User login
Aspirin alone preferred antithrombotic strategy after TAVI
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
ADHD and dyslexia may affect evaluation of concussion
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
From AAN Sports Concussion Conference
Twelve risk factors linked to 40% of world’s dementia cases
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
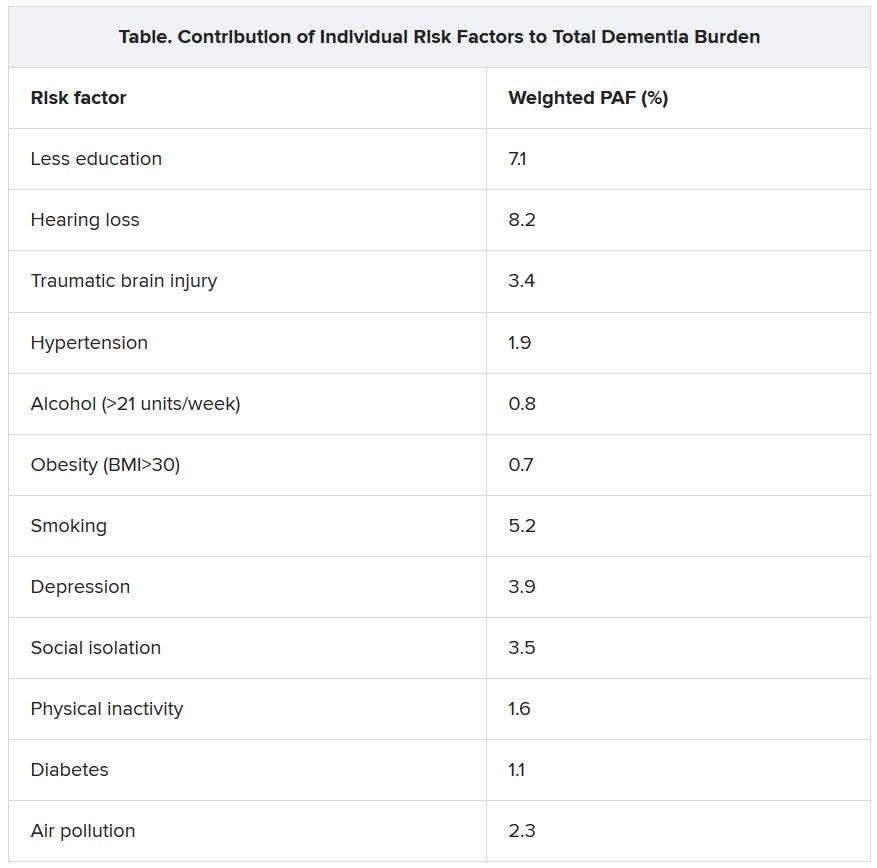
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
From AAIC 2020
FDA approves cannabidiol for tuberous sclerosis complex
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
Positive phase 3 top-line results for migraine prevention drug
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.
AMA urges change after dramatic increase in illicit opioid fatalities
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
Captopril questioned for diabetes patients in COVID-19 setting
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Intervention for AVM still too high risk: The latest from ARUBA
Enrollment into the trial, which compared medical management alone with medical management with interventional therapy (neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination), was stopped prematurely in 2013 after 33 months of follow-up because of a much higher rate of death and stroke in the intervention group.
Reaffirming the benefit of no intervention
Now the investigators are reporting extended follow-up to 50 months. The results were very similar to those at 33 months.
The current 50-month follow-up results show that 15 of 110 patients in the medical group had died or had a stroke (3.39 per 100 patient-years) versus 41 of 116 (12.32 per 100 patient-years) in the intervention group. The results reaffirm the strong benefit of not undergoing intervention (hazard ratio, 0.31; 95% confidence interval, 0.17-0.56).
These latest results were published in the July issue of the Lancet Neurology.
“With an AVM, the natural reflex is to try and fix it, but our trial shows that the tools we have to do that seem to be more damaging than just living with the AVM. If we try to take it out, the stroke risk is three to five times higher than just leaving it alone,” coauthor Christian Stapf, MD, a professor at the University of Montreal, said in an interview.
