐mg, 5‐day levofloxacin in the treatment of community‐acquired pneumonia caused by atypical pathogens.Curr Med Res Opin.2004;20:555–563.
- ,,, et al.Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study.BMJ.2006;332:1355.
- ,,,,.Gemifloxacin once daily for 5 days versus 7 days for the treatment of community‐acquired pneumonia: a randomized, multicentre, double‐blind study.J Antimicrob Chemother.2007;60:112–120.
- ,,.Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community‐acquired pneumonia of suspected pneumococcal origin.Respir Med.2004;98:708–720.
- ,,,.Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective, randomized, double‐blind study.Am J Ther.1999;6:217–222.
- ,,,,.Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community‐acquired pneumonia.J Antimicrob Chemother.2004;54:515–523.
- ,,,,,.Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis.Drugs.2008;68:1841–1854.
- ,,,.Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,,, et al.Low dosage and long treatment duration of beta‐lactam: risk factors for carriage of penicillin‐resistant Streptococcus pneumoniae.JAMA.1998;279:365–370.
- ,,, et al.Effect of short‐course, high‐dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial.JAMA.2001;286:49–56.
- ,,,.Antipneumococcal activity of ertapenem (MK‐0826) compared to those of other agents.Antimicrob Agents Chemother.2002;46:42–46.
- ,,.MIC and time‐kill study of antipneumococcal activities of RPR 106972 (a new oral streptogramin), RP 59500 (quinupristin‐dalfopristin), pyostacine (RP 7293), penicillin G, cefotaxime, erythromycin, and clarithromycin against 10 penicillin‐susceptible and ‐resistant pneumococci.Antimicrob Agents Chemother.1996;40:2071–2074.
- ,,.Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials.J Antimicrob Chemother.2003;51:443–446.
- ,,.Antipneumococcal activity of ertapenem compared to nine other compounds by time‐kill [abstract E‐800]. In:Program and Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago).Washington, DC:American Society for Microbiology,2001:184.
- ,,.Time‐kill analysis of the antipneumococcal activity of daptomycin compared with 8 other agents. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:161.
- ,,.Post‐antibiotic effect of garenoxacin against gram‐positive and gram‐negative organisms [abstract A‐496]. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:42.
- ,,, et al.Short‐course therapy of gemifloxacin effective against pneumococcal pneumonia in mice.J Chemother.2006;18:634–640.
- ,,.Animal models of Streptococcus pneumoniae disease.Clin Microbiol Rev.2008;21:666–685.
- ,,,,.Protective effect of trovafloxacin, ciprofloxacin and ampicillin against Streptococcus pneumoniae in a murine sepsis model.J Antimicrob Chemother.1999;44:477–481.
- ,,, et al.Follow‐up protected specimen brushes to assess treatment in nosocomial pneumonia.Am Rev Respir Dis.1993;147:38–44.
- ,.Antimicrobial therapy of community‐acquired pneumonia.Infect Dis Clin North Am.2004;18:993–1016, xi.
- ,,,,.Resolution of infectious parameters after antimicrobial therapy in patients with ventilator‐associated pneumonia.Am J Respir Crit Care Med.2001;163:1371–1375.
- ,,, et al.Clinical resolution in patients with suspicion of ventilator‐associated pneumonia: a cohort study comparing patients with and without acute respiratory distress syndrome.Crit Care Med.2005;33:1248–1253.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,, et al.Resolution of ventilator‐associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome.Crit Care Med.2003;31:676–682.
- .Pharmacoeconomic comparison of sequential IV/oral ciprofloxacin versus ceftazidime in the treatment of nosocomial pneumonia.Can J Hosp Pharm.1995;48:276–283.
- ,,, et al.Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics.Am J Med.1991;91:462–470.
- ,,, et al.A prospective, double‐blind, multicenter, randomized trial comparing ertapenem 3 vs > or = 5 days in community‐acquired intraabdominal infection.J Gastrointest Surg.2008;12:592–600.
- ,,,.Implications of leukocytosis and fever at conclusion of antibiotic therapy for intra‐abdominal sepsis.Ann Surg.1982;195:19–24.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,.Cannula‐associated Staphylococcus aureus bacteraemia: outcome in relation to treatment.Intern Med J.2005;35:319–330.
- ,.Optimal duration of therapy for catheter‐related Staphylococcus aureus bacteremia: a study of 55 cases and review.Clin Infect Dis.1992;14:75–82.
- ,,,,.Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin‐sensitive S aureus and methicillin‐resistant S aureus bacteremia.Am Heart J.2004;147:536–539.
- ,,, et al.Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients.J Am Coll Cardiol.1997;30:1072–1078.
- ,,, et al.Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow‐up.Clin Infect Dis.1999;28:106–114.
The appropriate duration of antimicrobial therapy for serious infections such as hospital‐ or healthcare‐associated pneumonia, complicated intra‐abdominal infection, and bacteremia has not been well studied. To the extent that guidelines for treatment duration exist, they are largely based on observational studies, clinical experience, and consensus, rather than data from well‐designed clinical studiesalthough such studies and data are beginning to emerge, more so in some areas (pneumonia) than others (intra‐abdominal infections and catheter‐related bacteremia). Additional studies supporting treatment durations for these and other important infections are encouraged, given the widely recognized relationships between antimicrobial use and development of antimicrobial resistance, and between antimicrobial resistance and increased morbidity, mortality, and healthcare costs.13 Duration is a component of antimicrobial exposure, and together with optimal dosing, has been linked with antimicrobial resistance and other adverse or unintended consequences of antimicrobial therapy. The general idea is to eradicate (kill) the pathogen as soon as possible, and then stop therapy, since dead bugs don't mutate.
An overwhelming body of work has established a link between antimicrobial use and emergence of antimicrobial‐resistant bacteria. This relationship holds for most, if not all, antimicrobial,47 but appears to be particularly strong for broader‐spectrum agents like fluoroquinolones,814 extended‐spectrum cephalosporins,1518 and carbapenems.4, 1822 Using an antimicrobial from a particular drug class typically promotes development of resistance to all members of the class, but can also lead to more broad‐based resistance including other drug classes, depending on the mechanisms of resistance. Emergence of resistance is expected to be especially high when a suboptimal antimicrobial regimen is administered for a prolonged time or duration,7, 23 as these conditions optimize pressure for selection of preexistent resistant strains or development of new ones.
Optimal efficacy and safety of antimicrobial therapy depends, first, on avoiding antimicrobials when they are not indicated, and second, when they are used, focusing on the 4 Ds of optimal antimicrobial therapy: right Drug, right Dose, De‐escalation to pathogen‐directed therapy, and right Duration of therapy.24 Corresponding articles in this supplement have focused on the first 3 Ds: Dr Syndman on selection of the right drug and dose, and Dr Kaye on de‐escalation of initial empiric therapy, when circumstances warrant it. The current article examines the rationale for reducing the duration of antimicrobial therapy (when possible), and current evidence or guidelines supporting the use of shorter courses of antimicrobial therapy for such infections as pneumonia (community‐, hospital‐, or healthcare‐acquired/associated), complicated intra‐abdominal infection, and bacteremia or sepsis. Key points will be illustrated through 3 case studies dealing with each of these general infection categories.
ADJUSTING DURATION TO OPTIMIZE ANTIMICROBIAL THERAPY
The ultimate goals of short‐course antimicrobial therapy are to rapidly eradicate pathogenic microorganisms and reduce selective pressure for emergence of resistance. The primary potential advantages of shorter duration antimicrobial therapy include lower cost, less toxicity, better adherence, reduced antimicrobial resistance, and reduced disruption of endogenous flora and risk of superinfections, such as Clostridium difficile‐associated disease.23 Other potential benefits of shorter antimicrobial durations include a shorter length of hospital stay and (perhaps) earlier removal of an intravenous catheter, which would be expected to reduce risk of iatrogenic complications and facilitate early mobility and earlier return to full health. Effective short‐course antimicrobial therapy also appears to better meet patient expectations of therapy than longer courses.25
Rapid or early eradication of pathogens depends not only on selecting an agent or combination of agents with activity against the causative pathogen, but also administering the agent in a manner that enables it to achieve its pharmacodynamic (PD) target for pathogen eradication in a rapid fashion.23, 26 The PD parameter that best predicts efficacy will vary for different antimicrobial classes, but the general idea is to use a dose, dosing schedule, and route of administration that rapidly achieves adequate tissue penetration and drug concentration at the infection site for a sufficient length of time for maximum efficacy. In brief, the general concept for short‐course antimicrobial therapy is to hit hard and fast then leave as soon as possible.23
The World Health Organization (WHO) 2000 report on overcoming antimicrobial resistance also recognizes that ideal antimicrobial usage includes using the correct drug, administered by the best route, in the right amount, at optimal intervals, for the appropriate period, after an accurate diagnosis.27 Administering antimicrobials for the wrong period of time (ie, duration) increases risk of resistance. In essence, the WHO report is another call to treat aggressively with shorter courses to help reduce antimicrobial resistance, and to avoid antimicrobial therapy when it is not warranted.
However, while there is general agreement about the utility of using as short an antimicrobial course as is consistent with efficacy, there has been a general dearth of information about exactly what the optimal duration is for particular agents (or drug classes) used to treat particular infections. This is especially the case for most infections occurring in critically ill patients in the hospital setting. Appropriate duration of therapy has been established for some infections, notably group A streptococcus pharyngitis, urinary tract infections, and some sexually transmitted diseases,2831 but treatment duration has not been firmly established for most serious infections. Furthermore, clinicians are often reluctant to shorten the duration of antimicrobial therapy in patients with serious infections for fear of incompletely eradicating the pathogen, thereby leading to relapses and significant morbidity or mortality.
Nevertheless, several studies have now been published that point to the effectiveness of shorter‐course antimicrobial therapy for community‐acquired pneumonia (CAP)3235 and hospital‐acquired pneumonia (HAP) or ventilator‐associated pneumonia (VAP),3645 and a more limited number pointing to the effectiveness of shorter‐course therapy for intra‐abdominal infections38, 46, 47 or bacteremia.4851 In addition, clinical practice guidelines recommend shorter‐course antimicrobial therapy for most patients with CAP,52 uncomplicated healthcare‐associated pneumonia (HCAP) or HAP/VAP,53 and complicated intra‐abdominal infections54and clinical practice guidelines for the management of intravascular catheter‐related infection, including bacteremia, specify a standard duration of therapy and conditions under which a shorter (or longer) course may be considered.55 Shorter‐course therapy can be best implemented based on clinical parameters (eg, resolution of fever, reduction of leukocytosis) along with clinical judgment of the well‐informed clinician with guidance from evidenced‐based guidelines.
The remainder of this section will examine some of the preclinical and clinical evidence supporting shorter‐course therapy for CAP. Subsequent sections of the article utilize 3 case studies to discuss current guidelines and supportive evidence for use of shorter‐course antimicrobial therapy in patients with HCAP or HAP/VAP, complicated intra‐abdominal infections, and bacteremia. The discussion of CAP is intended as an introduction that lays down some general concepts concerning shorter‐duration therapy before delving into the serious hospital‐ or healthcare‐related infections outlined above. Because there is more clinical research on duration of treatment for patients with HAP/VAP than for complicated intra‐abdominal infections or bacteremia, the section on HCAP/HAP/VAP is much longer and detailed than the ones for complicated intra‐abdominal infections or bacteremia.
CAP is defined as pneumonia developing in individuals who are not residents in a nursing home or extended‐care facility, and who have not recently been hospitalized or had significant exposure to the healthcare setting. Pneumonia developing after 48 hours of hospital admission, and that was not incubating at the time of admission, is known as HAP,53, 56 and VAP is a subset of HAP, more precisely defined as HAP that arises after endotracheal intubation.53 HCAP includes patients characterized by residence in a nursing home or extended‐care facility or hospitalization for 2 days in the preceding 90 days or other significant exposure to the healthcare setting.53, 57, 58
DURATION OF THERAPY FOR CAP
A number of studies have reported similar efficacy with shortened versus longer durations of antimicrobial therapy for CAP.33, 5964 Consistent with this, 2 recent meta‐analyses of studies comparing shorter‐ versus longer‐course therapy for mild‐to‐moderate CAP (22 randomized controlled trials and >8000 patients between them) reported similar efficacy and safety with shorter‐course therapy.65, 66 In addition, other studies have reported an association between longer durations of antimicrobial therapy and development of resistance by community respiratory pathogens, especially when lower doses have been used.67, 68 These findings are consistent with the belief that prolonged treatment with a suboptimal antimicrobial regimen creates particularly fertile conditions for selection or development of antimicrobial‐resistant strains.65, 66
Data from preclinical studies provide a basis for understanding the effectiveness of shorter‐dosing regimens of adequate antimicrobial therapy for CAP or other forms of pneumonia. In particular, in vitro time‐kill studies6974 and animal models of infection7577 have demonstrated that Streptococcus pneumoniae can be rapidly eradicated without use of long‐term therapy when appropriate antimicrobials are used. Consistent with these preclinical data, various clinical studies have also shown that S pneumoniae and other respiratory pathogens are rapidly eradicated from lower respiratory tract secretions after initiation of appropriate antimicrobial treatment. For example, Montravers et al. reported that 94% of respiratory pathogens were eradicated from the lungs of 76 patients with VAP after just 3 days of antimicrobial therapy.78
Based on the available data, the 2007 Infectious Diseases Society of America (IDSA)/America Thoracic Society (ATS) guidelines for CAP management recommend a minimum of 5 days of antimicrobial treatment, while noting that most patients become clinically stable within 3‐7 days of treatment onset and rarely require longer durations.52 The guidelines further recommend that CAP patients should be afebrile for 4872 hours and should have no more than 1 CAP‐associated sign of clinical instability before discontinuation of therapy. Although the general movement is toward use of shorter‐duration treatment courses than the traditional 710 days or longer, the IDSA/ATS guidelines acknowledge that longer durations may be needed in certain situations.79
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
Case 1 is a 72‐year‐old woman admitted with findings consistent with HCAP who was initiated on an empiric therapy regimen of vancomycin and piperacillin‐tazobactam. Results from blood and sputum cultures obtained prior to treatment initiation came back on day 3, and were negative for pathogenic bacteria. White blood cell (WBC) counts were trending downward, and the patient appeared to be stabilizing. She still had an elevated WBC count, slight fever (temperature maximum of 101.4F for the past 24 hours), and lung crackles at the right lung base. Because Gram stain failed to identify Gram‐positive cocci clusters, and there was no culture evidence of methicillin‐resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa, vancomycin treatment was terminated and the patient was switched to single‐agent therapy with intravenous ceftriaxone, a nonpseudomonal third‐generation cephalosporin. On hospital day 5, there was continuing evidence of response to antimicrobial therapy. The patient reported feeling better and she was breathing comfortably. Her cough was much improved, sputum production was markedly decreased, and her fever had resolved. Now, on day 7, the patient is still afebrile, her WBC count is normal, and she has 96% oxygen saturation on room air.
