User login
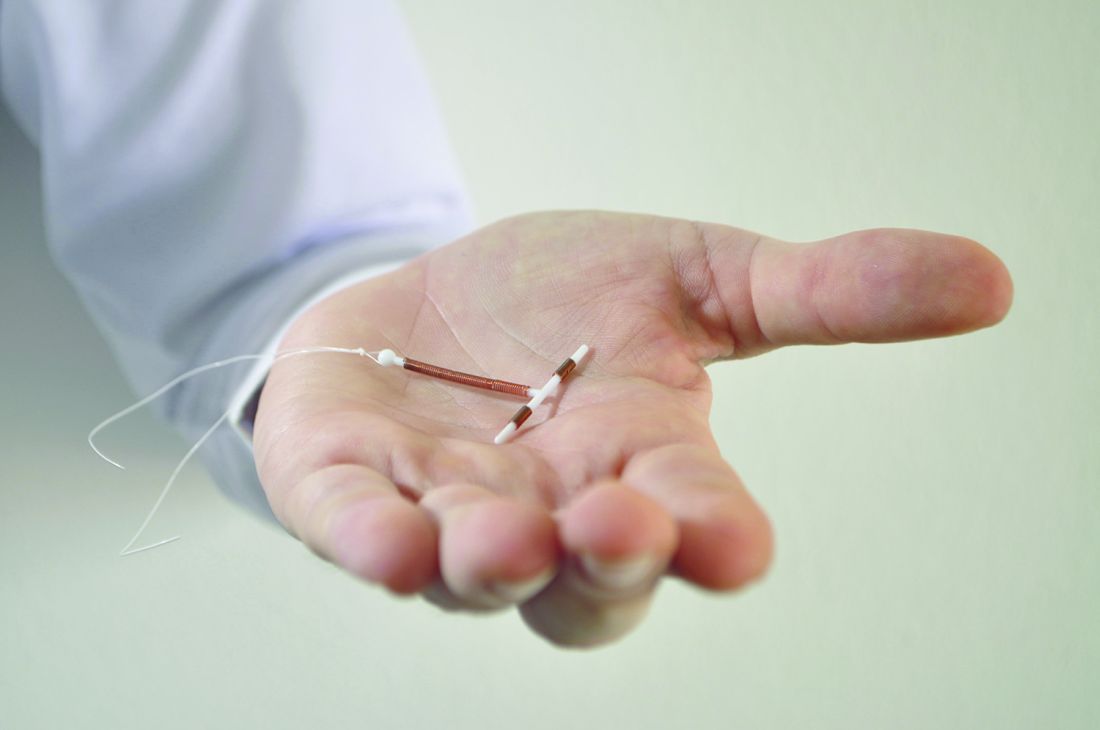
High continuation rates for IUDs, implants at 2 years
The large majority of women who start using a long-acting reversible contraceptive (LARC) find it acceptable and cost effective enough to continue using the method for at least 2 years, findings from a retrospective cohort study suggest.
Investigators performed a chart review to assess continuation rates in a real-world, mixed-payer setting among 8,603 women aged 15-44 years who had a device inserted between 2004 and 2012 at the University of Utah Healthcare System.
At 2 years, the proportion of women still using the device they had received was 77.8% for the hormonal IUD users, 73.1% for the copper IUD users, and 75.9% for the implant users, according to study findings reported online (Am J Obstet Gynecol. 2017 Feb 8. doi: 10.1016/j.ajog.2017.02.003).
“These data demonstrate a high rate of 2-year continuation of contraceptive devices in a mixed-payer system suggesting user acceptability and confirming that a majority of users reach the point of cost neutrality as demonstrated previously,” Jessica N. Sanders, PhD, of the University of Utah, Salt Lake City, and her colleagues wrote.
In analyses adjusted for potential confounders, compared with copper IUD users, the levonorgestrel IUD users and the implant users had higher adjusted 2-year continuation rates (incidence risk ratio, 1.08 for each).
Additionally, women’s likelihood of continuing use of their contraceptive device for at least 2 years increased with their age at the time of insertion. And women were more likely to still be using their method at that time if they paid for care themselves (incidence risk ratio, 1.13) or were covered by public health insurance (1.04), as compared with those covered by private insurance. Hispanic ethnicity was also associated with 2-year continuation.
“Differences by method type and patient characteristics were small and consistent with previous studies that have demonstrated LARC acceptability across patient demographics,” Dr. Sanders and her coauthors wrote.
Study limitations included possible bias and residual confounding, potentially limited generalizability to other populations of women, and lack of information on pregnancy and obstetric history for the majority of the cohort. But one of the study’s strengths is that it represents actual use, which may differ from prospective studies where participants are reimbursed to continue participation.
Dr. Sanders reported having no relevant conflicts of interest; some of the coauthors are employees of, have affiliations with, and/or own stock in companies that manufacture IUDs and implants. The study was funded in part by Bayer Healthcare.
The large majority of women who start using a long-acting reversible contraceptive (LARC) find it acceptable and cost effective enough to continue using the method for at least 2 years, findings from a retrospective cohort study suggest.
Investigators performed a chart review to assess continuation rates in a real-world, mixed-payer setting among 8,603 women aged 15-44 years who had a device inserted between 2004 and 2012 at the University of Utah Healthcare System.
At 2 years, the proportion of women still using the device they had received was 77.8% for the hormonal IUD users, 73.1% for the copper IUD users, and 75.9% for the implant users, according to study findings reported online (Am J Obstet Gynecol. 2017 Feb 8. doi: 10.1016/j.ajog.2017.02.003).
“These data demonstrate a high rate of 2-year continuation of contraceptive devices in a mixed-payer system suggesting user acceptability and confirming that a majority of users reach the point of cost neutrality as demonstrated previously,” Jessica N. Sanders, PhD, of the University of Utah, Salt Lake City, and her colleagues wrote.
In analyses adjusted for potential confounders, compared with copper IUD users, the levonorgestrel IUD users and the implant users had higher adjusted 2-year continuation rates (incidence risk ratio, 1.08 for each).
Additionally, women’s likelihood of continuing use of their contraceptive device for at least 2 years increased with their age at the time of insertion. And women were more likely to still be using their method at that time if they paid for care themselves (incidence risk ratio, 1.13) or were covered by public health insurance (1.04), as compared with those covered by private insurance. Hispanic ethnicity was also associated with 2-year continuation.
“Differences by method type and patient characteristics were small and consistent with previous studies that have demonstrated LARC acceptability across patient demographics,” Dr. Sanders and her coauthors wrote.
Study limitations included possible bias and residual confounding, potentially limited generalizability to other populations of women, and lack of information on pregnancy and obstetric history for the majority of the cohort. But one of the study’s strengths is that it represents actual use, which may differ from prospective studies where participants are reimbursed to continue participation.
Dr. Sanders reported having no relevant conflicts of interest; some of the coauthors are employees of, have affiliations with, and/or own stock in companies that manufacture IUDs and implants. The study was funded in part by Bayer Healthcare.
The large majority of women who start using a long-acting reversible contraceptive (LARC) find it acceptable and cost effective enough to continue using the method for at least 2 years, findings from a retrospective cohort study suggest.
Investigators performed a chart review to assess continuation rates in a real-world, mixed-payer setting among 8,603 women aged 15-44 years who had a device inserted between 2004 and 2012 at the University of Utah Healthcare System.
At 2 years, the proportion of women still using the device they had received was 77.8% for the hormonal IUD users, 73.1% for the copper IUD users, and 75.9% for the implant users, according to study findings reported online (Am J Obstet Gynecol. 2017 Feb 8. doi: 10.1016/j.ajog.2017.02.003).