Dr. Stapf explained that an AVM is a congenital abnormality in the linking of the arteries to the veins. “There are an excess number of arteries and veins. They usually sit there silently, but they can trigger seizures, as they can tickle the neurons in the vicinity.”
It is estimated that one to two AVMs are found spontaneously in every 100,000 persons every year, but this is dependent on the availability of MRI, and many go undetected, he noted. In MRI studies in healthy volunteers, the rate was about one AVM in every 2,000 individuals.
Challenging standard practice
With AVMs, rupture and intracerebral hemorrhage occur at a rate of about 1%-2% per year. Until the ARUBA results were published, the standard practice was to intervene to embolize or excise the malformation, Dr. Stapf said.
“The standard treatment was intervention. The experiment was not to do it. We were challenging standard practice, and the trial was not popular with interventionalists,” he said.
The initial study, which was published in 2014, received much criticism from the interventionalist community. Among the criticisms were that the selection criteria for enrollment limited its generalizability, fewer patients than expected in the intervention arm were referred for microvascular surgery, and the follow-up was too short to allow a meaningful comparison.
“The study received criticism, but this was mainly from interventionalists, who were having their income threatened,” Dr. Stapf said. “This was very unhappy news for them, especially in the U.S., with the fee-for-service system.”
But he says these longer-term results, together with additional analyses and data from other cohorts, reinforce their initial conclusions.
The current report also shows a benefit in functional outcome in the medical group. “After 5 years, patients are twice as likely to have a neurological handicap, defined as a score of 2 or higher on the modified Rankin scale in the intervention group,” he noted. “We also found that more patients in the intervention group had deficits not related to stroke, such as an increase in seizures.”
Results of subgroup analysis were consistent in all patient groups.
The “study was designed for 400 patients, but we only recruited about half that number. But even so, the effect of intervention on stroke is so strong there is no subgroup where it looks favorable,” Dr. Stapf said. “This result was not heterogeneous. The same effect is seen regardless of age, gender, presence of symptoms, size of AVM, location, anatomy, drainage. No matter how you look, there is no benefit for intervention.”
He also referred to a Scottish population-based cohort study that showed a similar risk reduction from not intervening. “This was an unselected population of every unruptured AVM patient in Scotland, which found a 65% relative reduction in death/stroke over 12 years. We found a 69% reduction. The Scottish study did not select any particular types of patients but showed the same result as us,” he noted. “It is hard to argue against these findings.”
Regarding the claim of selection bias, Dr. Stapf acknowledged that the study excluded patients who were judged to be in need of intervention and those judged to be at very low risk and who would not be considered for an intervention.
“But when we compared our cohort to two other unselected cohorts, they look very similar, apart from the fact that very large AVMs were not entered in our study, as they were considered too difficult to treat,” he said. “If there is a selection bias at all, it actually trends towards the intervention group, as we excluded those at the highest treatment risk, but we still showed more benefit of not intervening.”
He also says the microvascular surgery rates were consistent with real-world practice, with about 25% of patients undergoing such surgery. “This is similar to the Scottish population study. Our trial also showed a similar result in patients treated with the various different interventions – they all showed a much higher risk than not intervening,” he added.
He says practice has changed since the trial was first reported. “There are far fewer interventions now for unruptured AVMs. Most interventionalists have accepted the results now, although there are some who continue to find reasons to criticize the trial and carry on with the procedures.”
He says his advice to patients who have an unruptured AVM is to forget about it. “There doesn’t seem to be a trigger for rupture,” he said. “It doesn’t seem to be dependent on blood pressure or physical activity, and we can’t tell if it’s just about to go by looking at it. They are very different from an aneurysm in that regard.
“When I explain to patients that they are at an increased stroke risk and tell them about the results of the ARUBA study, they say they would prefer to get that stroke later in life than earlier. These patents can live for 30 or 40 years without a stroke.
