User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
Prognostic features could improve ALL outcomes
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
CHICAGO – New recognition of the prognostic value of cytogenetic factors, minimal residual disease activity, and Philadelphia chromosome–like signature could improve the treatment and outcomes of acute lymphoblastic leukemia (ALL), according to Anjali Advani, MD.
CD20
About 80% of ALL is B-cell ALL and the majority of patients have pre–B-cell ALL, Dr. Advani, a hematologist and director of the inpatient leukemia program at the Cleveland Clinic, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“And on the B lymphoblast, many antigens are expressed, including CD19, CD20, and CD52,” she said.
Rituximab, a drug often used for the treatment of lymphoma, is a chimeric monoclonal antibody against the protein CD20, which is expressed in 41% of ALL patients.
“Interestingly, CD20 expression in ALL has been associated with an adverse prognostic impact, which suggests that targeting this may potentially improve outcomes in these patients,” Dr. Advani said.
In fact, a recent randomized study by Sébastien Maury, MD, of the University of Paris-Est, and his colleagues, demonstrated that adding 16-18 doses of rituximab to a Berlin-Frankfurt-Münster (BFM)–based chemotherapy in Philadelphia chromosome (Ph)–negative patients aged 18-59 years with CD20-positive pre–B-cell ALL improved 2-year event-free survival from 52% to 65%. The data are consistent with those from prior studies, including a German study that showed a higher degree of minimal residual disease (MRD) negativity in patients treated with rituximab, she noted (N Engl J Med 2016;375:1044-53).
While the study by Dr. Maury and his colleagues didn’t look at MRD, that may offer an explanation for the improved event-free survival in their study, Dr. Advani suggested.
MRD
Minimal residual disease has become a standard part of practice in ALL, but pediatric ALL led the way in using early MRD measurement for risk-stratifying therapy, and it has taken a bit longer for it to be incorporated in the adult disease realm, Dr. Advani said.
Either flow cytometry or polymerase chain reaction (PCR) amplification can be used to measure MRD, she noted.
In one of the larger studies done in adults, researchers used PCR to look at MRD at two time points and stratified patients into three risk groups, including low, intermediate, and high risk (Blood. 2006 Feb 1;107:1116-23).
Measuring MRD at those two time points “clearly separated the prognosis of patients not only in terms of disease-free survival but [in] overall survival,” she said. “So that’s why, for ALL, this has really become very important.”
In the United States, where flow-based cytometry is used more, it is necessary to find a properly equipped laboratory that can provide reliable results, she added. “For example, at our center we actually send our MRD to Fred Hutchinson [Cancer Center in Seattle].”
Johns Hopkins [Baltimore] also has such a lab, and both can arrange to accept send-outs, she said.
The other “really exciting thing” in regard to MRD in ALL is the recent approval of blinatumomab for MRD-positive ALL, she said.
In a study of 113 evaluable patients who were treated with the monoclonal antibody, 78% achieved complete molecular response (Blood. 2018 Apr 5;131:1522-31).
“And probably most importantly, when they looked at those patients who responded to blinatumomab in terms of MRD, these patients had, again, not only improved relapse-free survival but also increased overall survival,” she said. “I think this really explains why in ALL, we are measuring MRD and how it can really impact these patients.”
One of the remaining questions that will be important to address going forward is whether patients with MRD-positive ALL should continue to be considered for transplant; some of these studies have shown “very, very good outcomes” in patients who have not been transplanted, she noted.
Ph-like signature
Another important new prognostic feature in ALL is the presence of the Ph-like signature, a gene expression signature that was initially described in children with poor-risk ALL, and which looks a lot like Ph-positive disease.
“When they delved in further, they identified that this signature actually correlated with multiple different [kinase] fusions ... and it turns out that 20%-25% of young adults have this signature,” she said.
Since event-free survival in young adults with ALL is usually in the 65%-70% range, most of the remaining 30%-35% likely have this signature, she explained.
The kinase fusions associated with the Ph-like signature retain intact tyrosine kinase domains, and the spectrum of the fusions changes across age groups.
Treatments targeting some of these – for example, dasatinib for patients with a Ph-like dasatinib-sensitive kinase mutation – are being investigated.