The question before the clinician is whether to terminate or continue antimicrobial therapy, and if continued, with what regimen and for how long? In addition, if a decision is made to continue antimicrobial therapy, there is a possibility of switching from an intravenous to oral treatment regimen. An examination of the literature and current treatment guidelines for HCAP/HAP/VAP should enable a more informed decision, one that optimally benefits not only this patient, but all subsequent ones who might be exposed and infected with a resistant pathogen that develops when treatment is continued longer than necessary.
Using Clinical Parameters to Shorten Antimicrobial Therapy
A prospective study by Dennesen et al., published 10 years ago, was one of the first suggesting the possibility of shortened duration of antimicrobial therapy for VAP.80 At the time, duration of antimicrobial therapy for VAP typically ranged from 7 to 21 days, and was most commonly 14 to 21 days. In this study, Dennesen and coworkers examined symptom resolution in 27 patients diagnosed with VAP based on clinical, radiologic, and microbiological criteria, each of whom received appropriate antimicrobial therapy based on culture susceptibility data.80 Significant improvements were observed for all clinical parameters examined (highest temperature, leukocyte count, pressure of arterial oxygen to fractional inspired oxygen [PaO2/FIO2] ratio, semiquantitative culture result of endotracheal aspirate), usually first appearing within the first 6 days of antimicrobial therapy. Furthermore, analyses of specific pathogens showed that appropriate antimicrobial therapy rapidly eradicated endotracheal colonization with S pneumoniae, Haemophilus influenzae, and S aureus, but not of P aeruginosa or Enterobacteriaceae. Moreover, endotracheal colonization with resistant pathogens tended to occur when antimicrobial therapy was continued beyond the first week. Taken together, these results suggested that prolonged antimicrobials beyond 7 days usually did not benefit VAP patients, and in fact increased risk of superinfection with a resistant strain. However, it is important to make a distinction between VAP and, for example, skin or bloodstream infections involving S aureus. While improved signs and symptoms generally indicate clinical cure for VAP, this reasoning should not be applied to S aureus bacteremia.
The findings from Dennesen et al. are generally consistent with those from Montravers et al., which showed that 94% of respiratory pathogens were eradicated from the lungs of VAP patients 3 days after initiation of antimicrobial therapy.78 They are also consistent with the findings from a 2005 study by Vidaur et al., which demonstrated resolution of fever (38C), PaO2/FIO2 (>250 mmHg), and WBC/leukocyte count (10,000) in 73%, 75%, and 53% of VAP patients, respectively, without acute respiratory distress syndrome (ARDS; n = 75) after 3 days of appropriate antimicrobial therapy.81 However, Vidaur et al. reported that fever took roughly twice as long to resolve in VAP patients with ARDS (n = 20) versus without ARDS, and that hypoxia resolution was less useful when evaluating treatment response in ARDS patients. As with the Dennesen et al. study,80 the results from Vidaur et al. suggest that measures of core body temperature and oxygenation can be useful guides for clinicians in determining whether to shorten the duration of antimicrobial therapy for patients with VAP, HAP, or HCAP.81
Along the same lines, the clinical pulmonary infection score (CPIS) has established itself as a means for the early termination (shortening) of initial empiric antimicrobial therapy in particular VAP patients. The CPIS is derived by scoring 57 clinical indices relevant for the diagnosis of VAP, as illustrated in Table 1.82 A score of >6 is considered suggestive of pneumonia, while one 6 implies low likelihood of pneumonia. A 2000 study by Singh et al. randomized 81 consecutive patients with pulmonary infiltrates and a CPIS 6 to receive either standard antimicrobial therapy (at discretion of the clinician) or ciprofloxacin monotherapy, with the intention of reevaluating patients at day 3.45 For patients in the ciprofloxacin (experimental) group, antimicrobial therapy was terminated at day 3 if the CPIS remained 6. As a result, only 28% of patients in the experimental group had antimicrobial therapy continued beyond day 3, compared with 90% of patients in the standard therapy group (P = 0.0001). More importantly, there were no significant differences in mortality between patients in the 2 treatment groups, despite a significantly shorter treatment duration for those in the experimental group (3.0 vs 9.8 days, P = 0.0001). In addition, mean length of intensive care unit (ICU) stay was significantly shorter (9.4 vs 14.7 days, P= 0.04) and mean antimicrobial cost was significantly lower ($259 vs $640, P = 0.0001) for patients in the experimental versus standard therapy group.
| Points | |
|---|---|
| |
| Temperature C | |
| 36.5 and 38.4 | 0 |
| 38.5 and 38.9 | 1 |
| 39 or 36.0 | 2 |
| Tracheal secretions | |
| Absence of secretions | 0 |
| Presence of non‐purulent secretions | 1 |
| Presence of purulent secretions | 2 |
| Pulmonary radiography (chest X‐ray) | |
| No infiltrate | 0 |
| Diffused (or patchy) infiltrate | 1 |
| Localized infiltrate | 2 |
| WBCs, leukocytes/mm3 | |
| 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 |
| +Band forms 500 | 2 |
| Oxygenation: PaO2/FIO2 mmHg | |
| >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 |
| Culture of tracheal aspirate (semiquantitative: 012 or 3+) | |
| Pathogenic bacteria cultured 1+ or no growth | 0 |
| Pathogenic bacteria cultured >1+ + same pathogenic bacteria seen on the gram stain >1+ | 1 2 |
| Progression of pulmonary infiltrate | |
| No radiographic progression | 0 |
| Radiographic progression (ARDS excluded) | 2 |
Furthermore, a significantly greater proportion of patients in the standard versus experimental therapy group exhibited evidence of antimicrobial resistance or superinfections (38% vs 14%, P = 0.017). The 2005 clinical practice guidelines for HAP, VAP, or HCAP state, A modified CPIS of 6 or less for 3 days, proposed by Singh and coworkers, is an objective criterion to select patients at low risk for early discontinuation of empiric treatment of HAP.53 While the Singh et al. study provides the rationale for shorter‐course therapy in ICU patients with pulmonary infiltrates who have low likelihood of pneumonia (CPIS 6), this criterion may or may not pertain to HAP/VAP more strictly, and still requires validation in patients with more severe forms of VAP. Incidentally, although the CPIS was designed to define VAP, and there are no data validating its use for other types of pneumonia, the clinical experience by this author indicates that it can be helpful in evaluating HCAP and non‐VAP HAP as well.
Clinical Trial to Support Shortened Duration of HCAP/HAP/VAP Therapy
A French study published in JAMA in 2003 provides more direct support that approximately 1 week of antimicrobial therapy produces effectiveness comparable to more traditional 23‐week therapy for most patients with VAP.37 In this prospective, multicenter, randomized, double‐blind (until day 8) clinical trial, 401 patients with microbiologically proven VAP were randomly assigned to receive either 8 days (n = 197) or 15 days (n = 204) of initial empiric antimicrobial therapy selected by the treating physician. No significant differences were observed between the 8‐day and 15‐day treatment groups for the 2 primary efficacy endpoints of death from any cause (18.8% vs 17.2%) and microbiologically documented pulmonary infection recurrence (28.9% vs 26.0%). There were also no differences between the groups for number of mechanical ventilation‐free days (8.7 vs 9.1 days), number of organ‐failure‐free days (8.7 vs 8.9 days), length of ICU stay (30.0 vs 27.5 days), unfavorable outcome (death, pulmonary infection recurrence, or prescription of a new antimicrobial) (46.2% vs 43.6%), mortality rate on day 60 (25.4% vs 27.9%), or in‐hospital mortality (32% vs 29.9%).
Conversely, patients in the 8‐day treatment group had significantly more antimicrobial‐free days (13.1 vs 8.7 days, P 0.001), and among patients who developed recurrent infections, multidrug‐resistant pathogens emerged more frequently in patients in the 15‐day versus 8‐day treatment group (62.0% vs 42.1%, P = 0.04). However, there was an apparent exception to the general comparable efficacy of the 8‐ and 15‐day treatment regimens for infections caused by nonfermenting Gram‐negative bacilli, including P aeruginosa. For primary infections caused by nonfermenting Gram‐negative bacilli, the 8‐day versus 15‐day regimen was associated with higher rates of pulmonary recurrence (40.6% vs 25.4%). Interestingly, the 8‐day regimen was not associated with more adverse outcomes here, just a higher recurrence rate. With respect to primary infections caused by MRSA, no differences were observed between the 2 treatment regimens for death for all causes (23.4% vs 30.2%) or pulmonary infection recurrence (33.3% vs 42.9%). Figure 1 presents the probability of survival data for the 8‐day and 15‐day treatment groups.

Hence, the data from the Chastre et al. study37 support use of an 8‐day (or shortened) regimen as standard antimicrobial therapy for most patients with VAP, with some possible exceptions. Additional studies provide further support for this general conclusion. For example, a prospective, randomized, controlled trial by Micek et al. evaluated the impact of using an antimicrobial discontinuation policy based on clinical criteria (discontinuation group; n = 150)versus the decision of treating physicians (conventional group, n = 140)to determine the duration of antimicrobial therapy for VAP, and observed a statistically shorter treatment duration in the discontinuation versus conventional management group (6.0 vs 8.0 days, P = 0.001), but no difference between the groups for hospital mortality (32.0% vs 37.1%), ICU length of stay (6.8 vs 7.0 days), or VAP recurrence (17.3% vs 19.3%).42 A prior study by the same group reported a shorter duration of antimicrobial therapy for VAP following implementation of an antimicrobial guideline (vs prior to implementation) (8.6 vs 14.8 days, P 0.001), and a lower rate of VAP recurrence among patients in the after period (7.7% vs 24.0%, P = 0.03). However, interpretation of the results was complicated by the fact that initial empiric therapy was more often appropriate during the after versus before guideline implementation period (94.2% vs 48.0%, P 0.001).40
A limited number of studies have focused further on shortened duration of therapy for patients with VAP caused by Gram‐negative bacteria, and particularly by nonfermenting Gram‐negative bacilli. A retrospective study by Hedrick et al. analyzed the relationship between antimicrobial duration and outcomes of 452 episodes of VAP in the ICU, 154 caused by nonfermenting Gram‐negative bacilli.39 In the study, 127 patients infected with a nonfermenting Gram‐negative bacillus received 9 days (mean 17.1 0.7 days) of antimicrobial therapy, while 27 received 3‐8 days (mean 6.4 0.3 days) of therapy. No significant differences were observed between the shorter‐ and longer‐duration groups for mortality (22% vs 14%, P = 0.38) or VAP recurrence (22% vs 34%, P = 0.27) for these patient populations. Table 2 provides the results for all 452 VAP episodes based on 8 days or 9 days of antimicrobial therapy.
| Patient Characteristic | 8 Days (n = 98) | 9 Days (n = 354) | P Value |
|---|---|---|---|
| |||
| Mean antimicrobial days | 6.2 | 16.8 | 0.0001 |
| Mean APACHE II | 18 | 20 | 0.0009 |
| % Trauma | 71 | 68 | 0.63 |
| Mean time to onset, days | 17.7 | 17.8 | 0.97 |
| Recurrence | 11% | 25% | 0.004 |
| Death | 13% | 11% | 0.59 |
| Nonfermenting Gram‐negative bacilli recurrence | 22% (n = 27) | 34% (n = 127) | 0.27 |
| Staphylococcus aureus recurrence | 20% (n = 10) | 38% (n = 47) | 0.47 |
The retrospective nature of the study limits the ability to more confidently interpret the results, but the data appear to be consistent with the conclusion that short‐duration therapy does not necessarily increase recurrence or worsen other outcomes in patients with VAP caused by nonfermenting Gram‐negative bacilli. The most common Gram‐negative bacilli associated with VAP in the study were P aeruginosa (18% of all infections), Enterobacter cloacae (11%), Acinetobacter spp (11%), Klebsiella pneumoniae (7%), Stenotrophomonas maltophilia (7%), Serratia spp (7%), H influenzae (6%), and Escherichia coli (4%). In addition, the study results suggest that short‐duration therapy is at least as effective as longer‐duration therapy for the overall VAP population, with potential benefits in terms of reduced antimicrobial use and lower rate of recurrence.