“These data demonstrate a high rate of 2-year continuation of contraceptive devices in a mixed-payer system suggesting user acceptability and confirming that a majority of users reach the point of cost neutrality as demonstrated previously,” Jessica N. Sanders, PhD, of the University of Utah, Salt Lake City, and her colleagues wrote.
In analyses adjusted for potential confounders, compared with copper IUD users, the levonorgestrel IUD users and the implant users had higher adjusted 2-year continuation rates (incidence risk ratio, 1.08 for each).
Additionally, women’s likelihood of continuing use of their contraceptive device for at least 2 years increased with their age at the time of insertion. And women were more likely to still be using their method at that time if they paid for care themselves (incidence risk ratio, 1.13) or were covered by public health insurance (1.04), as compared with those covered by private insurance. Hispanic ethnicity was also associated with 2-year continuation.
“Differences by method type and patient characteristics were small and consistent with previous studies that have demonstrated LARC acceptability across patient demographics,” Dr. Sanders and her coauthors wrote.
Study limitations included possible bias and residual confounding, potentially limited generalizability to other populations of women, and lack of information on pregnancy and obstetric history for the majority of the cohort. But one of the study’s strengths is that it represents actual use, which may differ from prospective studies where participants are reimbursed to continue participation.
Dr. Sanders reported having no relevant conflicts of interest; some of the coauthors are employees of, have affiliations with, and/or own stock in companies that manufacture IUDs and implants. The study was funded in part by Bayer Healthcare.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The 2-year continuation rate was 77.8% for the levonorgestrel 52 mg IUD, 73.1% for the copper T380A IUD, and 75.9% for the 68 mg etonogestrel implant.
Data source: A single-center retrospective cohort study of 8,603 women who had an IUD or contraceptive implant inserted during a 9-year period.
Disclosures: Dr. Sanders reported having no relevant conflicts of interest; some of the coauthors are employees of, have affiliations with, and/or own stock in companies that manufacture IUDs and implants. The study was funded in part by Bayer Healthcare.
Heart disease risk soars in young adults with coronary calcium
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
FROM JAMA CARDIOLOGY
Key clinical point:
Major finding: Individuals having any versus no coronary artery calcium when aged 32-46 years had elevated risks of CHD events (HR, 5.0) and death (HR, 1.6) by the age of 58 years.
Data source: A prospective community-based cohort study of 5,115 black and white adults (CARDIA Study).
Disclosures: Dr. Carr disclosed that he had no relevant conflicts of interest.
Resources and technologies are making teen drivers safer
SAN FRANCISCO – Clinicians and parents should capitalize on a variety of resources and new technologies that help keep teen drivers safe behind the wheel, according to Dr. Joseph O’Neil, a pediatrician at the Riley Hospital for Children in Indianapolis.
“As I like to share with parents, this is the one developmental milestone that parents really want their kids to have that is potentially lethal. This could really kill them,” he said at the annual meeting of the American Academy of Pediatrics. “Believe me, that’s a conversation stopper; they sort of look at you funny. But it’s true.”
“But there is some good news. We have been paying attention,” Dr. O’Neil said. Concerted safety efforts and campaigns led to a halving of young driver fatalities between 2005 and 2014, although a recent analysis suggesting a reversal of that trend has generated some concern.
Risk factors
Numerous factors increase the risk of crashes and deaths for teen drivers, beginning with their developmental stage, according to Dr. O’Neil. Youth are characterized by their striving for autonomy, impulsivity, risk taking, and greater susceptibility to peer influences, compounded by poor judgment of hazards.
“We know that their executive function is still improving, still maturing, They really don’t start getting to adult levels, if they ever do, until about 25,” he commented humorously.
Other risk factors include speeding, drinking and substance use, sleep deprivation, and distractions that range from cell phones, to eating and grooming, to all the gizmos on the dashboard today. Not wearing seat belts also plays a role, as teens are the age group least likely to buckle up, and risk rises with the number of young passengers in the vehicle.
The rate of fatal crashes among young drivers is more than twice as high at night, compared with during the day, with the hours of 9 p.m. to midnight being most hazardous. And the riskiest meteorologic conditions are, not surprisingly, snow and ice – something that parents should take into account in their typical rush to get driver’s education out of the way in the summer months, he said.
“Most of the evidence points to inexperience as probably the single most important risk factor because with inexperience, you’re going to use cell phones, you’re going to be distracted, you’re not going to be paying attention because you don’t have the experience to know that you should,” Dr. O’Neil said.
Graduated driver’s licenses
A key resource in addressing teen drivers’ inexperience and the fact that their crash rate is highest in their first year of driving are graduated driver licenses (GDLs). These licenses start with a learner’s permit mandating supervision and having many restrictions on conditions such as times when driving is permitted and number of passengers, and if there are no infractions, slowly lift these restrictions as the teen gains more driving experience, until he or she receives a full driver’s license.
Use of GDLs over the past 20 years or so been credited with a reduction of 10%-30% in the rate of motor vehicle fatalities among young teen drivers.
“The problem is that teens have smartened up; they are waiting until later, age 18, to start driving because they don’t want to go through the rigmarole of a GDL,” he said. “We know that that’s a problem because we have right shifted that curve, so we are not seeing as many 15- and 16-year-olds dying behind the wheel; we are seeing more of the 18- and 19-year-olds up to 25-year-olds.”
Clinicians should familiarize themselves with their state’s GDL, Dr. O’Neil recommended. As most states’ GDL laws end at age 18, legislators are now looking at options such as establishing a GDL requirement for all new drivers, regardless of age.
High-tech tools
Clinicians also should also be aware of a host of new high-tech tools designed to make teen drivers safer, often by extending parents’ supervisory role, Dr. O’Neil advised. “Your parents in your practices are going to ask you about these,” he said.
So-called black boxes on vehicles collect a wealth of data about driving and conditions inside the vehicle that can be made available to parents. If black boxes are used correctly, they can enable parents to give feedback to the young driver and reduce overall crash risk, he said.
New GPS monitors will track a vehicle’s speed and range, with an optional feature called geofencing whereby parents can prespecify geographic limits on where their teen driver can go. If the teen ventures outside those limits, the monitor sends a notification.
Video monitoring systems now on the market will record footage both inside and outside of the car. Some record continuously, whereas others capture only events. Parents can obtain a summary report, generally through a monthly subscription, delivered by telephone or email to see how their teen is driving when solo.
Other in-vehicle monitoring technologies include direct-feedback systems, such as the tones that sound when the driver fails to fasten his or her seat belt, changes lanes, or gets too close to another car. Some systems can be configured to send a text or email when these alerts are engaged.
Parents who want to be more proactive can, for certain vehicles, invest in smart keys that are programmed to control vehicle parameters, such as the vehicle speed or the volume on the radio, according to Dr. O’Neil.
Finally, downloadable apps for cell phones will block the user’s ability to call (except in an emergency), text, surf, and take selfies while driving. “This doesn’t mean the child won’t be able to use someone else’s phone, but it does do a nice job for that particular installation,” he commented.
Parent-teen driving agreements
“We’ve talked about a lot of neat things that are out there, but what it all boils down to in the end are the parents – mom and dad. Parents truly are the gatekeepers of the keys,” Dr. O’Neil asserted. “We know that they can have an influence on their teens’ behavior. Parents can set restrictions and regulations on driving, and make sure [teens] follow all the traffic laws and set limits on high-risk driving situations.”
However, parents often underestimate the risks that their teens take behind the wheel. “Everyone always thinks that it’s the other kid who’s going to be driving wildly,” he said. “It’s okay for us to say, ‘I know he’s a great kid, but it’s not the bad kids who get into crashes. All kids get into crashes,’” he said. “It’s important to remind parents that all kids are at risk.