Additionally, the Children’s Oncology Group has developed a clinically adaptable screening assay to identify the signature, she noted.
“So I would say that, and there is probably some difference in opinion, it is now becoming fairly standard that at diagnosis in an adult with ALL we’re sending the Ph-like signature,” she said. “And again, usually you’re going to have to send this out, and at our center we send it out to [Nationwide Children’s Hospital] in Columbus [Ohio].”
As important as it is to identify the Ph-like signature, given its association with poor prognosis, a number of questions remain, including whether transplant improves outcomes.
“The hope is it probably does, and that’s something that’s being evaluated in studies,” she said, noting that clinical studies are also specifically targeting these patients.
“So these patients should probably be enrolled on a clinical trial, because their outcome is clearly inferior,” she said.
Dr. Advani reported consultancy for Pfizer; research funding from Genzyme, Novartis, Pfizer, and Sigma Tau; and honoraria from Genzyme, Pfizer, and Sigma Tau. She is also on the speakers bureau for Sigma Tau.
EXPERT ANALYSIS FROM MHM 2018
Adding pembrolizumab to cisplatin-based CRT shows promise in HPV+ head and neck cancers
WASHINGTON – according to Steven F. Powell, MD.
Of 34 patients with a mean age of 59 years and stage III-IVb disease enrolled as part of an expansion cohort following a prior study demonstrating the safety of the regimen, 85% had a complete response (CR) at a median follow-up of 21 months based on imaging or salvage surgery, and an additional 2 patients had no clinical evidence of disease, Dr. Powell reported in a late-breaking oral abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
About 80% of the patients had intermediate-risk disease, which is “higher risk than your standard HPV-related cancers,” said Dr. Powell of Sanford Cancer Center, Sioux Falls, S.D.
“On posttreatment imaging ... we showed a 62% complete response rate based on [RECIST 1.1 CT criteria], with 11 patients having a partial response and 2 felt to have a partial response based on CT imaging. Looking at Hopkins criteria [for PET scans] alone – 78% of our patients had a complete response,” he said.
Of the patients with a partial response based on either criteria, 11 were negative for disease on PET and 1 that was positive based on Hopkins criteria underwent neck dissection and had only inflammatory tissue; these 12 patients were also considered to have had a CR.
Additionally, of the two patients with progressive disease, one had a positive PET scan, but all biopsies were negative for ongoing disease, thus that patient was also considered to have a CR, for the overall CR rate of 85%, Dr. Powell said.
Two of the four other patients with a partial response were found at surgery to have “nothing to biopsy or resect,” so it was felt that they had a complete response clinically, and the remaining two had partial responses locoregionally and had residual disease, including residual disease at the primary site in one patient, and nodal disease in one patient.
It is important to consider the challenges of PET imaging in this study, he noted, explaining that in one patient with progressive disease, a posttreatment PET appeared to show bone and dermal metastases, but biopsies of all the areas showed that those were granulomatous disease – most likely sarcoidosis that was not present prior to the treatment.
“This ended up resolving over a year and the PET scan became negative, so I think this highlights that as we move into the curative intent setting we need to be very careful that with PET scanning we need to confirm with biopsy [in patients treated with immuno-oncology] therapies,” Dr. Powell said.
As for survival, the early data are “very encouraging,” with only one patient progressing to date (progression-free survival, 97.1%), but he cautioned that follow-up is “still only 23 months.”
The patient who progressed developed distant metastases and died from their disease, he said.
Treatment in this study included 40 mg/m2 of cisplatin weekly (six planned doses), 200 mg of pembrolizumab every 3 weeks (eight planned doses) and radiation therapy at 2 Gy once daily for 35 fractions (total of 70 Gy). The primary efficacy endpoint was complete response at 100 days after completion of chemoradiotherapy (CRT).
“Looking at safety ... we did not see any new safety signals. We had two dose discontinuations due to immune-related adverse events, which resolved on their own without therapy. Two patients stopped early due to protocol reasons,” Dr. Powell said, noting that the discontinuation rate was comparable with that seen in pembrolizumab monotherapy studies.
Standard therapy compliance was also good, with the chemotherapy goal dose reached in 88% of patients. The CRT dose was reached in all patients with no major delays in treatment.
“So adding CRT did not impact the safety of giving standard therapy,” he said.