Another recent retrospective analysis examining an even shorter course of antimicrobial therapy (5 days) for patients with HAP associated with Gram‐negative bacteria reported a low overall recurrence rate (14%) and a critical care mortality rate (34.2%) in line with prior studies of short‐term therapy for VAP/HAP.44 However, the HAP relapse rate was significantly higher in patients with HAP caused by nonfermenting Gram‐negative bacilli versus other Gram‐negative species (17% vs 2%, P = 0.03).
A recent US pilot study explored the use of repeat bronchoalveolar lavage (BAL) to guide antimicrobial duration in 52 patients with VAP, and compared the results with a matched control group of 52 VAP patients treated before institution of the BAL pathway.43 Antimicrobial therapy in the pathway patients was discontinued if pathogen growth was 10,000 colony forming units/mL on the repeat BAL performed on day 4 of therapy. One objective was to determine whether a repeat BAL strategy, such as the one here, might be able to identify patients with VAP due to nonfermenting Gram‐negative bacilli or other microorganisms who could be safely and effectively treated with shorter‐duration therapy.
Results showed that the antimicrobial duration was significantly shorter for patients in the pathway group than the matched control group (9.8 vs 3.8 days, P 0.001), including the subset of patients with VAP associated with nonfermenting Gram‐negative bacilli (10.7 vs 14.4 days, P 0.001). No significant differences were observed between the overall treatment populations for VAP recurrence, mechanical ventilator‐free ICU days, ICU‐free hospital days, or mortality. Repeat BAL showed most VAP isolates in the study group (83%) responded to initial therapy with a mean duration of 8.8 days. Nonresponders without concomitant infections received significantly longer treatment than pure responding isolates (14.4 vs 7.3 days, P 0.001), and the most common nonresponding microorganisms were P aeruginosa (41% response rate) and S maltophilia (50% response rate), 2 nonfermenting Gram‐negative bacilli.
Most nonfermenting Gram‐negative bacilli‐associated VAP isolates in the study group did respond on repeat BAL (59%). These responders were treated for a mean duration of 8.2 days, and exhibited a similar recurrence rate versus that observed for the matched control group (12.0% vs 17.9%, P = 0.71). These pilot study results suggest that repeat BAL might be used to identify patients likely to benefit from short‐duration therapy, including patients infected with nonfermenting Gram‐negative bacilli. Further study on this is needed.
ATS/IDSA Guidelines for Duration of HCAP/HAP/VAP Therapy
Based largely on the studies by Dennesen et al.80 and Luna et al.83 indicating most VAP patients who respond to appropriate antimicrobial therapy do so within the first 6 days, and those by Chastre et al.37 and Singh et al.45 pointing to the efficacy and safety of shorter‐duration VAP therapy, the 2005 ATS/IDSA guidelines recommend the use of shorter‐duration antimicrobial therapy for most patients with HCAP or HAP/VAP.53 More specifically, the guidelines state, If patients receive an initially appropriate antimicrobial regimen, efforts should be made to shorten the duration of therapy from the traditional 14 to 21 days to periods as short as 7 days, provided that the etiologic pathogen is not P. aeruginosa, and that the patient has a good clinical response with resolution of clinical features of infection.53 Figure 2 presents an overview of the ATS/IDSA guidelines for HCAP/HAP/VAP management 48 to 72 hours after initiation of empiric antimicrobial therapy.53

Note that the clinician should consider terminating antimicrobial therapy in patients with clinical improvement and negative cultures or other evidence suggestive of a noninfectious cause. CPIS can also be helpful when deciding whether to terminate initial empiric therapy in a patient with clinical improvement after 23 days of therapy and negative cultures. If cultures are positive, the clinician should consider whether antimicrobial de‐escalation is possible (as discussed by Dr Kaye in the corresponding supplement article), and aim to treat selected patients with an antimicrobial course lasting 78 days. After 78 days, patients should be reassessed for treatment termination or other appropriate actions.
The ATS/IDSA guidelines also provide recommendations for route of drug administration, and if and when to switch from an intravenous to oral agent. In particular, the guidelines state that all patients with HCAP, HAP, or VAP should initially receive therapy intravenously, but conversion to oral/enteral therapy may be possible in certain responding patients, ie, those with a good clinical response and a functioning intestinal tract.53 Fluoroquinolones and linezolid have oral formulations with bioavailability equivalent to the intravenous form, meaning the oral formulations are capable of achieving high levels at the site of infection. This may facilitate conversion to oral therapy in select patients. Early step‐down is safe and effective with fluoroquinolones.84, 85
Based on the information just reviewed, the antimicrobial can be terminated on day 7 for case 1. She is afebrile, and her WBC and oxygenation are normal. In fact, since her records show she was responding at day 5, consideration could have been given to switching from intravenous to oral therapy at that time, and perhaps even discharging her to the rehabilitation center.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with sepsis and diverticular abscess with walled‐off perforation. Upon hospital arrival, Interventional Radiology inserted a drain, and the patient was initiated on ciprofloxacin and metronidazole therapy. Day 3 examination showed improvement in WBC count and normal vital signs, but the patient still had a low‐grade fever (100.9F). Abdominal examination results were improved, but with some diffuse tenderness. Initial cultures of the abdominal abscess isolated Gram‐negative rods, and the patient was continued on ciprofloxacin/metronidazole. Further cultures on day 4 identified an extended‐spectrum ‐lactamase (ESBL)‐producing E coli organism as the causative pathogen. The patient was switched from ciprofloxacin/metronidazole to ertapenem. It is now hospital day 8, and the patient continues to show good response to treatment. She is afebrile and WBC count is normal. The abscess catheter is no longer draining. Her abdominal pain is improved, and she is complaining that she is hungry. A repeat computed tomography scan shows resolution of the abscess and no evidence of bowel perforation. Should antimicrobial therapy be continued in this patient, and if so, with what agent and for how long?
Guidelines from the Surgical Infection Society and IDSA state that antimicrobial therapy of established or complicated intra‐abdominal infection in adults should be limited to 47 days, unless it is difficult to achieve adequate source control.54 This is because extended antimicrobial exposure increases antimicrobial cost and risk of resistance, superinfection, C difficile‐associated colitis, or other untoward and unintended consequences of antimicrobial therapy, and there is no evidence that longer treatment durations improve outcomes.46, 47, 54, 86 Runyon et al. randomized 90 patients with spontaneous bacterial peritonitis or culture‐negative neutrocytic ascites to receive 5 days or 10 days of cefotaxime monotherapy, and reported similar rates of infection‐related mortality (0% vs 4.3%), hospitalization mortality (33% vs 43%), bacteriologic cure (93% vs 92%), and recurrence of ascitic fluid infection (12% vs 13%).46 Furthermore, shorter‐course therapy was associated with significantly lower antimicrobial administration and costs. Similarly, a recent prospective, randomized, double‐blind trial comparing 3 versus 5 days of ertapenem therapy in 111 patients with community‐acquired intra‐abdominal infection reported similar cure (93% vs 90%) and eradication rates (95% vs 94%).86 However, it should be noted that the mean duration of antimicrobial therapy in the longer‐duration group was still relatively short (5.7 days, range of 5‐10 days).
Studies also indicate there is a very low risk of infection recurrence or treatment failure when antimicrobial therapy is terminated in a patient diagnosed with a complicated intra‐abdominal infection who no longer shows signs of continuing infection.38, 87 Lennard et al. compared postoperative outcomes in 65 patients with or without leukocytosis and fever at the conclusion of antimicrobial therapy for intra‐abdominal sepsis, and reported development of intra‐abdominal infection in 7 of 21 (33%) with persistent leukocytosis.87 None of the 30 patients with normal WBC counts at the end of therapy developed an intra‐abdominal infection postoperatively. Furthermore, intra‐abdominal infection occurred postoperatively in 11 of 14 patients (79%) who responded to treatment but were still febrile at the time of antimicrobial discontinuation.
Similar results were obtained in a much larger, more recent study that retrospectively analyzed the relationship between duration of antimicrobial therapy and infectious complications for patients with intra‐abdominal infections.38 In the study, 929 patients with intra‐abdominal infections associated with either fever or leukocytosis were organized into 4 quartiles based on total duration of antimicrobial therapy (quartile 1: 07 days, n = 218; quartile 2: 812 days, n = 217; quartile 3: 1317 days, n = 246; and quartile 4: >17 days, n = 248) or antimicrobial duration after resolution of leukocytosis (quartile 1: 05 days, n = 130; quartile 2: 610 days, n = 127; quartile 3: 1115 days, n = 124; and quartile 4: >15 days, n = 118). Based on either total duration of antimicrobial therapy or duration after leukocytosis resolution, risk of recurrence was significantly higher for patients in quartiles 3 or 4 versus those in quartile 1, and there was no difference between quartiles 1 and 2.
Taken together, these results suggest that antimicrobial therapy for intra‐abdominal sepsis can be shortened in patients exhibiting a clinical response to treatment, if there are no signs of persistent leukocytosis or fever. Hence, clinicians should use the resolution of clinical signs of infection as a guide to determine when during the 47‐day window antimicrobial therapy should be terminated.54 In practical terms, this usually means treatment can be terminated when the patient is afebrile, has normal WBC counts, and is able to tolerate an oral diet.
Based on the clinical status of case 2 after 8 days of antimicrobial therapy (afebrile with normal WBC counts and requesting oral diet), the ertapenem regimen should be stopped. There is no reason to consider further outpatient antimicrobial therapy for this particular patient, but the Surgical Infection Society and IDSA guidelines discuss the type of patient who should be considered for oral or outpatient antimicrobial therapy. According to the guidelines, the patient convalescing from a complicated intra‐abdominal infection may receive oral antimicrobial therapy, but that therapy should only be included as a component within the brief treatment duration already mentioned, ie, in total, it should rarely exceed 7 days.54 Such therapy is rarely indicated for patients who are afebrile, with normal peripheral WBC/leukocyte counts, and with return of bowel function. These recommendations make it clear that no further antimicrobial therapy is warranted for case 2.
However, for appropriate patients who are recovering from a complicated intra‐abdominal infection and are able to tolerate an oral diet, an oral antimicrobial regimen selected on the basis of identified primary isolates may be used for completion of therapy.54 In the absence of cultures, an oral regimen that covers commonly isolated pathogens (eg, E coli, streptococci, and Bacteroides fragilis) should be considered. Common regimens include an oral cephalosporin or fluoroquinolone with metronidazole, or amoxicillin‐clavulanic acid, assuming susceptibility studies do not demonstrate resistance. Given the identification of an ESBL‐producing E coli for case 2a pathogen relatively resistant to oral antimicrobialan oral regimen probably would not have been viable for this patient even earlier in the treatment course. Lastly, a repeat computed tomography scan was used for the case here. It should be noted that there are currently no well‐established criteria for determining when repeat imaging is needed to confirm resolution of fluid collections. This should be a clinical decision. A general practice is that the catheter is left in place until there is minimal drainage (eg, 10 mL/day); catheter sinograms can also be helpful in determining the status of the abscess.
CASE 3: CENTRAL LINE‐ASSOCIATED BACTEREMIA
Case 3 is a 56‐year‐old man with status epilepticus, intubation, and ICU stay. He was initially treated with vancomycin and piperacillin‐tazobactam for a fever of 103.4F on day 5 of hospitalization. Blood cultures grew Gram‐positive cocci. The central venous catheter was removed, and the initial antimicrobial regimen was de‐escalated to vancomycin monotherapy, which was associated with continued improvement in fever and WBC count, and clinical stability on hospital day 7. At that time, further blood culture analyses isolated methicillin‐susceptible S aureus (MSSA), and the antimicrobial regimen was switched/de‐escalated from vancomycin to cefazolin. It is now hospital day 9 (day 3 of cefazolin) and the patient continues to respond and is afebrile. Repeat blood cultures show no bacterial growth, and a transesophageal echocardiograph (TEE) was performed and revealed normal heart valves. Should the antimicrobial therapy be continued for this patient, and if so, with what agent and for how long?
The IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal and 46 weeks of antimicrobial therapy for patients with S aureus catheter‐related bloodstream infection (CRBSI), unless the patient has exceptions allowing consideration of shorter‐duration therapy (minimum of 14 days, with day 1 being the first day of negative blood culture results).55 These exceptions include absence of diabetes; immunocompetence (no immunosuppression); removal of the infected catheter; no prosthetic intravascular device (eg, pacemaker or recently placed vascular graft); no evidence of endocarditis or suppurative thrombophlebitis on TEE and ultrasound, respectively; fever and bacteremia resolved within 72 hours after initiation of appropriate antimicrobial therapy; and no evidence of metastatic infection on physical examination and sign‐ or symptom‐directed diagnostic tests.
Short‐duration (10‐16 day) antimicrobial therapy has been reported to yield similarly low recurrence or relapse rates as longer courses of therapy in patients with uncomplicated catheter‐associated S aureus bacteremia.50, 51, 88, 89 A small 1989 study by Ehni and Reller prospectively followed 13 patients with S aureus CRBSI who had received short‐course therapy (17 days), and reported only 1 case of relapse with endocarditis (8% relapse rate).50 A subsequent study by Malanoski et al. retrospectively analyzed the data from 55 patients with S aureus CRBSI.51 Excluding the 8 patients with early complications, the authors observed similar rates of relapse in patients treated for 1015 days and those receiving longer courses of antimicrobial therapy (0% vs 4.7%). The clinical characteristics of the 2 treatment duration groups were similar, and delayed catheter removal was linked with persistence of bacteremia (P = 0.01).