“One of the most valuable things that we can do as physicians to help parents navigate these crazy waters is talk about parent-teen driving agreements or contracts,” Dr. O’Neil said. “This has been shown time and time again to have a positive effect on driving behavior.”
These agreements list rules and expectations, and consequences for breaking the rules. “Both mom and dad, and the teen sign it. You put your name on the line, and that’s important because that really means something. This is probably the first contract this kid will ever sign, and it’s probably the most important one that [the teen] will ever sign.” He recommended that a paper version of the agreement be placed in a prominent location, such as on the refrigerator door, for maximal effectiveness.
A variety of parent-teen driving agreements are available online through initiatives such as the Checkpoints Program, Parents Are the Key to Safe Teen Drivers, I Drive Safely, and the AAP’s Parent-Teen Driving Agreement. Overall, their use has been shown to reduce the risks of traffic violations and crashes by 40%-50%.
Of note, these contracts complement rather than replace GDLs. Additionally, “the law of the land doesn’t trump the law of reality and the law of physics,” Dr. O’Neil pointed out. “We know that the laws in our states are not really always best practice, so as we advocate for best practice laws, what we can do is let the parents set better limits on the teen’s driving.”
Anticipatory guidance
“I usually start talking [with families] about driving when the child is 12 or 13,” Dr. O’Neil said. “Anticipatory guidance does work. We know that for a lot of other things that we do, but parents often need help in trying to figure out what to do.”
He recommended the AAP’s Healthy Children website as a source of good information and resources, including a Young Driver Tool for parents. “This has been vetted through the PROS [Pediatric Research in Office Settings] network, and it has been shown that parents do use it, parents do like it,” he noted. “And really it makes your job easier, because it takes time to talk about all these risk factors, and you can say, ‘Hey, I want you to go look at this website for teen driving. This will help.’ ”
Clinicians should generally cover with families the various risk factors, limit setting, use of GDLs, and parent-teen driving agreements. “Talk to parents about all these things. Talk to the teen; the teen will listen to you; you are an authority figure,” and “use interventional motivational techniques,” he said.
As parents control the vehicle their child drives, they should be counseled to give their teen the family’s safest car, preferably a newer, mid- to full-size vehicle with a small engine and modern safety features, according to Dr. O’Neil. “And we really do try to discourage teens buying their own cars because that sort of limits the parents’ leverage over them when they are starting to drive.”
Clinicians also should familiarize themselves with the driver’s education and similar resources in their community, including safe-driving initiatives spearheaded by groups such as Mothers Against Drunk Driving (MADD). They also should work with schools and the police to support “risky driving” prevention efforts.
Special anticipatory guidance is warranted when the new teen driver has a relevant condition such as attention-deficit/hyperactivity disorder. These youth are two to four times more likely to have a motor vehicle accident than typical teen drivers.
They may benefit from extended-release ADHD medication or a booster dose of their medication to keep them covered while driving, according to Dr. O’Neil.
“You may want to talk to them about holding off. Maybe their brain hasn’t matured enough yet, and you want to delay their driving. You may want to do a longer period of supervised driving or consider other things we’ve talked about – electronic resources or using a bigger, safer vehicle,” he suggested. “And always, always, always encourage limiting of distractions while driving.”
Dr. O’Neil said he had no relevant conflicts of interest.
SAN FRANCISCO – Clinicians and parents should capitalize on a variety of resources and new technologies that help keep teen drivers safe behind the wheel, according to Dr. Joseph O’Neil, a pediatrician at the Riley Hospital for Children in Indianapolis.
“As I like to share with parents, this is the one developmental milestone that parents really want their kids to have that is potentially lethal. This could really kill them,” he said at the annual meeting of the American Academy of Pediatrics. “Believe me, that’s a conversation stopper; they sort of look at you funny. But it’s true.”
“But there is some good news. We have been paying attention,” Dr. O’Neil said. Concerted safety efforts and campaigns led to a halving of young driver fatalities between 2005 and 2014, although a recent analysis suggesting a reversal of that trend has generated some concern.
Risk factors
Numerous factors increase the risk of crashes and deaths for teen drivers, beginning with their developmental stage, according to Dr. O’Neil. Youth are characterized by their striving for autonomy, impulsivity, risk taking, and greater susceptibility to peer influences, compounded by poor judgment of hazards.
“We know that their executive function is still improving, still maturing, They really don’t start getting to adult levels, if they ever do, until about 25,” he commented humorously.
Other risk factors include speeding, drinking and substance use, sleep deprivation, and distractions that range from cell phones, to eating and grooming, to all the gizmos on the dashboard today. Not wearing seat belts also plays a role, as teens are the age group least likely to buckle up, and risk rises with the number of young passengers in the vehicle.
The rate of fatal crashes among young drivers is more than twice as high at night, compared with during the day, with the hours of 9 p.m. to midnight being most hazardous. And the riskiest meteorologic conditions are, not surprisingly, snow and ice – something that parents should take into account in their typical rush to get driver’s education out of the way in the summer months, he said.
“Most of the evidence points to inexperience as probably the single most important risk factor because with inexperience, you’re going to use cell phones, you’re going to be distracted, you’re not going to be paying attention because you don’t have the experience to know that you should,” Dr. O’Neil said.
Graduated driver’s licenses
A key resource in addressing teen drivers’ inexperience and the fact that their crash rate is highest in their first year of driving are graduated driver licenses (GDLs). These licenses start with a learner’s permit mandating supervision and having many restrictions on conditions such as times when driving is permitted and number of passengers, and if there are no infractions, slowly lift these restrictions as the teen gains more driving experience, until he or she receives a full driver’s license.
Use of GDLs over the past 20 years or so been credited with a reduction of 10%-30% in the rate of motor vehicle fatalities among young teen drivers.
“The problem is that teens have smartened up; they are waiting until later, age 18, to start driving because they don’t want to go through the rigmarole of a GDL,” he said. “We know that that’s a problem because we have right shifted that curve, so we are not seeing as many 15- and 16-year-olds dying behind the wheel; we are seeing more of the 18- and 19-year-olds up to 25-year-olds.”
Clinicians should familiarize themselves with their state’s GDL, Dr. O’Neil recommended. As most states’ GDL laws end at age 18, legislators are now looking at options such as establishing a GDL requirement for all new drivers, regardless of age.
High-tech tools
Clinicians also should also be aware of a host of new high-tech tools designed to make teen drivers safer, often by extending parents’ supervisory role, Dr. O’Neil advised. “Your parents in your practices are going to ask you about these,” he said.
So-called black boxes on vehicles collect a wealth of data about driving and conditions inside the vehicle that can be made available to parents. If black boxes are used correctly, they can enable parents to give feedback to the young driver and reduce overall crash risk, he said.
New GPS monitors will track a vehicle’s speed and range, with an optional feature called geofencing whereby parents can prespecify geographic limits on where their teen driver can go. If the teen ventures outside those limits, the monitor sends a notification.
Video monitoring systems now on the market will record footage both inside and outside of the car. Some record continuously, whereas others capture only events. Parents can obtain a summary report, generally through a monthly subscription, delivered by telephone or email to see how their teen is driving when solo.
Other in-vehicle monitoring technologies include direct-feedback systems, such as the tones that sound when the driver fails to fasten his or her seat belt, changes lanes, or gets too close to another car. Some systems can be configured to send a text or email when these alerts are engaged.
Parents who want to be more proactive can, for certain vehicles, invest in smart keys that are programmed to control vehicle parameters, such as the vehicle speed or the volume on the radio, according to Dr. O’Neil.
Finally, downloadable apps for cell phones will block the user’s ability to call (except in an emergency), text, surf, and take selfies while driving. “This doesn’t mean the child won’t be able to use someone else’s phone, but it does do a nice job for that particular installation,” he commented.