Enrollment in this ongoing study was completed as of August, and data for the HPV-negative cohort should be available sometime in 2019. Several correlative research projects are also underway, he said.
The findings thus far show that pembrolizumab can be safely given with CRT in both HPV-positive and HPV-negative disease, with “encouraging response and progression-free survival in predominantly higher-risk patients,” Dr. Powell said.
“It is important to know that PET may pose challenges as we move into big phase 3, randomized trials, and I would strongly recommend biopsy to confirm PET findings,” he said, adding that it will be “interesting to see how this pans out in high-risk disease.
“I’m hopeful that our correlative research will help guide how we time therapy and how we move ahead in this field,” he said.
The Merck Investigator Studies Program provided grant support for this study. Dr. Powell has received research funding (to his institution) from Bristol-Myers Squibb, Genentech, Incyte, Merck, Novartis, and Pfizer.
WASHINGTON – according to Steven F. Powell, MD.
Of 34 patients with a mean age of 59 years and stage III-IVb disease enrolled as part of an expansion cohort following a prior study demonstrating the safety of the regimen, 85% had a complete response (CR) at a median follow-up of 21 months based on imaging or salvage surgery, and an additional 2 patients had no clinical evidence of disease, Dr. Powell reported in a late-breaking oral abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
About 80% of the patients had intermediate-risk disease, which is “higher risk than your standard HPV-related cancers,” said Dr. Powell of Sanford Cancer Center, Sioux Falls, S.D.
“On posttreatment imaging ... we showed a 62% complete response rate based on [RECIST 1.1 CT criteria], with 11 patients having a partial response and 2 felt to have a partial response based on CT imaging. Looking at Hopkins criteria [for PET scans] alone – 78% of our patients had a complete response,” he said.
Of the patients with a partial response based on either criteria, 11 were negative for disease on PET and 1 that was positive based on Hopkins criteria underwent neck dissection and had only inflammatory tissue; these 12 patients were also considered to have had a CR.
Additionally, of the two patients with progressive disease, one had a positive PET scan, but all biopsies were negative for ongoing disease, thus that patient was also considered to have a CR, for the overall CR rate of 85%, Dr. Powell said.
Two of the four other patients with a partial response were found at surgery to have “nothing to biopsy or resect,” so it was felt that they had a complete response clinically, and the remaining two had partial responses locoregionally and had residual disease, including residual disease at the primary site in one patient, and nodal disease in one patient.
It is important to consider the challenges of PET imaging in this study, he noted, explaining that in one patient with progressive disease, a posttreatment PET appeared to show bone and dermal metastases, but biopsies of all the areas showed that those were granulomatous disease – most likely sarcoidosis that was not present prior to the treatment.
“This ended up resolving over a year and the PET scan became negative, so I think this highlights that as we move into the curative intent setting we need to be very careful that with PET scanning we need to confirm with biopsy [in patients treated with immuno-oncology] therapies,” Dr. Powell said.
As for survival, the early data are “very encouraging,” with only one patient progressing to date (progression-free survival, 97.1%), but he cautioned that follow-up is “still only 23 months.”
The patient who progressed developed distant metastases and died from their disease, he said.
Treatment in this study included 40 mg/m2 of cisplatin weekly (six planned doses), 200 mg of pembrolizumab every 3 weeks (eight planned doses) and radiation therapy at 2 Gy once daily for 35 fractions (total of 70 Gy). The primary efficacy endpoint was complete response at 100 days after completion of chemoradiotherapy (CRT).
“Looking at safety ... we did not see any new safety signals. We had two dose discontinuations due to immune-related adverse events, which resolved on their own without therapy. Two patients stopped early due to protocol reasons,” Dr. Powell said, noting that the discontinuation rate was comparable with that seen in pembrolizumab monotherapy studies.
Standard therapy compliance was also good, with the chemotherapy goal dose reached in 88% of patients. The CRT dose was reached in all patients with no major delays in treatment.
“So adding CRT did not impact the safety of giving standard therapy,” he said.
Enrollment in this ongoing study was completed as of August, and data for the HPV-negative cohort should be available sometime in 2019. Several correlative research projects are also underway, he said.