A more recent multicenter, prospective observational study by Chang et al. examined recurrence and the impact of antimicrobial treatment in 505 consecutive patients with S aureus bacteremia, and determined that duration of antimicrobial therapy was not a factor associated with relapse.88 This was true both for patients with bacteremia resulting from endocarditis, bacteremia with no apparent source, or bacteremia due to a focus that could not be cured or removed (28 days therapy after defervescence, 28 days therapy, or 28 days therapy), or those with bacteremia resulting from a source amenable to definitive cure, such as an intravascular device that could be removed, an abscess that could be incised and drained, or an infected bone that could be resected (>14 days, 1014 days, or 10 days therapy). Similarly, a 2005 prospective study by Thomas and Morris determined there was no relationship between treatment duration (7 vs 8 days, 10 vs 10 days, or 14 vs 15 days; P = 0.62, 0.87, and 0.16, respectively) and rate of relapse for 276 patients with cannula‐associated S aureus bacteremia.89 Longer‐duration antimicrobial therapy is warranted in patients with CRBSI and an early complicated course, eg, fever and/or bacteremia persisting for >3 days after catheter removal.90
According to the IDSA guidelines, a TEE should be obtained for all patients with CRBSI involving S aureus who are being considered for a shorter duration of therapy, and the TEE should be performed at least 57 days after onset of bacteremia to minimize risk of false‐negative results.55 High rates of infective endocarditis are observed in patients with S aureus bacteremia,89, 9193 with higher rates in patients with MSSA versus MRSA bacteremia (43.4% vs 19.6%, P 0.009).91 TEE is essential to diagnose endocarditis and detect other complications of bacteremia.92, 93 This recommendation for use of TEE does not necessarily apply to all patients with CRBSI when S aureus is not involved.
Figure 3 summarizes the general recommendations from the IDSA guidelines for the management of CRBSI in patients with a short‐term catheter.55 The figure illustrates the varied recommendations for treatment duration depending on whether the infection is complicated or uncomplicated, and based on the pathogenic microorganism. Returning to case 3, the patient meets the general criteria for shorter duration of antimicrobial therapy: he is not diabetic or immunosuppressed, his catheter has been removed, he does not have any prosthetic intravascular devices, his fever and bacteremia (based on blood cultures) resolved within 3 days of initiating cefazolin therapy, and there is no evidence of endocarditis or other complications of bacteremia. Hence, he is an excellent example of a patient with uncomplicated MSSA CRBSI who meets the criteria for consideration of shortened antimicrobial therapy. Based on the clinical practice guidelines, the patient should continue on intravenous cefazolin for a 14‐day course of therapy, at which time he can be re‐evaluated. A recent review of bloodstream infections caused by various pathogens similarly concluded that the minimum treatment duration for low‐risk patients with S aureus CRBSI is 14 days.48 As a final point, it is also important to note that there is no role for oral therapy in patients with CRBSI, so whether shortened or not, the chosen regimen should be administered intravenously.

CONCLUSIONS
Shortening the duration of appropriate and adequate antimicrobial therapy represents one strategy for reducing pressure for selection or development of resistant pathogenic microorganisms. Other potential benefits of shorter courses of antimicrobial therapy include reduced risk of antimicrobial‐associated infections (superinfection, C difficile‐associated diarrhea) and other antimicrobial‐related adverse events, improved compliance, and reduced antimicrobial costs. Clinicians are sometimes concerned that reducing antimicrobial courses for patients with serious infections, such as HCAP/HAP/VAP, complicated intra‐abdominal infection, and CRBSI, will lead to incomplete eradication of pathogenic microorganisms, leading to disease recurrence and increased morbidity and mortality. When managing patients with these serious infections, clinicians often turn to the literature and recommendations from professional organizations for guidance. Available data from randomized controlled and nonrandomized clinical trials indicate that shorter‐course therapy is effective and safe for patients with CAP, HCAP/HAP/VAP, complicated intra‐abdominal infections, and CRBSI. Based on these data, and consensus/expert opinion, clinical practice guidelines have been developed that recommend specific durations of antimicrobial therapy for each of these infections.
Although greater study of antimicrobial therapy duration is needed, the current and developing literature and current treatment guidelines should enable clinicians to recognize patients who would benefit from shortened courses of antimicrobial therapy. In doing so, they would help to lower antimicrobial costs and reduce the growing problem of antimicrobial resistance, with its wide‐ranging, negative consequences for current and future patients, and the clinicians who treat them.
The appropriate duration of antimicrobial therapy for serious infections such as hospital‐ or healthcare‐associated pneumonia, complicated intra‐abdominal infection, and bacteremia has not been well studied. To the extent that guidelines for treatment duration exist, they are largely based on observational studies, clinical experience, and consensus, rather than data from well‐designed clinical studiesalthough such studies and data are beginning to emerge, more so in some areas (pneumonia) than others (intra‐abdominal infections and catheter‐related bacteremia). Additional studies supporting treatment durations for these and other important infections are encouraged, given the widely recognized relationships between antimicrobial use and development of antimicrobial resistance, and between antimicrobial resistance and increased morbidity, mortality, and healthcare costs.13 Duration is a component of antimicrobial exposure, and together with optimal dosing, has been linked with antimicrobial resistance and other adverse or unintended consequences of antimicrobial therapy. The general idea is to eradicate (kill) the pathogen as soon as possible, and then stop therapy, since dead bugs don't mutate.
An overwhelming body of work has established a link between antimicrobial use and emergence of antimicrobial‐resistant bacteria. This relationship holds for most, if not all, antimicrobial,47 but appears to be particularly strong for broader‐spectrum agents like fluoroquinolones,814 extended‐spectrum cephalosporins,1518 and carbapenems.4, 1822 Using an antimicrobial from a particular drug class typically promotes development of resistance to all members of the class, but can also lead to more broad‐based resistance including other drug classes, depending on the mechanisms of resistance. Emergence of resistance is expected to be especially high when a suboptimal antimicrobial regimen is administered for a prolonged time or duration,7, 23 as these conditions optimize pressure for selection of preexistent resistant strains or development of new ones.
Optimal efficacy and safety of antimicrobial therapy depends, first, on avoiding antimicrobials when they are not indicated, and second, when they are used, focusing on the 4 Ds of optimal antimicrobial therapy: right Drug, right Dose, De‐escalation to pathogen‐directed therapy, and right Duration of therapy.24 Corresponding articles in this supplement have focused on the first 3 Ds: Dr Syndman on selection of the right drug and dose, and Dr Kaye on de‐escalation of initial empiric therapy, when circumstances warrant it. The current article examines the rationale for reducing the duration of antimicrobial therapy (when possible), and current evidence or guidelines supporting the use of shorter courses of antimicrobial therapy for such infections as pneumonia (community‐, hospital‐, or healthcare‐acquired/associated), complicated intra‐abdominal infection, and bacteremia or sepsis. Key points will be illustrated through 3 case studies dealing with each of these general infection categories.
ADJUSTING DURATION TO OPTIMIZE ANTIMICROBIAL THERAPY
The ultimate goals of short‐course antimicrobial therapy are to rapidly eradicate pathogenic microorganisms and reduce selective pressure for emergence of resistance. The primary potential advantages of shorter duration antimicrobial therapy include lower cost, less toxicity, better adherence, reduced antimicrobial resistance, and reduced disruption of endogenous flora and risk of superinfections, such as Clostridium difficile‐associated disease.23 Other potential benefits of shorter antimicrobial durations include a shorter length of hospital stay and (perhaps) earlier removal of an intravenous catheter, which would be expected to reduce risk of iatrogenic complications and facilitate early mobility and earlier return to full health. Effective short‐course antimicrobial therapy also appears to better meet patient expectations of therapy than longer courses.25
Rapid or early eradication of pathogens depends not only on selecting an agent or combination of agents with activity against the causative pathogen, but also administering the agent in a manner that enables it to achieve its pharmacodynamic (PD) target for pathogen eradication in a rapid fashion.23, 26 The PD parameter that best predicts efficacy will vary for different antimicrobial classes, but the general idea is to use a dose, dosing schedule, and route of administration that rapidly achieves adequate tissue penetration and drug concentration at the infection site for a sufficient length of time for maximum efficacy. In brief, the general concept for short‐course antimicrobial therapy is to hit hard and fast then leave as soon as possible.23
The World Health Organization (WHO) 2000 report on overcoming antimicrobial resistance also recognizes that ideal antimicrobial usage includes using the correct drug, administered by the best route, in the right amount, at optimal intervals, for the appropriate period, after an accurate diagnosis.27 Administering antimicrobials for the wrong period of time (ie, duration) increases risk of resistance. In essence, the WHO report is another call to treat aggressively with shorter courses to help reduce antimicrobial resistance, and to avoid antimicrobial therapy when it is not warranted.
However, while there is general agreement about the utility of using as short an antimicrobial course as is consistent with efficacy, there has been a general dearth of information about exactly what the optimal duration is for particular agents (or drug classes) used to treat particular infections. This is especially the case for most infections occurring in critically ill patients in the hospital setting. Appropriate duration of therapy has been established for some infections, notably group A streptococcus pharyngitis, urinary tract infections, and some sexually transmitted diseases,2831 but treatment duration has not been firmly established for most serious infections. Furthermore, clinicians are often reluctant to shorten the duration of antimicrobial therapy in patients with serious infections for fear of incompletely eradicating the pathogen, thereby leading to relapses and significant morbidity or mortality.
Nevertheless, several studies have now been published that point to the effectiveness of shorter‐course antimicrobial therapy for community‐acquired pneumonia (CAP)3235 and hospital‐acquired pneumonia (HAP) or ventilator‐associated pneumonia (VAP),3645 and a more limited number pointing to the effectiveness of shorter‐course therapy for intra‐abdominal infections38, 46, 47 or bacteremia.4851 In addition, clinical practice guidelines recommend shorter‐course antimicrobial therapy for most patients with CAP,52 uncomplicated healthcare‐associated pneumonia (HCAP) or HAP/VAP,53 and complicated intra‐abdominal infections54and clinical practice guidelines for the management of intravascular catheter‐related infection, including bacteremia, specify a standard duration of therapy and conditions under which a shorter (or longer) course may be considered.55 Shorter‐course therapy can be best implemented based on clinical parameters (eg, resolution of fever, reduction of leukocytosis) along with clinical judgment of the well‐informed clinician with guidance from evidenced‐based guidelines.
The remainder of this section will examine some of the preclinical and clinical evidence supporting shorter‐course therapy for CAP. Subsequent sections of the article utilize 3 case studies to discuss current guidelines and supportive evidence for use of shorter‐course antimicrobial therapy in patients with HCAP or HAP/VAP, complicated intra‐abdominal infections, and bacteremia. The discussion of CAP is intended as an introduction that lays down some general concepts concerning shorter‐duration therapy before delving into the serious hospital‐ or healthcare‐related infections outlined above. Because there is more clinical research on duration of treatment for patients with HAP/VAP than for complicated intra‐abdominal infections or bacteremia, the section on HCAP/HAP/VAP is much longer and detailed than the ones for complicated intra‐abdominal infections or bacteremia.
CAP is defined as pneumonia developing in individuals who are not residents in a nursing home or extended‐care facility, and who have not recently been hospitalized or had significant exposure to the healthcare setting. Pneumonia developing after 48 hours of hospital admission, and that was not incubating at the time of admission, is known as HAP,53, 56 and VAP is a subset of HAP, more precisely defined as HAP that arises after endotracheal intubation.53 HCAP includes patients characterized by residence in a nursing home or extended‐care facility or hospitalization for 2 days in the preceding 90 days or other significant exposure to the healthcare setting.53, 57, 58
DURATION OF THERAPY FOR CAP
A number of studies have reported similar efficacy with shortened versus longer durations of antimicrobial therapy for CAP.33, 5964 Consistent with this, 2 recent meta‐analyses of studies comparing shorter‐ versus longer‐course therapy for mild‐to‐moderate CAP (22 randomized controlled trials and >8000 patients between them) reported similar efficacy and safety with shorter‐course therapy.65, 66 In addition, other studies have reported an association between longer durations of antimicrobial therapy and development of resistance by community respiratory pathogens, especially when lower doses have been used.67, 68 These findings are consistent with the belief that prolonged treatment with a suboptimal antimicrobial regimen creates particularly fertile conditions for selection or development of antimicrobial‐resistant strains.65, 66
Data from preclinical studies provide a basis for understanding the effectiveness of shorter‐dosing regimens of adequate antimicrobial therapy for CAP or other forms of pneumonia. In particular, in vitro time‐kill studies6974 and animal models of infection7577 have demonstrated that Streptococcus pneumoniae can be rapidly eradicated without use of long‐term therapy when appropriate antimicrobials are used. Consistent with these preclinical data, various clinical studies have also shown that S pneumoniae and other respiratory pathogens are rapidly eradicated from lower respiratory tract secretions after initiation of appropriate antimicrobial treatment. For example, Montravers et al. reported that 94% of respiratory pathogens were eradicated from the lungs of 76 patients with VAP after just 3 days of antimicrobial therapy.78
Based on the available data, the 2007 Infectious Diseases Society of America (IDSA)/America Thoracic Society (ATS) guidelines for CAP management recommend a minimum of 5 days of antimicrobial treatment, while noting that most patients become clinically stable within 3‐7 days of treatment onset and rarely require longer durations.52 The guidelines further recommend that CAP patients should be afebrile for 4872 hours and should have no more than 1 CAP‐associated sign of clinical instability before discontinuation of therapy. Although the general movement is toward use of shorter‐duration treatment courses than the traditional 710 days or longer, the IDSA/ATS guidelines acknowledge that longer durations may be needed in certain situations.79
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
Case 1 is a 72‐year‐old woman admitted with findings consistent with HCAP who was initiated on an empiric therapy regimen of vancomycin and piperacillin‐tazobactam. Results from blood and sputum cultures obtained prior to treatment initiation came back on day 3, and were negative for pathogenic bacteria. White blood cell (WBC) counts were trending downward, and the patient appeared to be stabilizing. She still had an elevated WBC count, slight fever (temperature maximum of 101.4F for the past 24 hours), and lung crackles at the right lung base. Because Gram stain failed to identify Gram‐positive cocci clusters, and there was no culture evidence of methicillin‐resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa, vancomycin treatment was terminated and the patient was switched to single‐agent therapy with intravenous ceftriaxone, a nonpseudomonal third‐generation cephalosporin. On hospital day 5, there was continuing evidence of response to antimicrobial therapy. The patient reported feeling better and she was breathing comfortably. Her cough was much improved, sputum production was markedly decreased, and her fever had resolved. Now, on day 7, the patient is still afebrile, her WBC count is normal, and she has 96% oxygen saturation on room air.