Parent-teen driving agreements
“We’ve talked about a lot of neat things that are out there, but what it all boils down to in the end are the parents – mom and dad. Parents truly are the gatekeepers of the keys,” Dr. O’Neil asserted. “We know that they can have an influence on their teens’ behavior. Parents can set restrictions and regulations on driving, and make sure [teens] follow all the traffic laws and set limits on high-risk driving situations.”
However, parents often underestimate the risks that their teens take behind the wheel. “Everyone always thinks that it’s the other kid who’s going to be driving wildly,” he said. “It’s okay for us to say, ‘I know he’s a great kid, but it’s not the bad kids who get into crashes. All kids get into crashes,’” he said. “It’s important to remind parents that all kids are at risk.
“One of the most valuable things that we can do as physicians to help parents navigate these crazy waters is talk about parent-teen driving agreements or contracts,” Dr. O’Neil said. “This has been shown time and time again to have a positive effect on driving behavior.”
These agreements list rules and expectations, and consequences for breaking the rules. “Both mom and dad, and the teen sign it. You put your name on the line, and that’s important because that really means something. This is probably the first contract this kid will ever sign, and it’s probably the most important one that [the teen] will ever sign.” He recommended that a paper version of the agreement be placed in a prominent location, such as on the refrigerator door, for maximal effectiveness.
A variety of parent-teen driving agreements are available online through initiatives such as the Checkpoints Program, Parents Are the Key to Safe Teen Drivers, I Drive Safely, and the AAP’s Parent-Teen Driving Agreement. Overall, their use has been shown to reduce the risks of traffic violations and crashes by 40%-50%.
Of note, these contracts complement rather than replace GDLs. Additionally, “the law of the land doesn’t trump the law of reality and the law of physics,” Dr. O’Neil pointed out. “We know that the laws in our states are not really always best practice, so as we advocate for best practice laws, what we can do is let the parents set better limits on the teen’s driving.”
Anticipatory guidance
“I usually start talking [with families] about driving when the child is 12 or 13,” Dr. O’Neil said. “Anticipatory guidance does work. We know that for a lot of other things that we do, but parents often need help in trying to figure out what to do.”
He recommended the AAP’s Healthy Children website as a source of good information and resources, including a Young Driver Tool for parents. “This has been vetted through the PROS [Pediatric Research in Office Settings] network, and it has been shown that parents do use it, parents do like it,” he noted. “And really it makes your job easier, because it takes time to talk about all these risk factors, and you can say, ‘Hey, I want you to go look at this website for teen driving. This will help.’ ”
Clinicians should generally cover with families the various risk factors, limit setting, use of GDLs, and parent-teen driving agreements. “Talk to parents about all these things. Talk to the teen; the teen will listen to you; you are an authority figure,” and “use interventional motivational techniques,” he said.
As parents control the vehicle their child drives, they should be counseled to give their teen the family’s safest car, preferably a newer, mid- to full-size vehicle with a small engine and modern safety features, according to Dr. O’Neil. “And we really do try to discourage teens buying their own cars because that sort of limits the parents’ leverage over them when they are starting to drive.”
Clinicians also should familiarize themselves with the driver’s education and similar resources in their community, including safe-driving initiatives spearheaded by groups such as Mothers Against Drunk Driving (MADD). They also should work with schools and the police to support “risky driving” prevention efforts.
Special anticipatory guidance is warranted when the new teen driver has a relevant condition such as attention-deficit/hyperactivity disorder. These youth are two to four times more likely to have a motor vehicle accident than typical teen drivers.
They may benefit from extended-release ADHD medication or a booster dose of their medication to keep them covered while driving, according to Dr. O’Neil.
“You may want to talk to them about holding off. Maybe their brain hasn’t matured enough yet, and you want to delay their driving. You may want to do a longer period of supervised driving or consider other things we’ve talked about – electronic resources or using a bigger, safer vehicle,” he suggested. “And always, always, always encourage limiting of distractions while driving.”
Dr. O’Neil said he had no relevant conflicts of interest.
SAN FRANCISCO – Clinicians and parents should capitalize on a variety of resources and new technologies that help keep teen drivers safe behind the wheel, according to Dr. Joseph O’Neil, a pediatrician at the Riley Hospital for Children in Indianapolis.
“As I like to share with parents, this is the one developmental milestone that parents really want their kids to have that is potentially lethal. This could really kill them,” he said at the annual meeting of the American Academy of Pediatrics. “Believe me, that’s a conversation stopper; they sort of look at you funny. But it’s true.”
“But there is some good news. We have been paying attention,” Dr. O’Neil said. Concerted safety efforts and campaigns led to a halving of young driver fatalities between 2005 and 2014, although a recent analysis suggesting a reversal of that trend has generated some concern.
Risk factors
Numerous factors increase the risk of crashes and deaths for teen drivers, beginning with their developmental stage, according to Dr. O’Neil. Youth are characterized by their striving for autonomy, impulsivity, risk taking, and greater susceptibility to peer influences, compounded by poor judgment of hazards.
“We know that their executive function is still improving, still maturing, They really don’t start getting to adult levels, if they ever do, until about 25,” he commented humorously.
Other risk factors include speeding, drinking and substance use, sleep deprivation, and distractions that range from cell phones, to eating and grooming, to all the gizmos on the dashboard today. Not wearing seat belts also plays a role, as teens are the age group least likely to buckle up, and risk rises with the number of young passengers in the vehicle.
The rate of fatal crashes among young drivers is more than twice as high at night, compared with during the day, with the hours of 9 p.m. to midnight being most hazardous. And the riskiest meteorologic conditions are, not surprisingly, snow and ice – something that parents should take into account in their typical rush to get driver’s education out of the way in the summer months, he said.
“Most of the evidence points to inexperience as probably the single most important risk factor because with inexperience, you’re going to use cell phones, you’re going to be distracted, you’re not going to be paying attention because you don’t have the experience to know that you should,” Dr. O’Neil said.
Graduated driver’s licenses
A key resource in addressing teen drivers’ inexperience and the fact that their crash rate is highest in their first year of driving are graduated driver licenses (GDLs). These licenses start with a learner’s permit mandating supervision and having many restrictions on conditions such as times when driving is permitted and number of passengers, and if there are no infractions, slowly lift these restrictions as the teen gains more driving experience, until he or she receives a full driver’s license.
Use of GDLs over the past 20 years or so been credited with a reduction of 10%-30% in the rate of motor vehicle fatalities among young teen drivers.
“The problem is that teens have smartened up; they are waiting until later, age 18, to start driving because they don’t want to go through the rigmarole of a GDL,” he said. “We know that that’s a problem because we have right shifted that curve, so we are not seeing as many 15- and 16-year-olds dying behind the wheel; we are seeing more of the 18- and 19-year-olds up to 25-year-olds.”
Clinicians should familiarize themselves with their state’s GDL, Dr. O’Neil recommended. As most states’ GDL laws end at age 18, legislators are now looking at options such as establishing a GDL requirement for all new drivers, regardless of age.
High-tech tools
Clinicians also should also be aware of a host of new high-tech tools designed to make teen drivers safer, often by extending parents’ supervisory role, Dr. O’Neil advised. “Your parents in your practices are going to ask you about these,” he said.
So-called black boxes on vehicles collect a wealth of data about driving and conditions inside the vehicle that can be made available to parents. If black boxes are used correctly, they can enable parents to give feedback to the young driver and reduce overall crash risk, he said.
New GPS monitors will track a vehicle’s speed and range, with an optional feature called geofencing whereby parents can prespecify geographic limits on where their teen driver can go. If the teen ventures outside those limits, the monitor sends a notification.
Video monitoring systems now on the market will record footage both inside and outside of the car. Some record continuously, whereas others capture only events. Parents can obtain a summary report, generally through a monthly subscription, delivered by telephone or email to see how their teen is driving when solo.