The findings thus far show that pembrolizumab can be safely given with CRT in both HPV-positive and HPV-negative disease, with “encouraging response and progression-free survival in predominantly higher-risk patients,” Dr. Powell said.
“It is important to know that PET may pose challenges as we move into big phase 3, randomized trials, and I would strongly recommend biopsy to confirm PET findings,” he said, adding that it will be “interesting to see how this pans out in high-risk disease.
“I’m hopeful that our correlative research will help guide how we time therapy and how we move ahead in this field,” he said.
The Merck Investigator Studies Program provided grant support for this study. Dr. Powell has received research funding (to his institution) from Bristol-Myers Squibb, Genentech, Incyte, Merck, Novartis, and Pfizer.
WASHINGTON – according to Steven F. Powell, MD.
Of 34 patients with a mean age of 59 years and stage III-IVb disease enrolled as part of an expansion cohort following a prior study demonstrating the safety of the regimen, 85% had a complete response (CR) at a median follow-up of 21 months based on imaging or salvage surgery, and an additional 2 patients had no clinical evidence of disease, Dr. Powell reported in a late-breaking oral abstract session at the annual meeting of the Society for Immunotherapy of Cancer.
About 80% of the patients had intermediate-risk disease, which is “higher risk than your standard HPV-related cancers,” said Dr. Powell of Sanford Cancer Center, Sioux Falls, S.D.
“On posttreatment imaging ... we showed a 62% complete response rate based on [RECIST 1.1 CT criteria], with 11 patients having a partial response and 2 felt to have a partial response based on CT imaging. Looking at Hopkins criteria [for PET scans] alone – 78% of our patients had a complete response,” he said.
Of the patients with a partial response based on either criteria, 11 were negative for disease on PET and 1 that was positive based on Hopkins criteria underwent neck dissection and had only inflammatory tissue; these 12 patients were also considered to have had a CR.
Additionally, of the two patients with progressive disease, one had a positive PET scan, but all biopsies were negative for ongoing disease, thus that patient was also considered to have a CR, for the overall CR rate of 85%, Dr. Powell said.
Two of the four other patients with a partial response were found at surgery to have “nothing to biopsy or resect,” so it was felt that they had a complete response clinically, and the remaining two had partial responses locoregionally and had residual disease, including residual disease at the primary site in one patient, and nodal disease in one patient.
It is important to consider the challenges of PET imaging in this study, he noted, explaining that in one patient with progressive disease, a posttreatment PET appeared to show bone and dermal metastases, but biopsies of all the areas showed that those were granulomatous disease – most likely sarcoidosis that was not present prior to the treatment.
“This ended up resolving over a year and the PET scan became negative, so I think this highlights that as we move into the curative intent setting we need to be very careful that with PET scanning we need to confirm with biopsy [in patients treated with immuno-oncology] therapies,” Dr. Powell said.
As for survival, the early data are “very encouraging,” with only one patient progressing to date (progression-free survival, 97.1%), but he cautioned that follow-up is “still only 23 months.”
The patient who progressed developed distant metastases and died from their disease, he said.
Treatment in this study included 40 mg/m2 of cisplatin weekly (six planned doses), 200 mg of pembrolizumab every 3 weeks (eight planned doses) and radiation therapy at 2 Gy once daily for 35 fractions (total of 70 Gy). The primary efficacy endpoint was complete response at 100 days after completion of chemoradiotherapy (CRT).
“Looking at safety ... we did not see any new safety signals. We had two dose discontinuations due to immune-related adverse events, which resolved on their own without therapy. Two patients stopped early due to protocol reasons,” Dr. Powell said, noting that the discontinuation rate was comparable with that seen in pembrolizumab monotherapy studies.
Standard therapy compliance was also good, with the chemotherapy goal dose reached in 88% of patients. The CRT dose was reached in all patients with no major delays in treatment.
“So adding CRT did not impact the safety of giving standard therapy,” he said.
Enrollment in this ongoing study was completed as of August, and data for the HPV-negative cohort should be available sometime in 2019. Several correlative research projects are also underway, he said.
The findings thus far show that pembrolizumab can be safely given with CRT in both HPV-positive and HPV-negative disease, with “encouraging response and progression-free survival in predominantly higher-risk patients,” Dr. Powell said.