“But, yes, there remains a major unmet need. We need to find a way to protect these patients. In future, we might find a better way of intervening, but at this point in time, the treatment we have is more dangerous than doing nothing,” he said.
Longer follow-up needed
In an editorial that accompanies the current study, Peter M. Rothwell, MD, of the University of Oxford, England, also dismisses much of the criticism of the ARUBA study. On the issue of external validity, he said: “I do not think that this is really any greater an issue for ARUBA than for most other similar trials.”
But Dr. Rothwell does believe that follow-up for longer than 5 years is needed. “To really understand the benefit/risk balance, we would need a 20- or 30-year follow-up. These patients are often in their 20s, 30s, or 40s, so we really need to know their cumulative risk over decades,” he said in an interview.
Noting that the study was funded by the National Institute of Neurological Disorders and Stroke (NINDS), Dr. Rothwell said funding should have been provided for much longer follow-up. “Patients who generously agreed to be randomly assigned in ARUBA and future similar patients have been let down by NINDS.
“We probably now won’t ever know the very–long-term impact, although the Scottish population study is following patients longer term,” he added.
“After this trial was first published, the guidelines recommended not to intervene. These latest results will not change that,” he said.
The ARUBA trial was funded internationally by the National Institutes of Health/NINDS. Dr. Stapf and Dr. Rothwell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Enrollment into the trial, which compared medical management alone with medical management with interventional therapy (neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination), was stopped prematurely in 2013 after 33 months of follow-up because of a much higher rate of death and stroke in the intervention group.
Reaffirming the benefit of no intervention
Now the investigators are reporting extended follow-up to 50 months. The results were very similar to those at 33 months.
The current 50-month follow-up results show that 15 of 110 patients in the medical group had died or had a stroke (3.39 per 100 patient-years) versus 41 of 116 (12.32 per 100 patient-years) in the intervention group. The results reaffirm the strong benefit of not undergoing intervention (hazard ratio, 0.31; 95% confidence interval, 0.17-0.56).
These latest results were published in the July issue of the Lancet Neurology.
“With an AVM, the natural reflex is to try and fix it, but our trial shows that the tools we have to do that seem to be more damaging than just living with the AVM. If we try to take it out, the stroke risk is three to five times higher than just leaving it alone,” coauthor Christian Stapf, MD, a professor at the University of Montreal, said in an interview.
Dr. Stapf explained that an AVM is a congenital abnormality in the linking of the arteries to the veins. “There are an excess number of arteries and veins. They usually sit there silently, but they can trigger seizures, as they can tickle the neurons in the vicinity.”
It is estimated that one to two AVMs are found spontaneously in every 100,000 persons every year, but this is dependent on the availability of MRI, and many go undetected, he noted. In MRI studies in healthy volunteers, the rate was about one AVM in every 2,000 individuals.
Challenging standard practice
With AVMs, rupture and intracerebral hemorrhage occur at a rate of about 1%-2% per year. Until the ARUBA results were published, the standard practice was to intervene to embolize or excise the malformation, Dr. Stapf said.
“The standard treatment was intervention. The experiment was not to do it. We were challenging standard practice, and the trial was not popular with interventionalists,” he said.
The initial study, which was published in 2014, received much criticism from the interventionalist community. Among the criticisms were that the selection criteria for enrollment limited its generalizability, fewer patients than expected in the intervention arm were referred for microvascular surgery, and the follow-up was too short to allow a meaningful comparison.
“The study received criticism, but this was mainly from interventionalists, who were having their income threatened,” Dr. Stapf said. “This was very unhappy news for them, especially in the U.S., with the fee-for-service system.”
But he says these longer-term results, together with additional analyses and data from other cohorts, reinforce their initial conclusions.
The current report also shows a benefit in functional outcome in the medical group. “After 5 years, patients are twice as likely to have a neurological handicap, defined as a score of 2 or higher on the modified Rankin scale in the intervention group,” he noted. “We also found that more patients in the intervention group had deficits not related to stroke, such as an increase in seizures.”
Results of subgroup analysis were consistent in all patient groups.