Other in-vehicle monitoring technologies include direct-feedback systems, such as the tones that sound when the driver fails to fasten his or her seat belt, changes lanes, or gets too close to another car. Some systems can be configured to send a text or email when these alerts are engaged.
Parents who want to be more proactive can, for certain vehicles, invest in smart keys that are programmed to control vehicle parameters, such as the vehicle speed or the volume on the radio, according to Dr. O’Neil.
Finally, downloadable apps for cell phones will block the user’s ability to call (except in an emergency), text, surf, and take selfies while driving. “This doesn’t mean the child won’t be able to use someone else’s phone, but it does do a nice job for that particular installation,” he commented.
Parent-teen driving agreements
“We’ve talked about a lot of neat things that are out there, but what it all boils down to in the end are the parents – mom and dad. Parents truly are the gatekeepers of the keys,” Dr. O’Neil asserted. “We know that they can have an influence on their teens’ behavior. Parents can set restrictions and regulations on driving, and make sure [teens] follow all the traffic laws and set limits on high-risk driving situations.”
However, parents often underestimate the risks that their teens take behind the wheel. “Everyone always thinks that it’s the other kid who’s going to be driving wildly,” he said. “It’s okay for us to say, ‘I know he’s a great kid, but it’s not the bad kids who get into crashes. All kids get into crashes,’” he said. “It’s important to remind parents that all kids are at risk.
“One of the most valuable things that we can do as physicians to help parents navigate these crazy waters is talk about parent-teen driving agreements or contracts,” Dr. O’Neil said. “This has been shown time and time again to have a positive effect on driving behavior.”
These agreements list rules and expectations, and consequences for breaking the rules. “Both mom and dad, and the teen sign it. You put your name on the line, and that’s important because that really means something. This is probably the first contract this kid will ever sign, and it’s probably the most important one that [the teen] will ever sign.” He recommended that a paper version of the agreement be placed in a prominent location, such as on the refrigerator door, for maximal effectiveness.
A variety of parent-teen driving agreements are available online through initiatives such as the Checkpoints Program, Parents Are the Key to Safe Teen Drivers, I Drive Safely, and the AAP’s Parent-Teen Driving Agreement. Overall, their use has been shown to reduce the risks of traffic violations and crashes by 40%-50%.
Of note, these contracts complement rather than replace GDLs. Additionally, “the law of the land doesn’t trump the law of reality and the law of physics,” Dr. O’Neil pointed out. “We know that the laws in our states are not really always best practice, so as we advocate for best practice laws, what we can do is let the parents set better limits on the teen’s driving.”
Anticipatory guidance
“I usually start talking [with families] about driving when the child is 12 or 13,” Dr. O’Neil said. “Anticipatory guidance does work. We know that for a lot of other things that we do, but parents often need help in trying to figure out what to do.”
He recommended the AAP’s Healthy Children website as a source of good information and resources, including a Young Driver Tool for parents. “This has been vetted through the PROS [Pediatric Research in Office Settings] network, and it has been shown that parents do use it, parents do like it,” he noted. “And really it makes your job easier, because it takes time to talk about all these risk factors, and you can say, ‘Hey, I want you to go look at this website for teen driving. This will help.’ ”
Clinicians should generally cover with families the various risk factors, limit setting, use of GDLs, and parent-teen driving agreements. “Talk to parents about all these things. Talk to the teen; the teen will listen to you; you are an authority figure,” and “use interventional motivational techniques,” he said.
As parents control the vehicle their child drives, they should be counseled to give their teen the family’s safest car, preferably a newer, mid- to full-size vehicle with a small engine and modern safety features, according to Dr. O’Neil. “And we really do try to discourage teens buying their own cars because that sort of limits the parents’ leverage over them when they are starting to drive.”
Clinicians also should familiarize themselves with the driver’s education and similar resources in their community, including safe-driving initiatives spearheaded by groups such as Mothers Against Drunk Driving (MADD). They also should work with schools and the police to support “risky driving” prevention efforts.
Special anticipatory guidance is warranted when the new teen driver has a relevant condition such as attention-deficit/hyperactivity disorder. These youth are two to four times more likely to have a motor vehicle accident than typical teen drivers.
They may benefit from extended-release ADHD medication or a booster dose of their medication to keep them covered while driving, according to Dr. O’Neil.
“You may want to talk to them about holding off. Maybe their brain hasn’t matured enough yet, and you want to delay their driving. You may want to do a longer period of supervised driving or consider other things we’ve talked about – electronic resources or using a bigger, safer vehicle,” he suggested. “And always, always, always encourage limiting of distractions while driving.”
Dr. O’Neil said he had no relevant conflicts of interest.
Ischemia-repairing cells fall short for treating intermittent claudication
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: At 6 months, peak walking time had increased by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, a nonsignificant difference (P = .238).
Data source: PACE, a randomized phase II trial of 82 patients with PAD and symptom-limiting intermittent claudication of the legs.
Disclosures: Dr. Perin received a research grant from the National Heart, Lung, and Blood Institute.
VIDEO: New antisense inhibitor nets impressive reductions in lipids
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Treatment was associated with reductions in levels of triglycerides (–66%), ApoC-III (–68%), LDL cholesterol (–35%), total cholesterol (–36%), and non-HDL cholesterol (–40%), as well as levels of HDL cholesterol (–25%).
Data source: A phase I/IIa ascending-dose trial among 32 healthy adults with hypertriglyceridemia.
Disclosures: Dr. Tsimikas is an employee of and shareholder in Ionis Pharmaceuticals, which sponsored the trial.
Regenerative medicine is likely game changer for cardiovascular disease
NEW ORLEANS – Regenerative medicine has much to offer the cardiovascular field, although there is still a way to go before it is ready for routine clinical application, according to Andre Terzic, MD, PhD, director of the Mayo Clinic Center for Regenerative Medicine and a professor in Cardiovascular Diseases Research at the Mayo Clinic, in Rochester, Minn.
Trials testing a variety of stem cell approaches in patients with conditions such as acute myocardial infarction, peripheral arterial disease, and dilated cardiomyopathy are providing important lessons about this novel strategy and showing its potential, he said in a state of the science talk at the American Heart Association scientific sessions.
Impact on therapeutic approaches and goals
“This is a true paradigm shift in how we are approaching patients from a more traditional fighting of disease, whether it’s in the vascular or cardiac arena, to ultimately really where regenerative medicine is driving: rebuilding vascular and heart health,” Dr. Terzic maintained.
Leaders in the field have put forth an associated value proposition, estimating that a decade from now, regenerative medicine will account for about 10% of all health care delivered globally, according to Dr. Terzic. And research presented at the meeting is a major step in that direction.
The potential applications in cardiovascular medicine are numerous, he proposed. Regenerative medicine might be used in prophylactic strategies, for protection against chronic disease, and in bridging strategies, to delay transplantation in patients with end organ damage. It might also be used in combination approaches, to augment the efficacy of primary therapy, and even to generate new organs for patients who have run out of options.
The specific therapeutic goals depend on the stage of disease. For example, the goal is to boost cardioprotection and limit inflammation in acute myocardial infarction; to augment myocardial regenerative reserve and improve survival in subacute disease; and to enhance the pro-reparative environment and restore structure and function in the setting of chronic cardiomyopathy.
Bringing regenerative medicine to the clinic
Ultimately, the health care field is striving to bring the science of regenerative medicine into the clinic, and turn the research pipeline into a clinical service line, Dr. Terzic said.
As might be expected, the whole process begins with identification of an unmet need in patients, which in turn generates a specific practice advancement goal. In the cardiovascular arena, the process has largely been driven by smaller collaborative groups and champions in the field, but is increasingly garnering new support from the business world and national networks.