“It is important to know that PET may pose challenges as we move into big phase 3, randomized trials, and I would strongly recommend biopsy to confirm PET findings,” he said, adding that it will be “interesting to see how this pans out in high-risk disease.
“I’m hopeful that our correlative research will help guide how we time therapy and how we move ahead in this field,” he said.
The Merck Investigator Studies Program provided grant support for this study. Dr. Powell has received research funding (to his institution) from Bristol-Myers Squibb, Genentech, Incyte, Merck, Novartis, and Pfizer.
REPORTING FROM SITC 2018
Key clinical point: Adding pembrolizumab to weekly low-dose cisplatin-based chemoradiotherapy shows promise in human papillomavirus–associated head and neck squamous cell carcinoma.
Major finding: A total of 85% of patients had a complete response at the 21-month follow-up; progression-free survival was 97.1%.
Study details: An expansion cohort of 34 patients.
Disclosures: The Merck Investigator Studies Program provided grant support for this study. Dr. Powell has received research funding (to his institution) from Bristol-Myers Squibb, Genentech, Incyte, Merck, Novartis, and Pfizer.
Refractory immune-mediated colitis: Fecal transplant may be the answer
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
WASHINGTON – , according to Yinghong Wang, MD.
In two patients who developed severe, refractory, immune-mediated colitis (IMC), FMT led to recovery, Dr. Wang of M.D. Anderson Cancer Center, Houston, reported at the annual meeting of the Society for Immunotherapy of Cancer.
Patient 1 was a woman with renal cell cancer who developed grade 2+ IMC within 1 month of initiation of treatment with combined ipilimumab and nivolumab. Infectious etiology was ruled out, and her symptoms and ulcers persisted despite 3 months of treatment with corticosteroids, two doses of infliximab, and one dose of vedolizumab.
A single FMT delivered via colonoscopy led to complete symptom resolution within 10 days, and a repeat colonoscopy showed “very nice healing of inflammation and ulcers,” Dr. Wang said.
Patient 2 was a man with prostate cancer who developed grade 2+ IMC 3 months after receiving two doses of ipilimumab. Infectious etiologies were ruled out, and like patient 1, his symptoms and mucosal ulcerations persisted despite 5 months of immunosuppression with corticosteroids, two doses of infliximab, and three doses of vedolizumab. He underwent two FMTs via colonoscopy.
“The first fecal transplant achieved partial response, and the second fecal transplant achieved complete clinical response, and this remission was sustained for a total of 8 months,” Dr. Wang said.
Immune checkpoint inhibitor–related IMC is typically treated with immunosuppressive therapy that is associated with significant morbidity, including a possible adverse impact on the antitumor effects of checkpoint inhibitors, Dr. Wang said.
However, studies have suggested that “the microbiome in healthy people potentially plays a very important and synergistic role for tumor regression in combination with immunotherapy,” and animal models also suggest that patients who develop IMC have differential bacterial signatures in their gut microbiome, she said.
“Based on that preliminary information, we performed fecal transplant as a compassionate treatment for cases refractory to all immunosuppression in June 2017 at M.D. Anderson,” she said.
Stool microbiome analyses showed successful engraftment of donor microbiome in recipient stool samples, and microbiome taxonomy showed increases in specific Escherichia species that “we think potentially play a role in this colitis recovery,” she said.
“Fecal transplant is safe and effective based on our preliminary study,” she said, adding that restoration of a healthy microbiome seems to reverse IMC. “Future large-scale studies are needed to evaluate this finding.”
Dr. Wang reported having no disclosures.
SOURCE: Wang Y et al. SITC 2018, Abstract P194.
REPORTING FROM SITC 2018
Key clinical point: FMT lead to recovery in two patients with refractory IMC.
Major finding: FMT was effective for the treatment of IMC in two patients.
Study details: Two case reports.
Disclosures: Dr. Wang reported having no disclosures.
Source: Wang Y et al. SITC 2018, Abstract P194.
MBDA score predicts, tracks RA patients’ responses to tofacitinib and rituximab
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Baseline PDUS and MBDA scores predicted 12-week CDAI and DAS28 responses to tofacitinib (P less than .01); MBDA scores correlated significantly with EULAR good or moderate response at 6 months (adjusted OR, 0.91).