The question before the clinician is whether to terminate or continue antimicrobial therapy, and if continued, with what regimen and for how long? In addition, if a decision is made to continue antimicrobial therapy, there is a possibility of switching from an intravenous to oral treatment regimen. An examination of the literature and current treatment guidelines for HCAP/HAP/VAP should enable a more informed decision, one that optimally benefits not only this patient, but all subsequent ones who might be exposed and infected with a resistant pathogen that develops when treatment is continued longer than necessary.
Using Clinical Parameters to Shorten Antimicrobial Therapy
A prospective study by Dennesen et al., published 10 years ago, was one of the first suggesting the possibility of shortened duration of antimicrobial therapy for VAP.80 At the time, duration of antimicrobial therapy for VAP typically ranged from 7 to 21 days, and was most commonly 14 to 21 days. In this study, Dennesen and coworkers examined symptom resolution in 27 patients diagnosed with VAP based on clinical, radiologic, and microbiological criteria, each of whom received appropriate antimicrobial therapy based on culture susceptibility data.80 Significant improvements were observed for all clinical parameters examined (highest temperature, leukocyte count, pressure of arterial oxygen to fractional inspired oxygen [PaO2/FIO2] ratio, semiquantitative culture result of endotracheal aspirate), usually first appearing within the first 6 days of antimicrobial therapy. Furthermore, analyses of specific pathogens showed that appropriate antimicrobial therapy rapidly eradicated endotracheal colonization with S pneumoniae, Haemophilus influenzae, and S aureus, but not of P aeruginosa or Enterobacteriaceae. Moreover, endotracheal colonization with resistant pathogens tended to occur when antimicrobial therapy was continued beyond the first week. Taken together, these results suggested that prolonged antimicrobials beyond 7 days usually did not benefit VAP patients, and in fact increased risk of superinfection with a resistant strain. However, it is important to make a distinction between VAP and, for example, skin or bloodstream infections involving S aureus. While improved signs and symptoms generally indicate clinical cure for VAP, this reasoning should not be applied to S aureus bacteremia.
The findings from Dennesen et al. are generally consistent with those from Montravers et al., which showed that 94% of respiratory pathogens were eradicated from the lungs of VAP patients 3 days after initiation of antimicrobial therapy.78 They are also consistent with the findings from a 2005 study by Vidaur et al., which demonstrated resolution of fever (38C), PaO2/FIO2 (>250 mmHg), and WBC/leukocyte count (10,000) in 73%, 75%, and 53% of VAP patients, respectively, without acute respiratory distress syndrome (ARDS; n = 75) after 3 days of appropriate antimicrobial therapy.81 However, Vidaur et al. reported that fever took roughly twice as long to resolve in VAP patients with ARDS (n = 20) versus without ARDS, and that hypoxia resolution was less useful when evaluating treatment response in ARDS patients. As with the Dennesen et al. study,80 the results from Vidaur et al. suggest that measures of core body temperature and oxygenation can be useful guides for clinicians in determining whether to shorten the duration of antimicrobial therapy for patients with VAP, HAP, or HCAP.81
Along the same lines, the clinical pulmonary infection score (CPIS) has established itself as a means for the early termination (shortening) of initial empiric antimicrobial therapy in particular VAP patients. The CPIS is derived by scoring 57 clinical indices relevant for the diagnosis of VAP, as illustrated in Table 1.82 A score of >6 is considered suggestive of pneumonia, while one 6 implies low likelihood of pneumonia. A 2000 study by Singh et al. randomized 81 consecutive patients with pulmonary infiltrates and a CPIS 6 to receive either standard antimicrobial therapy (at discretion of the clinician) or ciprofloxacin monotherapy, with the intention of reevaluating patients at day 3.45 For patients in the ciprofloxacin (experimental) group, antimicrobial therapy was terminated at day 3 if the CPIS remained 6. As a result, only 28% of patients in the experimental group had antimicrobial therapy continued beyond day 3, compared with 90% of patients in the standard therapy group (P = 0.0001). More importantly, there were no significant differences in mortality between patients in the 2 treatment groups, despite a significantly shorter treatment duration for those in the experimental group (3.0 vs 9.8 days, P = 0.0001). In addition, mean length of intensive care unit (ICU) stay was significantly shorter (9.4 vs 14.7 days, P= 0.04) and mean antimicrobial cost was significantly lower ($259 vs $640, P = 0.0001) for patients in the experimental versus standard therapy group.
| Points | |
|---|---|
| |
| Temperature C | |
| 36.5 and 38.4 | 0 |
| 38.5 and 38.9 | 1 |
| 39 or 36.0 | 2 |
| Tracheal secretions | |
| Absence of secretions | 0 |
| Presence of non‐purulent secretions | 1 |
| Presence of purulent secretions | 2 |
| Pulmonary radiography (chest X‐ray) | |
| No infiltrate | 0 |
| Diffused (or patchy) infiltrate | 1 |
| Localized infiltrate | 2 |
| WBCs, leukocytes/mm3 | |
| 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 |
| +Band forms 500 | 2 |
| Oxygenation: PaO2/FIO2 mmHg | |
| >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 |
| Culture of tracheal aspirate (semiquantitative: 012 or 3+) | |
| Pathogenic bacteria cultured 1+ or no growth | 0 |
| Pathogenic bacteria cultured >1+ + same pathogenic bacteria seen on the gram stain >1+ | 1 2 |
| Progression of pulmonary infiltrate | |
| No radiographic progression | 0 |
| Radiographic progression (ARDS excluded) | 2 |
Furthermore, a significantly greater proportion of patients in the standard versus experimental therapy group exhibited evidence of antimicrobial resistance or superinfections (38% vs 14%, P = 0.017). The 2005 clinical practice guidelines for HAP, VAP, or HCAP state, A modified CPIS of 6 or less for 3 days, proposed by Singh and coworkers, is an objective criterion to select patients at low risk for early discontinuation of empiric treatment of HAP.53 While the Singh et al. study provides the rationale for shorter‐course therapy in ICU patients with pulmonary infiltrates who have low likelihood of pneumonia (CPIS 6), this criterion may or may not pertain to HAP/VAP more strictly, and still requires validation in patients with more severe forms of VAP. Incidentally, although the CPIS was designed to define VAP, and there are no data validating its use for other types of pneumonia, the clinical experience by this author indicates that it can be helpful in evaluating HCAP and non‐VAP HAP as well.
Clinical Trial to Support Shortened Duration of HCAP/HAP/VAP Therapy
A French study published in JAMA in 2003 provides more direct support that approximately 1 week of antimicrobial therapy produces effectiveness comparable to more traditional 23‐week therapy for most patients with VAP.37 In this prospective, multicenter, randomized, double‐blind (until day 8) clinical trial, 401 patients with microbiologically proven VAP were randomly assigned to receive either 8 days (n = 197) or 15 days (n = 204) of initial empiric antimicrobial therapy selected by the treating physician. No significant differences were observed between the 8‐day and 15‐day treatment groups for the 2 primary efficacy endpoints of death from any cause (18.8% vs 17.2%) and microbiologically documented pulmonary infection recurrence (28.9% vs 26.0%). There were also no differences between the groups for number of mechanical ventilation‐free days (8.7 vs 9.1 days), number of organ‐failure‐free days (8.7 vs 8.9 days), length of ICU stay (30.0 vs 27.5 days), unfavorable outcome (death, pulmonary infection recurrence, or prescription of a new antimicrobial) (46.2% vs 43.6%), mortality rate on day 60 (25.4% vs 27.9%), or in‐hospital mortality (32% vs 29.9%).
Conversely, patients in the 8‐day treatment group had significantly more antimicrobial‐free days (13.1 vs 8.7 days, P 0.001), and among patients who developed recurrent infections, multidrug‐resistant pathogens emerged more frequently in patients in the 15‐day versus 8‐day treatment group (62.0% vs 42.1%, P = 0.04). However, there was an apparent exception to the general comparable efficacy of the 8‐ and 15‐day treatment regimens for infections caused by nonfermenting Gram‐negative bacilli, including P aeruginosa. For primary infections caused by nonfermenting Gram‐negative bacilli, the 8‐day versus 15‐day regimen was associated with higher rates of pulmonary recurrence (40.6% vs 25.4%). Interestingly, the 8‐day regimen was not associated with more adverse outcomes here, just a higher recurrence rate. With respect to primary infections caused by MRSA, no differences were observed between the 2 treatment regimens for death for all causes (23.4% vs 30.2%) or pulmonary infection recurrence (33.3% vs 42.9%). Figure 1 presents the probability of survival data for the 8‐day and 15‐day treatment groups.

Hence, the data from the Chastre et al. study37 support use of an 8‐day (or shortened) regimen as standard antimicrobial therapy for most patients with VAP, with some possible exceptions. Additional studies provide further support for this general conclusion. For example, a prospective, randomized, controlled trial by Micek et al. evaluated the impact of using an antimicrobial discontinuation policy based on clinical criteria (discontinuation group; n = 150)versus the decision of treating physicians (conventional group, n = 140)to determine the duration of antimicrobial therapy for VAP, and observed a statistically shorter treatment duration in the discontinuation versus conventional management group (6.0 vs 8.0 days, P = 0.001), but no difference between the groups for hospital mortality (32.0% vs 37.1%), ICU length of stay (6.8 vs 7.0 days), or VAP recurrence (17.3% vs 19.3%).42 A prior study by the same group reported a shorter duration of antimicrobial therapy for VAP following implementation of an antimicrobial guideline (vs prior to implementation) (8.6 vs 14.8 days, P 0.001), and a lower rate of VAP recurrence among patients in the after period (7.7% vs 24.0%, P = 0.03). However, interpretation of the results was complicated by the fact that initial empiric therapy was more often appropriate during the after versus before guideline implementation period (94.2% vs 48.0%, P 0.001).40
A limited number of studies have focused further on shortened duration of therapy for patients with VAP caused by Gram‐negative bacteria, and particularly by nonfermenting Gram‐negative bacilli. A retrospective study by Hedrick et al. analyzed the relationship between antimicrobial duration and outcomes of 452 episodes of VAP in the ICU, 154 caused by nonfermenting Gram‐negative bacilli.39 In the study, 127 patients infected with a nonfermenting Gram‐negative bacillus received 9 days (mean 17.1 0.7 days) of antimicrobial therapy, while 27 received 3‐8 days (mean 6.4 0.3 days) of therapy. No significant differences were observed between the shorter‐ and longer‐duration groups for mortality (22% vs 14%, P = 0.38) or VAP recurrence (22% vs 34%, P = 0.27) for these patient populations. Table 2 provides the results for all 452 VAP episodes based on 8 days or 9 days of antimicrobial therapy.
| Patient Characteristic | 8 Days (n = 98) | 9 Days (n = 354) | P Value |
|---|---|---|---|
| |||
| Mean antimicrobial days | 6.2 | 16.8 | 0.0001 |
| Mean APACHE II | 18 | 20 | 0.0009 |
| % Trauma | 71 | 68 | 0.63 |
| Mean time to onset, days | 17.7 | 17.8 | 0.97 |
| Recurrence | 11% | 25% | 0.004 |
| Death | 13% | 11% | 0.59 |
| Nonfermenting Gram‐negative bacilli recurrence | 22% (n = 27) | 34% (n = 127) | 0.27 |
| Staphylococcus aureus recurrence | 20% (n = 10) | 38% (n = 47) | 0.47 |
The retrospective nature of the study limits the ability to more confidently interpret the results, but the data appear to be consistent with the conclusion that short‐duration therapy does not necessarily increase recurrence or worsen other outcomes in patients with VAP caused by nonfermenting Gram‐negative bacilli. The most common Gram‐negative bacilli associated with VAP in the study were P aeruginosa (18% of all infections), Enterobacter cloacae (11%), Acinetobacter spp (11%), Klebsiella pneumoniae (7%), Stenotrophomonas maltophilia (7%), Serratia spp (7%), H influenzae (6%), and Escherichia coli (4%). In addition, the study results suggest that short‐duration therapy is at least as effective as longer‐duration therapy for the overall VAP population, with potential benefits in terms of reduced antimicrobial use and lower rate of recurrence.