“The importance at the moment is the experiences that are being built allow for a true iterative process in harnessing this new knowledge and essentially developing even more refined and more effective solutions as we look into the future,” Dr. Terzic commented.
“The cardiovascular field is clearly not alone, but is working in tandem with many other fields that are evolving rapidly in this area,” he added. “And I think the collective experience will be one of the main drivers as we build this shared vision for a practice not only for regenerative solutions, but for practice-integrated regenerative medicine, ultimately, as one of the models of care in the decade to come.”
A blueprint for moving forward
Dr. Terzic and his colleagues at the Mayo Clinic have developed a framework for advancing the field that is being used at their institution but could also be used elsewhere (Eur Heart J. 2016;37:1089-90).
“What we have historically been doing for the last decade or so is really building this discovery knowledge, and then moving it into translation and ultimately application,” he explained. “We call this a blueprint, a blueprint that has helped us all collectively get where we are today.”
For each of these three domains, investigators strive to build out the concept, starting with the specific regenerative technology or product, proceeding to its manufacture or production, and eventually devising ways to deliver it to patients through a clinical-grade supply chain available at the point of care.
“There is a new evolving concept of truly building a regenerative medicine care model,” summarized Dr. Terzic, who disclosed no relevant conflicts of interest. “Today, we are not fully there. We are really speaking about technological and translational readiness. But ultimately, the goal is to ensure clinical readiness.”
NEW ORLEANS – Regenerative medicine has much to offer the cardiovascular field, although there is still a way to go before it is ready for routine clinical application, according to Andre Terzic, MD, PhD, director of the Mayo Clinic Center for Regenerative Medicine and a professor in Cardiovascular Diseases Research at the Mayo Clinic, in Rochester, Minn.
Trials testing a variety of stem cell approaches in patients with conditions such as acute myocardial infarction, peripheral arterial disease, and dilated cardiomyopathy are providing important lessons about this novel strategy and showing its potential, he said in a state of the science talk at the American Heart Association scientific sessions.
Impact on therapeutic approaches and goals
“This is a true paradigm shift in how we are approaching patients from a more traditional fighting of disease, whether it’s in the vascular or cardiac arena, to ultimately really where regenerative medicine is driving: rebuilding vascular and heart health,” Dr. Terzic maintained.
Leaders in the field have put forth an associated value proposition, estimating that a decade from now, regenerative medicine will account for about 10% of all health care delivered globally, according to Dr. Terzic. And research presented at the meeting is a major step in that direction.
The potential applications in cardiovascular medicine are numerous, he proposed. Regenerative medicine might be used in prophylactic strategies, for protection against chronic disease, and in bridging strategies, to delay transplantation in patients with end organ damage. It might also be used in combination approaches, to augment the efficacy of primary therapy, and even to generate new organs for patients who have run out of options.
The specific therapeutic goals depend on the stage of disease. For example, the goal is to boost cardioprotection and limit inflammation in acute myocardial infarction; to augment myocardial regenerative reserve and improve survival in subacute disease; and to enhance the pro-reparative environment and restore structure and function in the setting of chronic cardiomyopathy.
Bringing regenerative medicine to the clinic
Ultimately, the health care field is striving to bring the science of regenerative medicine into the clinic, and turn the research pipeline into a clinical service line, Dr. Terzic said.
As might be expected, the whole process begins with identification of an unmet need in patients, which in turn generates a specific practice advancement goal. In the cardiovascular arena, the process has largely been driven by smaller collaborative groups and champions in the field, but is increasingly garnering new support from the business world and national networks.
“The importance at the moment is the experiences that are being built allow for a true iterative process in harnessing this new knowledge and essentially developing even more refined and more effective solutions as we look into the future,” Dr. Terzic commented.
“The cardiovascular field is clearly not alone, but is working in tandem with many other fields that are evolving rapidly in this area,” he added. “And I think the collective experience will be one of the main drivers as we build this shared vision for a practice not only for regenerative solutions, but for practice-integrated regenerative medicine, ultimately, as one of the models of care in the decade to come.”
A blueprint for moving forward
Dr. Terzic and his colleagues at the Mayo Clinic have developed a framework for advancing the field that is being used at their institution but could also be used elsewhere (Eur Heart J. 2016;37:1089-90).
“What we have historically been doing for the last decade or so is really building this discovery knowledge, and then moving it into translation and ultimately application,” he explained. “We call this a blueprint, a blueprint that has helped us all collectively get where we are today.”
For each of these three domains, investigators strive to build out the concept, starting with the specific regenerative technology or product, proceeding to its manufacture or production, and eventually devising ways to deliver it to patients through a clinical-grade supply chain available at the point of care.
“There is a new evolving concept of truly building a regenerative medicine care model,” summarized Dr. Terzic, who disclosed no relevant conflicts of interest. “Today, we are not fully there. We are really speaking about technological and translational readiness. But ultimately, the goal is to ensure clinical readiness.”
NEW ORLEANS – Regenerative medicine has much to offer the cardiovascular field, although there is still a way to go before it is ready for routine clinical application, according to Andre Terzic, MD, PhD, director of the Mayo Clinic Center for Regenerative Medicine and a professor in Cardiovascular Diseases Research at the Mayo Clinic, in Rochester, Minn.
Trials testing a variety of stem cell approaches in patients with conditions such as acute myocardial infarction, peripheral arterial disease, and dilated cardiomyopathy are providing important lessons about this novel strategy and showing its potential, he said in a state of the science talk at the American Heart Association scientific sessions.
Impact on therapeutic approaches and goals
“This is a true paradigm shift in how we are approaching patients from a more traditional fighting of disease, whether it’s in the vascular or cardiac arena, to ultimately really where regenerative medicine is driving: rebuilding vascular and heart health,” Dr. Terzic maintained.
Leaders in the field have put forth an associated value proposition, estimating that a decade from now, regenerative medicine will account for about 10% of all health care delivered globally, according to Dr. Terzic. And research presented at the meeting is a major step in that direction.
The potential applications in cardiovascular medicine are numerous, he proposed. Regenerative medicine might be used in prophylactic strategies, for protection against chronic disease, and in bridging strategies, to delay transplantation in patients with end organ damage. It might also be used in combination approaches, to augment the efficacy of primary therapy, and even to generate new organs for patients who have run out of options.
The specific therapeutic goals depend on the stage of disease. For example, the goal is to boost cardioprotection and limit inflammation in acute myocardial infarction; to augment myocardial regenerative reserve and improve survival in subacute disease; and to enhance the pro-reparative environment and restore structure and function in the setting of chronic cardiomyopathy.
Bringing regenerative medicine to the clinic
Ultimately, the health care field is striving to bring the science of regenerative medicine into the clinic, and turn the research pipeline into a clinical service line, Dr. Terzic said.
As might be expected, the whole process begins with identification of an unmet need in patients, which in turn generates a specific practice advancement goal. In the cardiovascular arena, the process has largely been driven by smaller collaborative groups and champions in the field, but is increasingly garnering new support from the business world and national networks.
“The importance at the moment is the experiences that are being built allow for a true iterative process in harnessing this new knowledge and essentially developing even more refined and more effective solutions as we look into the future,” Dr. Terzic commented.
“The cardiovascular field is clearly not alone, but is working in tandem with many other fields that are evolving rapidly in this area,” he added. “And I think the collective experience will be one of the main drivers as we build this shared vision for a practice not only for regenerative solutions, but for practice-integrated regenerative medicine, ultimately, as one of the models of care in the decade to come.”
A blueprint for moving forward
Dr. Terzic and his colleagues at the Mayo Clinic have developed a framework for advancing the field that is being used at their institution but could also be used elsewhere (Eur Heart J. 2016;37:1089-90).