Study details: An open-label study of 25 patients and a post-hoc analysis of three studies including 57 patients.
Disclosures: The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures. Dr. Hitraya is an employee of Myriad Genetics.
Source: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
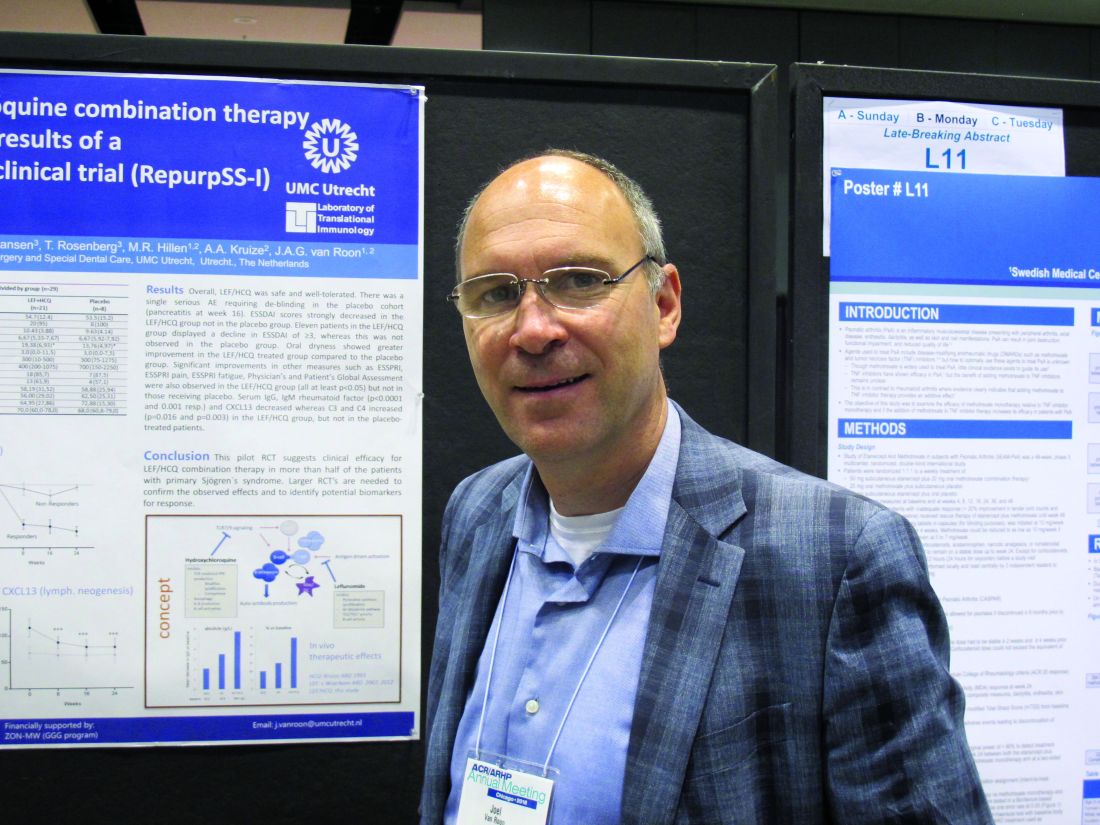
Etanercept bests methotrexate for PsA; combo adds little benefit
CHICAGO – Etanercept monotherapy showed greater efficacy, compared with methotrexate monotherapy for the treatment of psoriatic arthritis, and combining the two agents provided no benefit over etanercept alone for most outcomes in the randomized, controlled, international, phase 3 SEAM-PsA study.
A 20% improvement in American College of Rheumatology criteria at week 24 – the primary endpoint of the study – was significantly greater in 284 patients treated with etanercept monotherapy and in 283 patients treated with combination etanercept and methotrexate than in 284 patients treated with methotrexate monotherapy (60.9% and 65.0% vs. 50.7%, respectively), Philip J. Mease, MD, of the Swedish Medical Center and the University of Washington, Seattle, and his colleagues reported in a late-breaking poster on the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study at the annual meeting of the American College of Rheumatology.
