User login
A Raw Deal
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
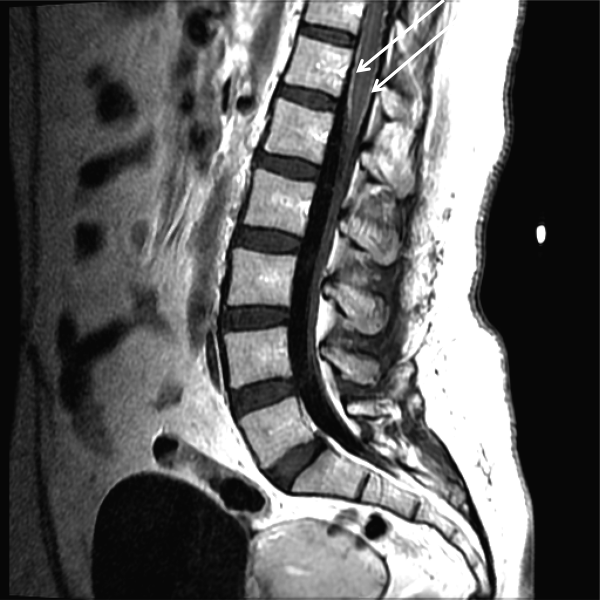
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).
The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
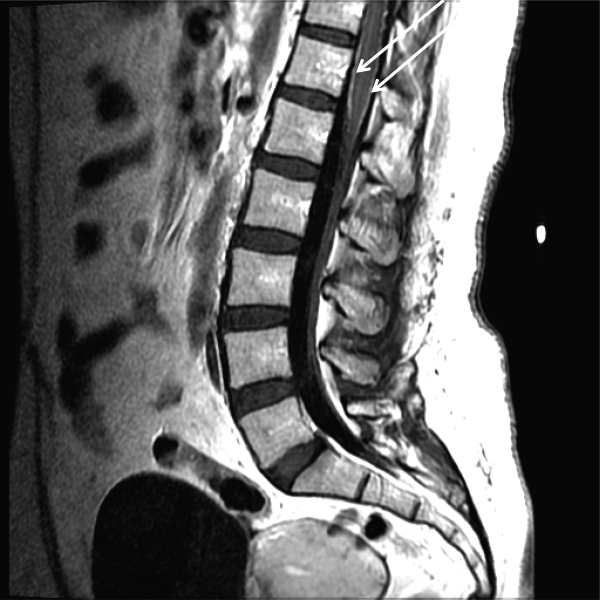
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.