Another recent retrospective analysis examining an even shorter course of antimicrobial therapy (5 days) for patients with HAP associated with Gram‐negative bacteria reported a low overall recurrence rate (14%) and a critical care mortality rate (34.2%) in line with prior studies of short‐term therapy for VAP/HAP.44 However, the HAP relapse rate was significantly higher in patients with HAP caused by nonfermenting Gram‐negative bacilli versus other Gram‐negative species (17% vs 2%, P = 0.03).
A recent US pilot study explored the use of repeat bronchoalveolar lavage (BAL) to guide antimicrobial duration in 52 patients with VAP, and compared the results with a matched control group of 52 VAP patients treated before institution of the BAL pathway.43 Antimicrobial therapy in the pathway patients was discontinued if pathogen growth was 10,000 colony forming units/mL on the repeat BAL performed on day 4 of therapy. One objective was to determine whether a repeat BAL strategy, such as the one here, might be able to identify patients with VAP due to nonfermenting Gram‐negative bacilli or other microorganisms who could be safely and effectively treated with shorter‐duration therapy.
Results showed that the antimicrobial duration was significantly shorter for patients in the pathway group than the matched control group (9.8 vs 3.8 days, P 0.001), including the subset of patients with VAP associated with nonfermenting Gram‐negative bacilli (10.7 vs 14.4 days, P 0.001). No significant differences were observed between the overall treatment populations for VAP recurrence, mechanical ventilator‐free ICU days, ICU‐free hospital days, or mortality. Repeat BAL showed most VAP isolates in the study group (83%) responded to initial therapy with a mean duration of 8.8 days. Nonresponders without concomitant infections received significantly longer treatment than pure responding isolates (14.4 vs 7.3 days, P 0.001), and the most common nonresponding microorganisms were P aeruginosa (41% response rate) and S maltophilia (50% response rate), 2 nonfermenting Gram‐negative bacilli.
Most nonfermenting Gram‐negative bacilli‐associated VAP isolates in the study group did respond on repeat BAL (59%). These responders were treated for a mean duration of 8.2 days, and exhibited a similar recurrence rate versus that observed for the matched control group (12.0% vs 17.9%, P = 0.71). These pilot study results suggest that repeat BAL might be used to identify patients likely to benefit from short‐duration therapy, including patients infected with nonfermenting Gram‐negative bacilli. Further study on this is needed.
ATS/IDSA Guidelines for Duration of HCAP/HAP/VAP Therapy
Based largely on the studies by Dennesen et al.80 and Luna et al.83 indicating most VAP patients who respond to appropriate antimicrobial therapy do so within the first 6 days, and those by Chastre et al.37 and Singh et al.45 pointing to the efficacy and safety of shorter‐duration VAP therapy, the 2005 ATS/IDSA guidelines recommend the use of shorter‐duration antimicrobial therapy for most patients with HCAP or HAP/VAP.53 More specifically, the guidelines state, If patients receive an initially appropriate antimicrobial regimen, efforts should be made to shorten the duration of therapy from the traditional 14 to 21 days to periods as short as 7 days, provided that the etiologic pathogen is not P. aeruginosa, and that the patient has a good clinical response with resolution of clinical features of infection.53 Figure 2 presents an overview of the ATS/IDSA guidelines for HCAP/HAP/VAP management 48 to 72 hours after initiation of empiric antimicrobial therapy.53

Note that the clinician should consider terminating antimicrobial therapy in patients with clinical improvement and negative cultures or other evidence suggestive of a noninfectious cause. CPIS can also be helpful when deciding whether to terminate initial empiric therapy in a patient with clinical improvement after 23 days of therapy and negative cultures. If cultures are positive, the clinician should consider whether antimicrobial de‐escalation is possible (as discussed by Dr Kaye in the corresponding supplement article), and aim to treat selected patients with an antimicrobial course lasting 78 days. After 78 days, patients should be reassessed for treatment termination or other appropriate actions.
The ATS/IDSA guidelines also provide recommendations for route of drug administration, and if and when to switch from an intravenous to oral agent. In particular, the guidelines state that all patients with HCAP, HAP, or VAP should initially receive therapy intravenously, but conversion to oral/enteral therapy may be possible in certain responding patients, ie, those with a good clinical response and a functioning intestinal tract.53 Fluoroquinolones and linezolid have oral formulations with bioavailability equivalent to the intravenous form, meaning the oral formulations are capable of achieving high levels at the site of infection. This may facilitate conversion to oral therapy in select patients. Early step‐down is safe and effective with fluoroquinolones.84, 85
Based on the information just reviewed, the antimicrobial can be terminated on day 7 for case 1. She is afebrile, and her WBC and oxygenation are normal. In fact, since her records show she was responding at day 5, consideration could have been given to switching from intravenous to oral therapy at that time, and perhaps even discharging her to the rehabilitation center.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with sepsis and diverticular abscess with walled‐off perforation. Upon hospital arrival, Interventional Radiology inserted a drain, and the patient was initiated on ciprofloxacin and metronidazole therapy. Day 3 examination showed improvement in WBC count and normal vital signs, but the patient still had a low‐grade fever (100.9F). Abdominal examination results were improved, but with some diffuse tenderness. Initial cultures of the abdominal abscess isolated Gram‐negative rods, and the patient was continued on ciprofloxacin/metronidazole. Further cultures on day 4 identified an extended‐spectrum ‐lactamase (ESBL)‐producing E coli organism as the causative pathogen. The patient was switched from ciprofloxacin/metronidazole to ertapenem. It is now hospital day 8, and the patient continues to show good response to treatment. She is afebrile and WBC count is normal. The abscess catheter is no longer draining. Her abdominal pain is improved, and she is complaining that she is hungry. A repeat computed tomography scan shows resolution of the abscess and no evidence of bowel perforation. Should antimicrobial therapy be continued in this patient, and if so, with what agent and for how long?
Guidelines from the Surgical Infection Society and IDSA state that antimicrobial therapy of established or complicated intra‐abdominal infection in adults should be limited to 47 days, unless it is difficult to achieve adequate source control.54 This is because extended antimicrobial exposure increases antimicrobial cost and risk of resistance, superinfection, C difficile‐associated colitis, or other untoward and unintended consequences of antimicrobial therapy, and there is no evidence that longer treatment durations improve outcomes.46, 47, 54, 86 Runyon et al. randomized 90 patients with spontaneous bacterial peritonitis or culture‐negative neutrocytic ascites to receive 5 days or 10 days of cefotaxime monotherapy, and reported similar rates of infection‐related mortality (0% vs 4.3%), hospitalization mortality (33% vs 43%), bacteriologic cure (93% vs 92%), and recurrence of ascitic fluid infection (12% vs 13%).46 Furthermore, shorter‐course therapy was associated with significantly lower antimicrobial administration and costs. Similarly, a recent prospective, randomized, double‐blind trial comparing 3 versus 5 days of ertapenem therapy in 111 patients with community‐acquired intra‐abdominal infection reported similar cure (93% vs 90%) and eradication rates (95% vs 94%).86 However, it should be noted that the mean duration of antimicrobial therapy in the longer‐duration group was still relatively short (5.7 days, range of 5‐10 days).
Studies also indicate there is a very low risk of infection recurrence or treatment failure when antimicrobial therapy is terminated in a patient diagnosed with a complicated intra‐abdominal infection who no longer shows signs of continuing infection.38, 87 Lennard et al. compared postoperative outcomes in 65 patients with or without leukocytosis and fever at the conclusion of antimicrobial therapy for intra‐abdominal sepsis, and reported development of intra‐abdominal infection in 7 of 21 (33%) with persistent leukocytosis.87 None of the 30 patients with normal WBC counts at the end of therapy developed an intra‐abdominal infection postoperatively. Furthermore, intra‐abdominal infection occurred postoperatively in 11 of 14 patients (79%) who responded to treatment but were still febrile at the time of antimicrobial discontinuation.
Similar results were obtained in a much larger, more recent study that retrospectively analyzed the relationship between duration of antimicrobial therapy and infectious complications for patients with intra‐abdominal infections.38 In the study, 929 patients with intra‐abdominal infections associated with either fever or leukocytosis were organized into 4 quartiles based on total duration of antimicrobial therapy (quartile 1: 07 days, n = 218; quartile 2: 812 days, n = 217; quartile 3: 1317 days, n = 246; and quartile 4: >17 days, n = 248) or antimicrobial duration after resolution of leukocytosis (quartile 1: 05 days, n = 130; quartile 2: 610 days, n = 127; quartile 3: 1115 days, n = 124; and quartile 4: >15 days, n = 118). Based on either total duration of antimicrobial therapy or duration after leukocytosis resolution, risk of recurrence was significantly higher for patients in quartiles 3 or 4 versus those in quartile 1, and there was no difference between quartiles 1 and 2.
Taken together, these results suggest that antimicrobial therapy for intra‐abdominal sepsis can be shortened in patients exhibiting a clinical response to treatment, if there are no signs of persistent leukocytosis or fever. Hence, clinicians should use the resolution of clinical signs of infection as a guide to determine when during the 47‐day window antimicrobial therapy should be terminated.54 In practical terms, this usually means treatment can be terminated when the patient is afebrile, has normal WBC counts, and is able to tolerate an oral diet.
Based on the clinical status of case 2 after 8 days of antimicrobial therapy (afebrile with normal WBC counts and requesting oral diet), the ertapenem regimen should be stopped. There is no reason to consider further outpatient antimicrobial therapy for this particular patient, but the Surgical Infection Society and IDSA guidelines discuss the type of patient who should be considered for oral or outpatient antimicrobial therapy. According to the guidelines, the patient convalescing from a complicated intra‐abdominal infection may receive oral antimicrobial therapy, but that therapy should only be included as a component within the brief treatment duration already mentioned, ie, in total, it should rarely exceed 7 days.54 Such therapy is rarely indicated for patients who are afebrile, with normal peripheral WBC/leukocyte counts, and with return of bowel function. These recommendations make it clear that no further antimicrobial therapy is warranted for case 2.
However, for appropriate patients who are recovering from a complicated intra‐abdominal infection and are able to tolerate an oral diet, an oral antimicrobial regimen selected on the basis of identified primary isolates may be used for completion of therapy.54 In the absence of cultures, an oral regimen that covers commonly isolated pathogens (eg, E coli, streptococci, and Bacteroides fragilis) should be considered. Common regimens include an oral cephalosporin or fluoroquinolone with metronidazole, or amoxicillin‐clavulanic acid, assuming susceptibility studies do not demonstrate resistance. Given the identification of an ESBL‐producing E coli for case 2a pathogen relatively resistant to oral antimicrobialan oral regimen probably would not have been viable for this patient even earlier in the treatment course. Lastly, a repeat computed tomography scan was used for the case here. It should be noted that there are currently no well‐established criteria for determining when repeat imaging is needed to confirm resolution of fluid collections. This should be a clinical decision. A general practice is that the catheter is left in place until there is minimal drainage (eg, 10 mL/day); catheter sinograms can also be helpful in determining the status of the abscess.
CASE 3: CENTRAL LINE‐ASSOCIATED BACTEREMIA
Case 3 is a 56‐year‐old man with status epilepticus, intubation, and ICU stay. He was initially treated with vancomycin and piperacillin‐tazobactam for a fever of 103.4F on day 5 of hospitalization. Blood cultures grew Gram‐positive cocci. The central venous catheter was removed, and the initial antimicrobial regimen was de‐escalated to vancomycin monotherapy, which was associated with continued improvement in fever and WBC count, and clinical stability on hospital day 7. At that time, further blood culture analyses isolated methicillin‐susceptible S aureus (MSSA), and the antimicrobial regimen was switched/de‐escalated from vancomycin to cefazolin. It is now hospital day 9 (day 3 of cefazolin) and the patient continues to respond and is afebrile. Repeat blood cultures show no bacterial growth, and a transesophageal echocardiograph (TEE) was performed and revealed normal heart valves. Should the antimicrobial therapy be continued for this patient, and if so, with what agent and for how long?
The IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal and 46 weeks of antimicrobial therapy for patients with S aureus catheter‐related bloodstream infection (CRBSI), unless the patient has exceptions allowing consideration of shorter‐duration therapy (minimum of 14 days, with day 1 being the first day of negative blood culture results).55 These exceptions include absence of diabetes; immunocompetence (no immunosuppression); removal of the infected catheter; no prosthetic intravascular device (eg, pacemaker or recently placed vascular graft); no evidence of endocarditis or suppurative thrombophlebitis on TEE and ultrasound, respectively; fever and bacteremia resolved within 72 hours after initiation of appropriate antimicrobial therapy; and no evidence of metastatic infection on physical examination and sign‐ or symptom‐directed diagnostic tests.
Short‐duration (10‐16 day) antimicrobial therapy has been reported to yield similarly low recurrence or relapse rates as longer courses of therapy in patients with uncomplicated catheter‐associated S aureus bacteremia.50, 51, 88, 89 A small 1989 study by Ehni and Reller prospectively followed 13 patients with S aureus CRBSI who had received short‐course therapy (17 days), and reported only 1 case of relapse with endocarditis (8% relapse rate).50 A subsequent study by Malanoski et al. retrospectively analyzed the data from 55 patients with S aureus CRBSI.51 Excluding the 8 patients with early complications, the authors observed similar rates of relapse in patients treated for 1015 days and those receiving longer courses of antimicrobial therapy (0% vs 4.7%). The clinical characteristics of the 2 treatment duration groups were similar, and delayed catheter removal was linked with persistence of bacteremia (P = 0.01).