The “study was designed for 400 patients, but we only recruited about half that number. But even so, the effect of intervention on stroke is so strong there is no subgroup where it looks favorable,” Dr. Stapf said. “This result was not heterogeneous. The same effect is seen regardless of age, gender, presence of symptoms, size of AVM, location, anatomy, drainage. No matter how you look, there is no benefit for intervention.”
He also referred to a Scottish population-based cohort study that showed a similar risk reduction from not intervening. “This was an unselected population of every unruptured AVM patient in Scotland, which found a 65% relative reduction in death/stroke over 12 years. We found a 69% reduction. The Scottish study did not select any particular types of patients but showed the same result as us,” he noted. “It is hard to argue against these findings.”
Regarding the claim of selection bias, Dr. Stapf acknowledged that the study excluded patients who were judged to be in need of intervention and those judged to be at very low risk and who would not be considered for an intervention.
“But when we compared our cohort to two other unselected cohorts, they look very similar, apart from the fact that very large AVMs were not entered in our study, as they were considered too difficult to treat,” he said. “If there is a selection bias at all, it actually trends towards the intervention group, as we excluded those at the highest treatment risk, but we still showed more benefit of not intervening.”
He also says the microvascular surgery rates were consistent with real-world practice, with about 25% of patients undergoing such surgery. “This is similar to the Scottish population study. Our trial also showed a similar result in patients treated with the various different interventions – they all showed a much higher risk than not intervening,” he added.
He says practice has changed since the trial was first reported. “There are far fewer interventions now for unruptured AVMs. Most interventionalists have accepted the results now, although there are some who continue to find reasons to criticize the trial and carry on with the procedures.”
He says his advice to patients who have an unruptured AVM is to forget about it. “There doesn’t seem to be a trigger for rupture,” he said. “It doesn’t seem to be dependent on blood pressure or physical activity, and we can’t tell if it’s just about to go by looking at it. They are very different from an aneurysm in that regard.
“When I explain to patients that they are at an increased stroke risk and tell them about the results of the ARUBA study, they say they would prefer to get that stroke later in life than earlier. These patents can live for 30 or 40 years without a stroke.
“But, yes, there remains a major unmet need. We need to find a way to protect these patients. In future, we might find a better way of intervening, but at this point in time, the treatment we have is more dangerous than doing nothing,” he said.
Longer follow-up needed
In an editorial that accompanies the current study, Peter M. Rothwell, MD, of the University of Oxford, England, also dismisses much of the criticism of the ARUBA study. On the issue of external validity, he said: “I do not think that this is really any greater an issue for ARUBA than for most other similar trials.”
But Dr. Rothwell does believe that follow-up for longer than 5 years is needed. “To really understand the benefit/risk balance, we would need a 20- or 30-year follow-up. These patients are often in their 20s, 30s, or 40s, so we really need to know their cumulative risk over decades,” he said in an interview.
Noting that the study was funded by the National Institute of Neurological Disorders and Stroke (NINDS), Dr. Rothwell said funding should have been provided for much longer follow-up. “Patients who generously agreed to be randomly assigned in ARUBA and future similar patients have been let down by NINDS.
“We probably now won’t ever know the very–long-term impact, although the Scottish population study is following patients longer term,” he added.
“After this trial was first published, the guidelines recommended not to intervene. These latest results will not change that,” he said.
The ARUBA trial was funded internationally by the National Institutes of Health/NINDS. Dr. Stapf and Dr. Rothwell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Enrollment into the trial, which compared medical management alone with medical management with interventional therapy (neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination), was stopped prematurely in 2013 after 33 months of follow-up because of a much higher rate of death and stroke in the intervention group.
Reaffirming the benefit of no intervention
Now the investigators are reporting extended follow-up to 50 months. The results were very similar to those at 33 months.
The current 50-month follow-up results show that 15 of 110 patients in the medical group had died or had a stroke (3.39 per 100 patient-years) versus 41 of 116 (12.32 per 100 patient-years) in the intervention group. The results reaffirm the strong benefit of not undergoing intervention (hazard ratio, 0.31; 95% confidence interval, 0.17-0.56).