“What we have historically been doing for the last decade or so is really building this discovery knowledge, and then moving it into translation and ultimately application,” he explained. “We call this a blueprint, a blueprint that has helped us all collectively get where we are today.”
For each of these three domains, investigators strive to build out the concept, starting with the specific regenerative technology or product, proceeding to its manufacture or production, and eventually devising ways to deliver it to patients through a clinical-grade supply chain available at the point of care.
“There is a new evolving concept of truly building a regenerative medicine care model,” summarized Dr. Terzic, who disclosed no relevant conflicts of interest. “Today, we are not fully there. We are really speaking about technological and translational readiness. But ultimately, the goal is to ensure clinical readiness.”
Key clinical point:
Major finding: Trials testing a variety of stem cell approaches in patients with conditions such as acute MI, PAD, and dilated cardiomyopathy are providing important lessons.
Data source: Expert analysis of current trials by Dr. Terzic.
Disclosures: Dr. Terzic disclosed that he had no relevant conflicts of interest.
Stem cell therapy for STEMI falls short at 2 years
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: At 2 years, differences in LV ejection fraction, infarct size, and end-diastolic volume index between the cell therapy and placebo groups remained nonsignificant.
Data source: TIME, a randomized, phase II trial of intracoronary delivery of autologous bone marrow mononuclear cells in 120 patients with ST-segment acute MI and LV dysfunction.
Disclosures: Dr. Traverse has received a research grant from the National Heart, Lung, and Blood Institute.
VIDEO: MILANO-PILOT marks end of the road for HDL mimetic
NEW ORLEANS – The future doesn’t look bright at the moment for use of agents that mimic HDL cholesterol to reverse coronary disease, based on disappointing results of the randomized, phase II MILANO-PILOT trial.
Among 120 patients with recent acute coronary syndrome, a mimetic known as MDCO-216 was no better than placebo at reducing coronary plaque measured by intravascular ultrasound (IVUS), according to data presented at the American Heart Association scientific sessions.
The median reduction in percent atheroma volume, the trial’s primary endpoint, was 0.5% with the mimetic and 0.8% with placebo, reported Stephen J. Nicholls, MD, of the South Australian Health and Medical Research Institute, University of Adelaide (Australia). Findings were similar for secondary outcomes assessing other measures of disease regression.
These data bring to an end a story that began in the 1980s, with discovery that a family in a town in northern Italy had a naturally occurring apolipoprotein A-I variant that mimics the actions of HDL cholesterol, subsequently named ApoA-IMilano. Despite having low levels of HDL, family members have a reduced risk of coronary disease.
In a small randomized trial, patients with acute coronary syndrome given an early recombinant form of this apolipoprotein saw a reduction in IVUS-measured coronary atherosclerosis (JAMA. 2003;290:2292-300). This prompted development of the refined recombinant form, MDCO-216, tested in the new trial.
“MDCO-216 did not produce a significant effect on coronary disease progression as measured by IVUS,” said Dr. Nicholls, who discussed the findings in a video interview. “These results occurred on a background of contemporary therapy in the post-ACS setting, which in 2016 is very effective for many patients.”
“The findings from this pilot study do not provide the evidence required to proceed with further development,” he concluded.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hope hinges on wild type
“This trial was extremely well done, world-class investigators carried out this trial, it was three times the size of the previous IVUS trial, and I think it is convincingly negative,” said discussant Daniel J. Rader, MD, associate director of the Institute for Translational Medicine and Therapeutics and director of the Preventive Cardiovascular Program at Penn Medicine, Philadelphia. “The sponsor has elected to stop the development of this product, and I think we can probably write the obituary for ApoA-IMilano, even though it was never studied in cardiovascular outcomes.”
“At the end of the day, my message is that this has been a wild ride with ApoA-IMilano, and it’s sad to see that, at least when it comes to coronary atherosclerosis, this trial does not support that ApoA-IMilano has an effect,” Dr. Rader concluded. “But I do think we need to see through the other products with wild-type ApoA-I given the marked difference in structure before we can conclude that this general approach of infusing a reconstituted ApoA-I particle is not going to work.”
Trial details
Patients from 22 centers globally were randomized in MILANO-PILOT. All patients had experienced acute coronary syndrome in the past 14 days and had maximum stenosis of 20%-50% of a target vessel on coronary angiography.
Results showed – as expected based on past experience – that HDL cholesterol levels increased by 8.0% with placebo and decreased by 7.8% with MDCO-216 (P less than .001), Dr. Nicholls reported. Apolipoprotein A-I levels increased by 5.6% in the former and decreased by 5.3% in the latter (P less than .001). Changes in a variety of other lipid measures did not differ significantly between groups.
“These study patients were extraordinarily well treated. We achieved LDL cholesterol levels in both treatment groups that were less than 70 mg/dL,” he pointed out.
In addition to the lack of difference in the reduction in percent atheroma volume, there was also no significant difference between MDCO-216 and placebo in the secondary endpoints of median change in total atheroma volume, either in the entire vessel length imaged (–4.7 vs. –6.9 mm3) or in the most diseased 10-mm segment (–2.4 vs. –2.4 mm3).
The percentage of patients having any degree of disease regression was 55.8% with MDCO-216 and 67.2% with placebo, another nonsignificant difference.
“The majority of patients in this study demonstrated some degree of regression, and I believe that really highlights the impact of contemporary treatment guidelines have on coronary atherosclerosis in the early post-ACS setting in the contemporary era,” Dr. Nicholls maintained.
Exploratory analyses did not indicate any clear differential impact of MDCO-216 versus placebo according to whether patients had already been taking statins at baseline.
MDCO-216 was generally safe and well tolerated. “We saw no differences between the groups in terms of a range of biochemical adverse events, serious adverse events, and infusion site reactions,” reported Dr. Nicholls, who disclosed that he received a research grant from The Medicines Company, which sponsored the trial.
NEW ORLEANS – The future doesn’t look bright at the moment for use of agents that mimic HDL cholesterol to reverse coronary disease, based on disappointing results of the randomized, phase II MILANO-PILOT trial.
Among 120 patients with recent acute coronary syndrome, a mimetic known as MDCO-216 was no better than placebo at reducing coronary plaque measured by intravascular ultrasound (IVUS), according to data presented at the American Heart Association scientific sessions.
The median reduction in percent atheroma volume, the trial’s primary endpoint, was 0.5% with the mimetic and 0.8% with placebo, reported Stephen J. Nicholls, MD, of the South Australian Health and Medical Research Institute, University of Adelaide (Australia). Findings were similar for secondary outcomes assessing other measures of disease regression.
These data bring to an end a story that began in the 1980s, with discovery that a family in a town in northern Italy had a naturally occurring apolipoprotein A-I variant that mimics the actions of HDL cholesterol, subsequently named ApoA-IMilano. Despite having low levels of HDL, family members have a reduced risk of coronary disease.
In a small randomized trial, patients with acute coronary syndrome given an early recombinant form of this apolipoprotein saw a reduction in IVUS-measured coronary atherosclerosis (JAMA. 2003;290:2292-300). This prompted development of the refined recombinant form, MDCO-216, tested in the new trial.
“MDCO-216 did not produce a significant effect on coronary disease progression as measured by IVUS,” said Dr. Nicholls, who discussed the findings in a video interview. “These results occurred on a background of contemporary therapy in the post-ACS setting, which in 2016 is very effective for many patients.”
“The findings from this pilot study do not provide the evidence required to proceed with further development,” he concluded.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hope hinges on wild type
“This trial was extremely well done, world-class investigators carried out this trial, it was three times the size of the previous IVUS trial, and I think it is convincingly negative,” said discussant Daniel J. Rader, MD, associate director of the Institute for Translational Medicine and Therapeutics and director of the Preventive Cardiovascular Program at Penn Medicine, Philadelphia. “The sponsor has elected to stop the development of this product, and I think we can probably write the obituary for ApoA-IMilano, even though it was never studied in cardiovascular outcomes.”