A more recent multicenter, prospective observational study by Chang et al. examined recurrence and the impact of antimicrobial treatment in 505 consecutive patients with S aureus bacteremia, and determined that duration of antimicrobial therapy was not a factor associated with relapse.88 This was true both for patients with bacteremia resulting from endocarditis, bacteremia with no apparent source, or bacteremia due to a focus that could not be cured or removed (28 days therapy after defervescence, 28 days therapy, or 28 days therapy), or those with bacteremia resulting from a source amenable to definitive cure, such as an intravascular device that could be removed, an abscess that could be incised and drained, or an infected bone that could be resected (>14 days, 1014 days, or 10 days therapy). Similarly, a 2005 prospective study by Thomas and Morris determined there was no relationship between treatment duration (7 vs 8 days, 10 vs 10 days, or 14 vs 15 days; P = 0.62, 0.87, and 0.16, respectively) and rate of relapse for 276 patients with cannula‐associated S aureus bacteremia.89 Longer‐duration antimicrobial therapy is warranted in patients with CRBSI and an early complicated course, eg, fever and/or bacteremia persisting for >3 days after catheter removal.90
According to the IDSA guidelines, a TEE should be obtained for all patients with CRBSI involving S aureus who are being considered for a shorter duration of therapy, and the TEE should be performed at least 57 days after onset of bacteremia to minimize risk of false‐negative results.55 High rates of infective endocarditis are observed in patients with S aureus bacteremia,89, 9193 with higher rates in patients with MSSA versus MRSA bacteremia (43.4% vs 19.6%, P 0.009).91 TEE is essential to diagnose endocarditis and detect other complications of bacteremia.92, 93 This recommendation for use of TEE does not necessarily apply to all patients with CRBSI when S aureus is not involved.
Figure 3 summarizes the general recommendations from the IDSA guidelines for the management of CRBSI in patients with a short‐term catheter.55 The figure illustrates the varied recommendations for treatment duration depending on whether the infection is complicated or uncomplicated, and based on the pathogenic microorganism. Returning to case 3, the patient meets the general criteria for shorter duration of antimicrobial therapy: he is not diabetic or immunosuppressed, his catheter has been removed, he does not have any prosthetic intravascular devices, his fever and bacteremia (based on blood cultures) resolved within 3 days of initiating cefazolin therapy, and there is no evidence of endocarditis or other complications of bacteremia. Hence, he is an excellent example of a patient with uncomplicated MSSA CRBSI who meets the criteria for consideration of shortened antimicrobial therapy. Based on the clinical practice guidelines, the patient should continue on intravenous cefazolin for a 14‐day course of therapy, at which time he can be re‐evaluated. A recent review of bloodstream infections caused by various pathogens similarly concluded that the minimum treatment duration for low‐risk patients with S aureus CRBSI is 14 days.48 As a final point, it is also important to note that there is no role for oral therapy in patients with CRBSI, so whether shortened or not, the chosen regimen should be administered intravenously.

CONCLUSIONS
Shortening the duration of appropriate and adequate antimicrobial therapy represents one strategy for reducing pressure for selection or development of resistant pathogenic microorganisms. Other potential benefits of shorter courses of antimicrobial therapy include reduced risk of antimicrobial‐associated infections (superinfection, C difficile‐associated diarrhea) and other antimicrobial‐related adverse events, improved compliance, and reduced antimicrobial costs. Clinicians are sometimes concerned that reducing antimicrobial courses for patients with serious infections, such as HCAP/HAP/VAP, complicated intra‐abdominal infection, and CRBSI, will lead to incomplete eradication of pathogenic microorganisms, leading to disease recurrence and increased morbidity and mortality. When managing patients with these serious infections, clinicians often turn to the literature and recommendations from professional organizations for guidance. Available data from randomized controlled and nonrandomized clinical trials indicate that shorter‐course therapy is effective and safe for patients with CAP, HCAP/HAP/VAP, complicated intra‐abdominal infections, and CRBSI. Based on these data, and consensus/expert opinion, clinical practice guidelines have been developed that recommend specific durations of antimicrobial therapy for each of these infections.
Although greater study of antimicrobial therapy duration is needed, the current and developing literature and current treatment guidelines should enable clinicians to recognize patients who would benefit from shortened courses of antimicrobial therapy. In doing so, they would help to lower antimicrobial costs and reduce the growing problem of antimicrobial resistance, with its wide‐ranging, negative consequences for current and future patients, and the clinicians who treat them.
- .The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs.Clin Infect Dis.2006;42(suppl 2):S82–S89.
- ,,.Clinical and economic burden of antimicrobial resistance.Expert Rev Anti Infect Ther.2008;6:751–763.
- .Economics of antibiotic resistance.Expert Rev Anti Infect Ther.2008;6:523–539.
- ,,,.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43:1379–1382.
- ,,,,.Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis.BMJ.2010;340:c2096.
- ,,,,,.Risk factors for invasive pneumococcal disease in children: a population‐based case‐control study in North America.Pediatrics.1999;103:E28.
- ,,.Antimicrobial resistance: consideration as an adverse drug event.Crit Care Med.2010;38:S155–S161.
- ,,,,,.Fluoroquinolone‐resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact.Am J Med.2006;119:526.e519–526.525.
- ,,, et al.Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates.Am J Med.2008;121:876–884.
- ,,,,,.Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes.Infect Control Hosp Epidemiol.2006;27:893–900.
- ,,,,.Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals.Clin Infect Dis.2005;41:435–440.
- ,,,.Evaluation of an intervention designed to decrease the rate of nosocomial methicillin‐resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use.Infect Control Hosp Epidemiol.2006;27:155–169.
- ,,,,,.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals.Infect Control Hosp Epidemiol.2007;28:1389–1395.
- ,,, et al.Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned.Arch Intern Med.2002;162:1515–1520.
- ,,,,.Nosocomial outbreak of Klebsiella infection resistant to late‐generation cephalosporins.Ann Intern Med.1993;119:353–358.
- ,,.The effect of an antimicrobial restriction program on Pseudomonas aeruginosa resistance to beta‐lactams in a large teaching hospital.Pharmacotherapy.2003;23:618–624.
- ,,,.Association between antibiotic usage and subsequent colonization or infection of extensive drug‐resistant Acinetobacter baumannii: a matched case‐control study in intensive care units.Diagn Microbiol Infect Dis.2008;62:298–305.
- ,,.Relationships between antimicrobial use and antimicrobial resistance in Gram‐negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan.Int J Antimicrob Agents.2005;26:463–472.
- ,,, et al.Risk factors for acquisition of imipenem‐resistant Acinetobacter baumannii: a case‐control study.Antimicrob Agents Chemother.2004;48:224–228.
- ,,,,.Consumption of imipenem correlates with beta‐lactam resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother.2002;46:2920–2925.
- ,,.Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem‐resistant Pseudomonas aeruginosa.Antimicrob Agents Chemother.2009;53:1983–1986.
- .Clinical efficacy of newer agents in short‐duration therapy for community‐acquired pneumonia.Clin Infect Dis.2004;39(suppl 3):S159–S164.
- ,.The role of carbapenems in the treatment of severe nosocomial respiratory tract infections.Expert Opin Pharmacother.2008;9:561–575.
- ,.Does short‐course antibiotic therapy better meet patient expectations?Int J Antimicrob Agents.2003;21:222–228.
- ,,,.Tackling empirical antibiotic therapy for ventilator‐associated pneumonia in your ICU: guidance for implementing the guidelines.Semin Respir Crit Care Med.2009;30:102–115.
- World Health Organization (WHO) report on infectious diseases 2000. Overcoming antimicrobial resistance. Chapter 5. Call to action: A massive effort to provide proper treatment. Available at: http://www.who.int/infectious‐disease‐report/2000/index.html. Accessed January 14,2011.
- ,,,,.Diagnosis and management of group A streptococcal pharyngitis: a practice guideline. Infectious Diseases Society of America.Clin Infect Dis.1997;25:574–583.
- ,,,,,.Shorter‐course antibiotic therapy (SCAT): principles, current data, and caveats. In: Owens RCJ, Lautenbach E, editors.Antimicrobial Resistance: Problem Pathogens and Clinical Countermeasures.New York, NY:Informa Healthcare;2008:337–370.
- ,,, et al.International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases.Clin Infect Dis.2011;52:e103–e120.
- ,.Sexually transmitted diseases treatment guidelines, 2006.MMWR Recomm Rep.2006;55:1–94.
- ,,,,.Novel, single‐dose microsphere formulation of azithromycin versus 7‐day levofloxacin therapy for treatment of mild to moderate community‐acquired pneumonia in adults.Antimicrob Agents Chemother.2005;49:4035–4041.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,.Randomized, multicentre study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of adults with mild to moderate community‐acquired pneumonia. Azithromycin Study Group.Eur J Clin Microbiol Infect Dis.1998;17:828–833.
- ,,,,,.A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia. The optimal duration of therapy.Chest.1996;110:965–971.
- ,,,.Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator‐associated pneumonia to decrease resistance in the intensive care unit.Clin Infect Dis.2006;43(suppl 2):S75–S81.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- ,,, et al.Can we define the ideal duration of antibiotic therapy?Surg Infect (Larchmt).2006;7:419–432.
- ,,,,,.Duration of antibiotic therapy for ventilator‐associated pneumonia caused by non‐fermentative gram‐negative bacilli.Surg Infect (Larchmt).2007;8:589–597.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,.Antibiotic utilization and outcomes for patients with clinically suspected ventilator‐associated pneumonia and negative quantitative BAL culture results.Chest.2005;128:2706–2713.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Repeat bronchoalveolar lavage to guide antibiotic duration for ventilator‐associated pneumonia.J Trauma.2007;63:1329–1337.
- ,,.Short course antibiotic therapy for Gram‐negative hospital‐acquired pneumonia in the critically ill.J Hosp Infect.2010;74:337–343.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,.Short‐course versus long‐course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients.Gastroenterology.1991;100:1737–1742.
- ,,,,.Complicated appendicitis: is there a minimum intravenous antibiotic requirement? A prospective randomized trial.Am Surg.2000;66:887–890.
- ,,.Short‐course therapy for bloodstream infections in immunocompetent adults.Int J Antimicrob Agents.2009;34(suppl 4):S47–S51.
- ,,,.Short‐course monotherapy strategy for treating bacteremia in the critically ill.Minerva Anestesiol.2006;72:841–857.
- ,.Short‐course therapy for catheter‐associated Staphylococcus aureus bacteremia.Arch Intern Med.1989;149:533–536.
- ,,,.Staphylococcus aureus catheter‐associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters.Arch Intern Med.1995;155:1161–1166.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44(suppl 2):S27–S72.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,.Hospital‐acquired pneumonia: pathophysiology, diagnosis, and treatment.Surg Clin North Am.2009;89:439–461, ix.
- ,,.Healthcare‐associated pneumonia in adults: management principles to improve outcomes.Infect Dis Clin North Am.2004;18:939–962.
- ,,.Healthcare‐associated infections. A useful concept?Curr Opin Crit Care.2009;15:419–424.
- ,,,,,.Efficacy of 750‐mg, 5‐day levofloxacin in the treatment of community‐acquired pneumonia caused by atypical pathogens.Curr Med Res Opin.2004;20:555–563.
- ,,, et al.Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study.BMJ.2006;332:1355.
- ,,,,.Gemifloxacin once daily for 5 days versus 7 days for the treatment of community‐acquired pneumonia: a randomized, multicentre, double‐blind study.J Antimicrob Chemother.2007;60:112–120.
- ,,.Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community‐acquired pneumonia of suspected pneumococcal origin.Respir Med.2004;98:708–720.
- ,,,.Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective, randomized, double‐blind study.Am J Ther.1999;6:217–222.
- ,,,,.Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community‐acquired pneumonia.J Antimicrob Chemother.2004;54:515–523.
- ,,,,,.Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis.Drugs.2008;68:1841–1854.
- ,,,.Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,,, et al.Low dosage and long treatment duration of beta‐lactam: risk factors for carriage of penicillin‐resistant Streptococcus pneumoniae.JAMA.1998;279:365–370.
- ,,, et al.Effect of short‐course, high‐dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial.JAMA.2001;286:49–56.
- ,,,.Antipneumococcal activity of ertapenem (MK‐0826) compared to those of other agents.Antimicrob Agents Chemother.2002;46:42–46.
- ,,.MIC and time‐kill study of antipneumococcal activities of RPR 106972 (a new oral streptogramin), RP 59500 (quinupristin‐dalfopristin), pyostacine (RP 7293), penicillin G, cefotaxime, erythromycin, and clarithromycin against 10 penicillin‐susceptible and ‐resistant pneumococci.Antimicrob Agents Chemother.1996;40:2071–2074.
- ,,.Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials.J Antimicrob Chemother.2003;51:443–446.
- ,,.Antipneumococcal activity of ertapenem compared to nine other compounds by time‐kill [abstract E‐800]. In:Program and Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago).Washington, DC:American Society for Microbiology,2001:184.
- ,,.Time‐kill analysis of the antipneumococcal activity of daptomycin compared with 8 other agents. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:161.
- ,,.Post‐antibiotic effect of garenoxacin against gram‐positive and gram‐negative organisms [abstract A‐496]. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:42.
- ,,, et al.Short‐course therapy of gemifloxacin effective against pneumococcal pneumonia in mice.J Chemother.2006;18:634–640.
- ,,.Animal models of Streptococcus pneumoniae disease.Clin Microbiol Rev.2008;21:666–685.
- ,,,,.Protective effect of trovafloxacin, ciprofloxacin and ampicillin against Streptococcus pneumoniae in a murine sepsis model.J Antimicrob Chemother.1999;44:477–481.