These latest results were published in the July issue of the Lancet Neurology.
“With an AVM, the natural reflex is to try and fix it, but our trial shows that the tools we have to do that seem to be more damaging than just living with the AVM. If we try to take it out, the stroke risk is three to five times higher than just leaving it alone,” coauthor Christian Stapf, MD, a professor at the University of Montreal, said in an interview.
Dr. Stapf explained that an AVM is a congenital abnormality in the linking of the arteries to the veins. “There are an excess number of arteries and veins. They usually sit there silently, but they can trigger seizures, as they can tickle the neurons in the vicinity.”
It is estimated that one to two AVMs are found spontaneously in every 100,000 persons every year, but this is dependent on the availability of MRI, and many go undetected, he noted. In MRI studies in healthy volunteers, the rate was about one AVM in every 2,000 individuals.
Challenging standard practice
With AVMs, rupture and intracerebral hemorrhage occur at a rate of about 1%-2% per year. Until the ARUBA results were published, the standard practice was to intervene to embolize or excise the malformation, Dr. Stapf said.
“The standard treatment was intervention. The experiment was not to do it. We were challenging standard practice, and the trial was not popular with interventionalists,” he said.
The initial study, which was published in 2014, received much criticism from the interventionalist community. Among the criticisms were that the selection criteria for enrollment limited its generalizability, fewer patients than expected in the intervention arm were referred for microvascular surgery, and the follow-up was too short to allow a meaningful comparison.
“The study received criticism, but this was mainly from interventionalists, who were having their income threatened,” Dr. Stapf said. “This was very unhappy news for them, especially in the U.S., with the fee-for-service system.”
But he says these longer-term results, together with additional analyses and data from other cohorts, reinforce their initial conclusions.
The current report also shows a benefit in functional outcome in the medical group. “After 5 years, patients are twice as likely to have a neurological handicap, defined as a score of 2 or higher on the modified Rankin scale in the intervention group,” he noted. “We also found that more patients in the intervention group had deficits not related to stroke, such as an increase in seizures.”
Results of subgroup analysis were consistent in all patient groups.
The “study was designed for 400 patients, but we only recruited about half that number. But even so, the effect of intervention on stroke is so strong there is no subgroup where it looks favorable,” Dr. Stapf said. “This result was not heterogeneous. The same effect is seen regardless of age, gender, presence of symptoms, size of AVM, location, anatomy, drainage. No matter how you look, there is no benefit for intervention.”
He also referred to a Scottish population-based cohort study that showed a similar risk reduction from not intervening. “This was an unselected population of every unruptured AVM patient in Scotland, which found a 65% relative reduction in death/stroke over 12 years. We found a 69% reduction. The Scottish study did not select any particular types of patients but showed the same result as us,” he noted. “It is hard to argue against these findings.”
Regarding the claim of selection bias, Dr. Stapf acknowledged that the study excluded patients who were judged to be in need of intervention and those judged to be at very low risk and who would not be considered for an intervention.
“But when we compared our cohort to two other unselected cohorts, they look very similar, apart from the fact that very large AVMs were not entered in our study, as they were considered too difficult to treat,” he said. “If there is a selection bias at all, it actually trends towards the intervention group, as we excluded those at the highest treatment risk, but we still showed more benefit of not intervening.”
He also says the microvascular surgery rates were consistent with real-world practice, with about 25% of patients undergoing such surgery. “This is similar to the Scottish population study. Our trial also showed a similar result in patients treated with the various different interventions – they all showed a much higher risk than not intervening,” he added.
He says practice has changed since the trial was first reported. “There are far fewer interventions now for unruptured AVMs. Most interventionalists have accepted the results now, although there are some who continue to find reasons to criticize the trial and carry on with the procedures.”
He says his advice to patients who have an unruptured AVM is to forget about it. “There doesn’t seem to be a trigger for rupture,” he said. “It doesn’t seem to be dependent on blood pressure or physical activity, and we can’t tell if it’s just about to go by looking at it. They are very different from an aneurysm in that regard.