“At the end of the day, my message is that this has been a wild ride with ApoA-IMilano, and it’s sad to see that, at least when it comes to coronary atherosclerosis, this trial does not support that ApoA-IMilano has an effect,” Dr. Rader concluded. “But I do think we need to see through the other products with wild-type ApoA-I given the marked difference in structure before we can conclude that this general approach of infusing a reconstituted ApoA-I particle is not going to work.”
Trial details
Patients from 22 centers globally were randomized in MILANO-PILOT. All patients had experienced acute coronary syndrome in the past 14 days and had maximum stenosis of 20%-50% of a target vessel on coronary angiography.
Results showed – as expected based on past experience – that HDL cholesterol levels increased by 8.0% with placebo and decreased by 7.8% with MDCO-216 (P less than .001), Dr. Nicholls reported. Apolipoprotein A-I levels increased by 5.6% in the former and decreased by 5.3% in the latter (P less than .001). Changes in a variety of other lipid measures did not differ significantly between groups.
“These study patients were extraordinarily well treated. We achieved LDL cholesterol levels in both treatment groups that were less than 70 mg/dL,” he pointed out.
In addition to the lack of difference in the reduction in percent atheroma volume, there was also no significant difference between MDCO-216 and placebo in the secondary endpoints of median change in total atheroma volume, either in the entire vessel length imaged (–4.7 vs. –6.9 mm3) or in the most diseased 10-mm segment (–2.4 vs. –2.4 mm3).
The percentage of patients having any degree of disease regression was 55.8% with MDCO-216 and 67.2% with placebo, another nonsignificant difference.
“The majority of patients in this study demonstrated some degree of regression, and I believe that really highlights the impact of contemporary treatment guidelines have on coronary atherosclerosis in the early post-ACS setting in the contemporary era,” Dr. Nicholls maintained.
Exploratory analyses did not indicate any clear differential impact of MDCO-216 versus placebo according to whether patients had already been taking statins at baseline.
MDCO-216 was generally safe and well tolerated. “We saw no differences between the groups in terms of a range of biochemical adverse events, serious adverse events, and infusion site reactions,” reported Dr. Nicholls, who disclosed that he received a research grant from The Medicines Company, which sponsored the trial.
NEW ORLEANS – The future doesn’t look bright at the moment for use of agents that mimic HDL cholesterol to reverse coronary disease, based on disappointing results of the randomized, phase II MILANO-PILOT trial.
Among 120 patients with recent acute coronary syndrome, a mimetic known as MDCO-216 was no better than placebo at reducing coronary plaque measured by intravascular ultrasound (IVUS), according to data presented at the American Heart Association scientific sessions.
The median reduction in percent atheroma volume, the trial’s primary endpoint, was 0.5% with the mimetic and 0.8% with placebo, reported Stephen J. Nicholls, MD, of the South Australian Health and Medical Research Institute, University of Adelaide (Australia). Findings were similar for secondary outcomes assessing other measures of disease regression.
These data bring to an end a story that began in the 1980s, with discovery that a family in a town in northern Italy had a naturally occurring apolipoprotein A-I variant that mimics the actions of HDL cholesterol, subsequently named ApoA-IMilano. Despite having low levels of HDL, family members have a reduced risk of coronary disease.
In a small randomized trial, patients with acute coronary syndrome given an early recombinant form of this apolipoprotein saw a reduction in IVUS-measured coronary atherosclerosis (JAMA. 2003;290:2292-300). This prompted development of the refined recombinant form, MDCO-216, tested in the new trial.
“MDCO-216 did not produce a significant effect on coronary disease progression as measured by IVUS,” said Dr. Nicholls, who discussed the findings in a video interview. “These results occurred on a background of contemporary therapy in the post-ACS setting, which in 2016 is very effective for many patients.”
“The findings from this pilot study do not provide the evidence required to proceed with further development,” he concluded.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hope hinges on wild type
“This trial was extremely well done, world-class investigators carried out this trial, it was three times the size of the previous IVUS trial, and I think it is convincingly negative,” said discussant Daniel J. Rader, MD, associate director of the Institute for Translational Medicine and Therapeutics and director of the Preventive Cardiovascular Program at Penn Medicine, Philadelphia. “The sponsor has elected to stop the development of this product, and I think we can probably write the obituary for ApoA-IMilano, even though it was never studied in cardiovascular outcomes.”
“At the end of the day, my message is that this has been a wild ride with ApoA-IMilano, and it’s sad to see that, at least when it comes to coronary atherosclerosis, this trial does not support that ApoA-IMilano has an effect,” Dr. Rader concluded. “But I do think we need to see through the other products with wild-type ApoA-I given the marked difference in structure before we can conclude that this general approach of infusing a reconstituted ApoA-I particle is not going to work.”
Trial details
Patients from 22 centers globally were randomized in MILANO-PILOT. All patients had experienced acute coronary syndrome in the past 14 days and had maximum stenosis of 20%-50% of a target vessel on coronary angiography.
Results showed – as expected based on past experience – that HDL cholesterol levels increased by 8.0% with placebo and decreased by 7.8% with MDCO-216 (P less than .001), Dr. Nicholls reported. Apolipoprotein A-I levels increased by 5.6% in the former and decreased by 5.3% in the latter (P less than .001). Changes in a variety of other lipid measures did not differ significantly between groups.
“These study patients were extraordinarily well treated. We achieved LDL cholesterol levels in both treatment groups that were less than 70 mg/dL,” he pointed out.
In addition to the lack of difference in the reduction in percent atheroma volume, there was also no significant difference between MDCO-216 and placebo in the secondary endpoints of median change in total atheroma volume, either in the entire vessel length imaged (–4.7 vs. –6.9 mm3) or in the most diseased 10-mm segment (–2.4 vs. –2.4 mm3).
The percentage of patients having any degree of disease regression was 55.8% with MDCO-216 and 67.2% with placebo, another nonsignificant difference.
“The majority of patients in this study demonstrated some degree of regression, and I believe that really highlights the impact of contemporary treatment guidelines have on coronary atherosclerosis in the early post-ACS setting in the contemporary era,” Dr. Nicholls maintained.
Exploratory analyses did not indicate any clear differential impact of MDCO-216 versus placebo according to whether patients had already been taking statins at baseline.
MDCO-216 was generally safe and well tolerated. “We saw no differences between the groups in terms of a range of biochemical adverse events, serious adverse events, and infusion site reactions,” reported Dr. Nicholls, who disclosed that he received a research grant from The Medicines Company, which sponsored the trial.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The percent atheroma volume decreased by 0.5% with the mimetic and 0.8% with placebo, a nonsignificant difference.
Data source: A randomized phase II trial among 120 patients with recent ACS and coronary stenosis (MILANO-PILOT trial).
Disclosures: Dr. Nicholls has received a research grant from The Medicines Company, which sponsored the study.
Bone marrow cells prove limb-saving for some
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The 52-week rate of amputation-free survival events was 20.2% with the aspirate and 30.5% with placebo (P = .224).
Data source: A randomized phase III trial among 152 patients with critical limb ischemia who were not candidates for revascularization surgery (MOBILE trial).
Disclosures: Dr. Murphy disclosed that he had no relevant conflicts of interest. The trial was sponsored by Biomet Biologics, LLC.
Allogeneic stem cells show promise for treating nonischemic dilated cardiomyopathy
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
Data source: A randomized phase I/II trial among 37 patients with nonischemic dilated cardiomyopathy (POSEIDON-DCM trial).
Disclosures: Dr. Hare disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.