- ,,, et al.Follow‐up protected specimen brushes to assess treatment in nosocomial pneumonia.Am Rev Respir Dis.1993;147:38–44.
- ,.Antimicrobial therapy of community‐acquired pneumonia.Infect Dis Clin North Am.2004;18:993–1016, xi.
- ,,,,.Resolution of infectious parameters after antimicrobial therapy in patients with ventilator‐associated pneumonia.Am J Respir Crit Care Med.2001;163:1371–1375.
- ,,, et al.Clinical resolution in patients with suspicion of ventilator‐associated pneumonia: a cohort study comparing patients with and without acute respiratory distress syndrome.Crit Care Med.2005;33:1248–1253.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,, et al.Resolution of ventilator‐associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome.Crit Care Med.2003;31:676–682.
- .Pharmacoeconomic comparison of sequential IV/oral ciprofloxacin versus ceftazidime in the treatment of nosocomial pneumonia.Can J Hosp Pharm.1995;48:276–283.
- ,,, et al.Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics.Am J Med.1991;91:462–470.
- ,,, et al.A prospective, double‐blind, multicenter, randomized trial comparing ertapenem 3 vs > or = 5 days in community‐acquired intraabdominal infection.J Gastrointest Surg.2008;12:592–600.
- ,,,.Implications of leukocytosis and fever at conclusion of antibiotic therapy for intra‐abdominal sepsis.Ann Surg.1982;195:19–24.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,.Cannula‐associated Staphylococcus aureus bacteraemia: outcome in relation to treatment.Intern Med J.2005;35:319–330.
- ,.Optimal duration of therapy for catheter‐related Staphylococcus aureus bacteremia: a study of 55 cases and review.Clin Infect Dis.1992;14:75–82.
- ,,,,.Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin‐sensitive S aureus and methicillin‐resistant S aureus bacteremia.Am Heart J.2004;147:536–539.
- ,,, et al.Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients.J Am Coll Cardiol.1997;30:1072–1078.
- ,,, et al.Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow‐up.Clin Infect Dis.1999;28:106–114.
- .The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs.Clin Infect Dis.2006;42(suppl 2):S82–S89.
- ,,.Clinical and economic burden of antimicrobial resistance.Expert Rev Anti Infect Ther.2008;6:751–763.
- .Economics of antibiotic resistance.Expert Rev Anti Infect Ther.2008;6:523–539.
- ,,,.Emergence of antibiotic‐resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents.Antimicrob Agents Chemother.1999;43:1379–1382.
- ,,,,.Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis.BMJ.2010;340:c2096.
- ,,,,,.Risk factors for invasive pneumococcal disease in children: a population‐based case‐control study in North America.Pediatrics.1999;103:E28.
- ,,.Antimicrobial resistance: consideration as an adverse drug event.Crit Care Med.2010;38:S155–S161.
- ,,,,,.Fluoroquinolone‐resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact.Am J Med.2006;119:526.e519–526.525.
- ,,, et al.Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates.Am J Med.2008;121:876–884.
- ,,,,,.Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes.Infect Control Hosp Epidemiol.2006;27:893–900.
- ,,,,.Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals.Clin Infect Dis.2005;41:435–440.
- ,,,.Evaluation of an intervention designed to decrease the rate of nosocomial methicillin‐resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use.Infect Control Hosp Epidemiol.2006;27:155–169.
- ,,,,,.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals.Infect Control Hosp Epidemiol.2007;28:1389–1395.
- ,,, et al.Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned.Arch Intern Med.2002;162:1515–1520.
- ,,,,.Nosocomial outbreak of Klebsiella infection resistant to late‐generation cephalosporins.Ann Intern Med.1993;119:353–358.
- ,,.The effect of an antimicrobial restriction program on Pseudomonas aeruginosa resistance to beta‐lactams in a large teaching hospital.Pharmacotherapy.2003;23:618–624.
- ,,,.Association between antibiotic usage and subsequent colonization or infection of extensive drug‐resistant Acinetobacter baumannii: a matched case‐control study in intensive care units.Diagn Microbiol Infect Dis.2008;62:298–305.
- ,,.Relationships between antimicrobial use and antimicrobial resistance in Gram‐negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan.Int J Antimicrob Agents.2005;26:463–472.
- ,,, et al.Risk factors for acquisition of imipenem‐resistant Acinetobacter baumannii: a case‐control study.Antimicrob Agents Chemother.2004;48:224–228.
- ,,,,.Consumption of imipenem correlates with beta‐lactam resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother.2002;46:2920–2925.
- ,,.Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem‐resistant Pseudomonas aeruginosa.Antimicrob Agents Chemother.2009;53:1983–1986.
- .Clinical efficacy of newer agents in short‐duration therapy for community‐acquired pneumonia.Clin Infect Dis.2004;39(suppl 3):S159–S164.
- ,.The role of carbapenems in the treatment of severe nosocomial respiratory tract infections.Expert Opin Pharmacother.2008;9:561–575.
- ,.Does short‐course antibiotic therapy better meet patient expectations?Int J Antimicrob Agents.2003;21:222–228.
- ,,,.Tackling empirical antibiotic therapy for ventilator‐associated pneumonia in your ICU: guidance for implementing the guidelines.Semin Respir Crit Care Med.2009;30:102–115.
- World Health Organization (WHO) report on infectious diseases 2000. Overcoming antimicrobial resistance. Chapter 5. Call to action: A massive effort to provide proper treatment. Available at: http://www.who.int/infectious‐disease‐report/2000/index.html. Accessed January 14,2011.
- ,,,,.Diagnosis and management of group A streptococcal pharyngitis: a practice guideline. Infectious Diseases Society of America.Clin Infect Dis.1997;25:574–583.
- ,,,,,.Shorter‐course antibiotic therapy (SCAT): principles, current data, and caveats. In: Owens RCJ, Lautenbach E, editors.Antimicrobial Resistance: Problem Pathogens and Clinical Countermeasures.New York, NY:Informa Healthcare;2008:337–370.
- ,,, et al.International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases.Clin Infect Dis.2011;52:e103–e120.
- ,.Sexually transmitted diseases treatment guidelines, 2006.MMWR Recomm Rep.2006;55:1–94.
- ,,,,.Novel, single‐dose microsphere formulation of azithromycin versus 7‐day levofloxacin therapy for treatment of mild to moderate community‐acquired pneumonia in adults.Antimicrob Agents Chemother.2005;49:4035–4041.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,.Randomized, multicentre study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of adults with mild to moderate community‐acquired pneumonia. Azithromycin Study Group.Eur J Clin Microbiol Infect Dis.1998;17:828–833.
- ,,,,,.A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia. The optimal duration of therapy.Chest.1996;110:965–971.
- ,,,.Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator‐associated pneumonia to decrease resistance in the intensive care unit.Clin Infect Dis.2006;43(suppl 2):S75–S81.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- ,,, et al.Can we define the ideal duration of antibiotic therapy?Surg Infect (Larchmt).2006;7:419–432.
- ,,,,,.Duration of antibiotic therapy for ventilator‐associated pneumonia caused by non‐fermentative gram‐negative bacilli.Surg Infect (Larchmt).2007;8:589–597.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,.Antibiotic utilization and outcomes for patients with clinically suspected ventilator‐associated pneumonia and negative quantitative BAL culture results.Chest.2005;128:2706–2713.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Repeat bronchoalveolar lavage to guide antibiotic duration for ventilator‐associated pneumonia.J Trauma.2007;63:1329–1337.
- ,,.Short course antibiotic therapy for Gram‐negative hospital‐acquired pneumonia in the critically ill.J Hosp Infect.2010;74:337–343.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,.Short‐course versus long‐course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients.Gastroenterology.1991;100:1737–1742.
- ,,,,.Complicated appendicitis: is there a minimum intravenous antibiotic requirement? A prospective randomized trial.Am Surg.2000;66:887–890.
- ,,.Short‐course therapy for bloodstream infections in immunocompetent adults.Int J Antimicrob Agents.2009;34(suppl 4):S47–S51.
- ,,,.Short‐course monotherapy strategy for treating bacteremia in the critically ill.Minerva Anestesiol.2006;72:841–857.
- ,.Short‐course therapy for catheter‐associated Staphylococcus aureus bacteremia.Arch Intern Med.1989;149:533–536.
- ,,,.Staphylococcus aureus catheter‐associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters.Arch Intern Med.1995;155:1161–1166.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44(suppl 2):S27–S72.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,.Hospital‐acquired pneumonia: pathophysiology, diagnosis, and treatment.Surg Clin North Am.2009;89:439–461, ix.
- ,,.Healthcare‐associated pneumonia in adults: management principles to improve outcomes.Infect Dis Clin North Am.2004;18:939–962.
- ,,.Healthcare‐associated infections. A useful concept?Curr Opin Crit Care.2009;15:419–424.
- ,,,,,.Efficacy of 750‐mg, 5‐day levofloxacin in the treatment of community‐acquired pneumonia caused by atypical pathogens.Curr Med Res Opin.2004;20:555–563.
- ,,, et al.Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study.BMJ.2006;332:1355.
- ,,,,.Gemifloxacin once daily for 5 days versus 7 days for the treatment of community‐acquired pneumonia: a randomized, multicentre, double‐blind study.J Antimicrob Chemother.2007;60:112–120.
- ,,.Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community‐acquired pneumonia of suspected pneumococcal origin.Respir Med.2004;98:708–720.
- ,,,.Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective, randomized, double‐blind study.Am J Ther.1999;6:217–222.
- ,,,,.Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community‐acquired pneumonia.J Antimicrob Chemother.2004;54:515–523.
- ,,,,,.Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis.Drugs.2008;68:1841–1854.
- ,,,.Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,,, et al.Low dosage and long treatment duration of beta‐lactam: risk factors for carriage of penicillin‐resistant Streptococcus pneumoniae.JAMA.1998;279:365–370.
- ,,, et al.Effect of short‐course, high‐dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial.JAMA.2001;286:49–56.
- ,,,.Antipneumococcal activity of ertapenem (MK‐0826) compared to those of other agents.Antimicrob Agents Chemother.2002;46:42–46.
- ,,.MIC and time‐kill study of antipneumococcal activities of RPR 106972 (a new oral streptogramin), RP 59500 (quinupristin‐dalfopristin), pyostacine (RP 7293), penicillin G, cefotaxime, erythromycin, and clarithromycin against 10 penicillin‐susceptible and ‐resistant pneumococci.Antimicrob Agents Chemother.1996;40:2071–2074.
- ,,.Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials.J Antimicrob Chemother.2003;51:443–446.
- ,,.Antipneumococcal activity of ertapenem compared to nine other compounds by time‐kill [abstract E‐800]. In:Program and Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago).Washington, DC:American Society for Microbiology,2001:184.
- ,,.Time‐kill analysis of the antipneumococcal activity of daptomycin compared with 8 other agents. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:161.
- ,,.Post‐antibiotic effect of garenoxacin against gram‐positive and gram‐negative organisms [abstract A‐496]. In:Program and Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy (San Diego).Washington, DC:American Society for Microbiology,2002:42.
- ,,, et al.Short‐course therapy of gemifloxacin effective against pneumococcal pneumonia in mice.J Chemother.2006;18:634–640.
- ,,.Animal models of Streptococcus pneumoniae disease.Clin Microbiol Rev.2008;21:666–685.
- ,,,,.Protective effect of trovafloxacin, ciprofloxacin and ampicillin against Streptococcus pneumoniae in a murine sepsis model.J Antimicrob Chemother.1999;44:477–481.
- ,,, et al.Follow‐up protected specimen brushes to assess treatment in nosocomial pneumonia.Am Rev Respir Dis.1993;147:38–44.
- ,.Antimicrobial therapy of community‐acquired pneumonia.Infect Dis Clin North Am.2004;18:993–1016, xi.
- ,,,,.Resolution of infectious parameters after antimicrobial therapy in patients with ventilator‐associated pneumonia.Am J Respir Crit Care Med.2001;163:1371–1375.
- ,,, et al.Clinical resolution in patients with suspicion of ventilator‐associated pneumonia: a cohort study comparing patients with and without acute respiratory distress syndrome.Crit Care Med.2005;33:1248–1253.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,, et al.Resolution of ventilator‐associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome.Crit Care Med.2003;31:676–682.
- .Pharmacoeconomic comparison of sequential IV/oral ciprofloxacin versus ceftazidime in the treatment of nosocomial pneumonia.Can J Hosp Pharm.1995;48:276–283.
- ,,, et al.Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics.Am J Med.1991;91:462–470.
- ,,, et al.A prospective, double‐blind, multicenter, randomized trial comparing ertapenem 3 vs > or = 5 days in community‐acquired intraabdominal infection.J Gastrointest Surg.2008;12:592–600.
- ,,,.Implications of leukocytosis and fever at conclusion of antibiotic therapy for intra‐abdominal sepsis.Ann Surg.1982;195:19–24.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,.Cannula‐associated Staphylococcus aureus bacteraemia: outcome in relation to treatment.Intern Med J.2005;35:319–330.
- ,.Optimal duration of therapy for catheter‐related Staphylococcus aureus bacteremia: a study of 55 cases and review.Clin Infect Dis.1992;14:75–82.
- ,,,,.Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin‐sensitive S aureus and methicillin‐resistant S aureus bacteremia.Am Heart J.2004;147:536–539.
- ,,, et al.Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients.J Am Coll Cardiol.1997;30:1072–1078.
- ,,, et al.Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow‐up.Clin Infect Dis.1999;28:106–114.