“When I explain to patients that they are at an increased stroke risk and tell them about the results of the ARUBA study, they say they would prefer to get that stroke later in life than earlier. These patents can live for 30 or 40 years without a stroke.
“But, yes, there remains a major unmet need. We need to find a way to protect these patients. In future, we might find a better way of intervening, but at this point in time, the treatment we have is more dangerous than doing nothing,” he said.
Longer follow-up needed
In an editorial that accompanies the current study, Peter M. Rothwell, MD, of the University of Oxford, England, also dismisses much of the criticism of the ARUBA study. On the issue of external validity, he said: “I do not think that this is really any greater an issue for ARUBA than for most other similar trials.”
But Dr. Rothwell does believe that follow-up for longer than 5 years is needed. “To really understand the benefit/risk balance, we would need a 20- or 30-year follow-up. These patients are often in their 20s, 30s, or 40s, so we really need to know their cumulative risk over decades,” he said in an interview.
Noting that the study was funded by the National Institute of Neurological Disorders and Stroke (NINDS), Dr. Rothwell said funding should have been provided for much longer follow-up. “Patients who generously agreed to be randomly assigned in ARUBA and future similar patients have been let down by NINDS.
“We probably now won’t ever know the very–long-term impact, although the Scottish population study is following patients longer term,” he added.
“After this trial was first published, the guidelines recommended not to intervene. These latest results will not change that,” he said.
The ARUBA trial was funded internationally by the National Institutes of Health/NINDS. Dr. Stapf and Dr. Rothwell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM LANCET NEUROLOGY
Circadian rhythm changes linked to future Parkinson’s disease risk
a new study suggests. “We found that men with abnormal circadian rhythms had three times the risk of developing Parkinson’s disease over an 11-year follow-up period,” lead author, Yue Leng, MD, University of California, San Francisco, said in an interview.
“If confirmed to be a risk factor for Parkinson’s disease, then circadian rhythmicity could be a promising intervention target and will open new opportunities for the prevention and management of Parkinson’s disease,” the researchers concluded.
The study was published online in JAMA Neurology on June 15.
Circadian disruption is very common in neurodegenerative diseases such as Parkinson’s disease, but there isn’t much information on how it may predict the disease, Dr. Leng explained. “We wanted to see whether circadian abnormalities may predict Parkinson’s disease,” she said. “Parkinson’s disease has a long prodromal phase where brain changes have started to occur but no clinical symptoms have become evident. It would be useful to be able to identify these patients, and maybe changes in circadian rhythms may help us to do that,” she added.
For the study, the researchers analyzed data from 2,930 community-dwelling men aged 65 years or older (mean age, 76 years) who participated in the Osteoporotic Fractures in Men Study, in which they underwent comprehensive sleep and rest-activity rhythms assessment. “Patterns of rest and activity were measured with an actigraph device, which is worn on the wrist like a watch and captures movements which are translated into a rest-activity rhythm model – one of the most commonly used and evidence-based measures of circadian rhythm,” Dr. Leng said. Men were asked to wear the actigraphs continuously for a minimum of three 24-hour periods.
Results showed that 78 men (2.7%) developed Parkinson’s disease during the 11-year follow-up. After accounting for all covariates, the risk of Parkinson’s disease increased with decreasing circadian amplitude (strength of the rhythm) with an odds ratio of 1.77 per each decrease by one standard deviation; mesor (mean level of activity) with an odds ratio of 1.64; or robustness (how closely activity follows a 24-hour pattern) with an odds ratio of 1.54.
Those in the lowest quartile of amplitude, mesor, or robustness had approximately three times the risk of developing Parkinson’s disease compared with those in the highest quartile of amplitude. The association remained after further adjustment for nighttime sleep disturbances.
“It has previously been shown that daytime napping has been linked to risk of developing Parkinson’s disease. Now we have shown that abnormalities in the overall 24-hour circadian rest activity rhythm are also present in the prodromal phase of Parkinson’s disease, and this association was independent of several confounders, including nighttime sleep disturbances,” Dr. Leng said.
“This raises awareness of the importance of circadian rhythm in older individuals and changes in their 24-hour pattern of behavior could be an early signal of Parkinson’s disease,” she said.
“This study does not tell us whether these circadian changes are causal for Parkinson’s or not,” Dr. Leng noted.
Future studies are needed to explore underlying mechanisms and to determine whether circadian disruption itself might contribute to the development of Parkinson’s disease, the researchers said.
“If there is a causal link, then using techniques to improve circadian rhythm could help to prevent or slow the onset of Parkinson’s disease,” Dr. Leng suggested. There are many established therapies that act on circadian rhythm including bright light therapy, melatonin, and chronotherapy, she added.
Support for this study was provided by the National Institute on Aging (NIA); the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and the Weill Pilot Award. Dr. Leng reported grants from the NIA and the University of California, San Francisco, Weill Institute for Neurosciences during the conduct of the study; and grants from Global Brain Health Institute, the Alzheimer’s Association, and the Alzheimer’s Society outside the submitted work.
A version of this article originally appeared on Medscape.com.
a new study suggests. “We found that men with abnormal circadian rhythms had three times the risk of developing Parkinson’s disease over an 11-year follow-up period,” lead author, Yue Leng, MD, University of California, San Francisco, said in an interview.
“If confirmed to be a risk factor for Parkinson’s disease, then circadian rhythmicity could be a promising intervention target and will open new opportunities for the prevention and management of Parkinson’s disease,” the researchers concluded.
The study was published online in JAMA Neurology on June 15.
Circadian disruption is very common in neurodegenerative diseases such as Parkinson’s disease, but there isn’t much information on how it may predict the disease, Dr. Leng explained. “We wanted to see whether circadian abnormalities may predict Parkinson’s disease,” she said. “Parkinson’s disease has a long prodromal phase where brain changes have started to occur but no clinical symptoms have become evident. It would be useful to be able to identify these patients, and maybe changes in circadian rhythms may help us to do that,” she added.
For the study, the researchers analyzed data from 2,930 community-dwelling men aged 65 years or older (mean age, 76 years) who participated in the Osteoporotic Fractures in Men Study, in which they underwent comprehensive sleep and rest-activity rhythms assessment. “Patterns of rest and activity were measured with an actigraph device, which is worn on the wrist like a watch and captures movements which are translated into a rest-activity rhythm model – one of the most commonly used and evidence-based measures of circadian rhythm,” Dr. Leng said. Men were asked to wear the actigraphs continuously for a minimum of three 24-hour periods.
Results showed that 78 men (2.7%) developed Parkinson’s disease during the 11-year follow-up. After accounting for all covariates, the risk of Parkinson’s disease increased with decreasing circadian amplitude (strength of the rhythm) with an odds ratio of 1.77 per each decrease by one standard deviation; mesor (mean level of activity) with an odds ratio of 1.64; or robustness (how closely activity follows a 24-hour pattern) with an odds ratio of 1.54.
Those in the lowest quartile of amplitude, mesor, or robustness had approximately three times the risk of developing Parkinson’s disease compared with those in the highest quartile of amplitude. The association remained after further adjustment for nighttime sleep disturbances.
“It has previously been shown that daytime napping has been linked to risk of developing Parkinson’s disease. Now we have shown that abnormalities in the overall 24-hour circadian rest activity rhythm are also present in the prodromal phase of Parkinson’s disease, and this association was independent of several confounders, including nighttime sleep disturbances,” Dr. Leng said.
“This raises awareness of the importance of circadian rhythm in older individuals and changes in their 24-hour pattern of behavior could be an early signal of Parkinson’s disease,” she said.
“This study does not tell us whether these circadian changes are causal for Parkinson’s or not,” Dr. Leng noted.
Future studies are needed to explore underlying mechanisms and to determine whether circadian disruption itself might contribute to the development of Parkinson’s disease, the researchers said.
“If there is a causal link, then using techniques to improve circadian rhythm could help to prevent or slow the onset of Parkinson’s disease,” Dr. Leng suggested. There are many established therapies that act on circadian rhythm including bright light therapy, melatonin, and chronotherapy, she added.
Support for this study was provided by the National Institute on Aging (NIA); the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and the Weill Pilot Award. Dr. Leng reported grants from the NIA and the University of California, San Francisco, Weill Institute for Neurosciences during the conduct of the study; and grants from Global Brain Health Institute, the Alzheimer’s Association, and the Alzheimer’s Society outside the submitted work.
A version of this article originally appeared on Medscape.com.
a new study suggests. “We found that men with abnormal circadian rhythms had three times the risk of developing Parkinson’s disease over an 11-year follow-up period,” lead author, Yue Leng, MD, University of California, San Francisco, said in an interview.
“If confirmed to be a risk factor for Parkinson’s disease, then circadian rhythmicity could be a promising intervention target and will open new opportunities for the prevention and management of Parkinson’s disease,” the researchers concluded.
The study was published online in JAMA Neurology on June 15.
Circadian disruption is very common in neurodegenerative diseases such as Parkinson’s disease, but there isn’t much information on how it may predict the disease, Dr. Leng explained. “We wanted to see whether circadian abnormalities may predict Parkinson’s disease,” she said. “Parkinson’s disease has a long prodromal phase where brain changes have started to occur but no clinical symptoms have become evident. It would be useful to be able to identify these patients, and maybe changes in circadian rhythms may help us to do that,” she added.
For the study, the researchers analyzed data from 2,930 community-dwelling men aged 65 years or older (mean age, 76 years) who participated in the Osteoporotic Fractures in Men Study, in which they underwent comprehensive sleep and rest-activity rhythms assessment. “Patterns of rest and activity were measured with an actigraph device, which is worn on the wrist like a watch and captures movements which are translated into a rest-activity rhythm model – one of the most commonly used and evidence-based measures of circadian rhythm,” Dr. Leng said. Men were asked to wear the actigraphs continuously for a minimum of three 24-hour periods.
Results showed that 78 men (2.7%) developed Parkinson’s disease during the 11-year follow-up. After accounting for all covariates, the risk of Parkinson’s disease increased with decreasing circadian amplitude (strength of the rhythm) with an odds ratio of 1.77 per each decrease by one standard deviation; mesor (mean level of activity) with an odds ratio of 1.64; or robustness (how closely activity follows a 24-hour pattern) with an odds ratio of 1.54.
Those in the lowest quartile of amplitude, mesor, or robustness had approximately three times the risk of developing Parkinson’s disease compared with those in the highest quartile of amplitude. The association remained after further adjustment for nighttime sleep disturbances.
“It has previously been shown that daytime napping has been linked to risk of developing Parkinson’s disease. Now we have shown that abnormalities in the overall 24-hour circadian rest activity rhythm are also present in the prodromal phase of Parkinson’s disease, and this association was independent of several confounders, including nighttime sleep disturbances,” Dr. Leng said.
“This raises awareness of the importance of circadian rhythm in older individuals and changes in their 24-hour pattern of behavior could be an early signal of Parkinson’s disease,” she said.
“This study does not tell us whether these circadian changes are causal for Parkinson’s or not,” Dr. Leng noted.
Future studies are needed to explore underlying mechanisms and to determine whether circadian disruption itself might contribute to the development of Parkinson’s disease, the researchers said.
“If there is a causal link, then using techniques to improve circadian rhythm could help to prevent or slow the onset of Parkinson’s disease,” Dr. Leng suggested. There are many established therapies that act on circadian rhythm including bright light therapy, melatonin, and chronotherapy, she added.
Support for this study was provided by the National Institute on Aging (NIA); the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and the Weill Pilot Award. Dr. Leng reported grants from the NIA and the University of California, San Francisco, Weill Institute for Neurosciences during the conduct of the study; and grants from Global Brain Health Institute, the Alzheimer’s Association, and the Alzheimer’s Society outside the submitted work.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
CVD risk continues to fall down to systolic BP of 90 mm HG
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.