The key secondary endpoint of minimal disease activity response at week 24 also was significantly greater in the etanercept monotherapy and combination groups than in the methotrexate monotherapy group (35.9% and 35.7% vs. 22.9%, respectively), the investigators noted.
Additionally, at week 48, the etanercept monotherapy group and combination group both showed less radiographic progression than did the methotrexate monotherapy arm (mean change in modified total Sharp score from baseline, –0.04 and –0.01 vs. 0.08).
Overall, the etanercept monotherapy group and combination therapy group had similar results, with some differences in skin outcomes. Treatment was well tolerated, and except for more nausea occurring with methotrexate, adverse event rates were similar in the three study arms. No new safety signals were observed.
“The most common serious adverse events were infections and infestations, which occurred in 1.1% of patients in the methotrexate monotherapy arm, 2.8% of patients in the etanercept monotherapy arm, and 2.5% of patients in the combination therapy arm,” they wrote.
Study participants were biologic-naive adults with active PsA and no prior methotrexate treatment for their disease. They had a mean age of 48.4 years, most were white, and median disease duration was 0.6 years.
They were randomized to receive either 50 mg subcutaneous injections of etanercept plus oral placebo weekly, 50 mg subcutaneous etanercept plus 20 mg oral methotrexate weekly, or 20 mg oral methotrexate plus placebo injections weekly; the groups were well balanced with respect to baseline characteristics, the investigators said.
Rescue therapy of etanercept plus methotrexate was given after 24 weeks in patients with less than 20% improvement in tender joint counts and swollen joint counts from baseline.
“Agents used to treat PsA include disease-modifying antirheumatic drugs such as methotrexate and tumor necrosis factor inhibitors, but how to optimally use these agents to treat PsA is unknown,” they wrote, explaining that while methotrexate is widely used in this setting, little clinical evidence exists to guide its use, and that while tumor necrosis factor inhibitors have shown efficacy in PsA, the benefit of adding methotrexate remains unclear.
The current findings, however, demonstrate that adding methotrexate does not appear to increase the efficacy of etanercept monotherapy for most outcomes.
An exception was with combination therapy for some skin-related outcomes, including percent improvement in psoriasis-affected body surface area and percentage of patients with “status clear or almost clear,” they said.
Further, methotrexate monotherapy in this study appeared to have some “meaningful efficacy for both articular and nonarticular PsA symptoms,” the investigators noted.
“These results provide information of practical value for clinical practice when considering treatment option for PsA,” they concluded.
The study was supported by Amgen. Dr. Mease reported receiving research grants, speaker fees, and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, and UCB.
SOURCE: Mease PJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L11.
CHICAGO – Etanercept monotherapy showed greater efficacy, compared with methotrexate monotherapy for the treatment of psoriatic arthritis, and combining the two agents provided no benefit over etanercept alone for most outcomes in the randomized, controlled, international, phase 3 SEAM-PsA study.
A 20% improvement in American College of Rheumatology criteria at week 24 – the primary endpoint of the study – was significantly greater in 284 patients treated with etanercept monotherapy and in 283 patients treated with combination etanercept and methotrexate than in 284 patients treated with methotrexate monotherapy (60.9% and 65.0% vs. 50.7%, respectively), Philip J. Mease, MD, of the Swedish Medical Center and the University of Washington, Seattle, and his colleagues reported in a late-breaking poster on the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study at the annual meeting of the American College of Rheumatology.
The key secondary endpoint of minimal disease activity response at week 24 also was significantly greater in the etanercept monotherapy and combination groups than in the methotrexate monotherapy group (35.9% and 35.7% vs. 22.9%, respectively), the investigators noted.
Additionally, at week 48, the etanercept monotherapy group and combination group both showed less radiographic progression than did the methotrexate monotherapy arm (mean change in modified total Sharp score from baseline, –0.04 and –0.01 vs. 0.08).
Overall, the etanercept monotherapy group and combination therapy group had similar results, with some differences in skin outcomes. Treatment was well tolerated, and except for more nausea occurring with methotrexate, adverse event rates were similar in the three study arms. No new safety signals were observed.
“The most common serious adverse events were infections and infestations, which occurred in 1.1% of patients in the methotrexate monotherapy arm, 2.8% of patients in the etanercept monotherapy arm, and 2.5% of patients in the combination therapy arm,” they wrote.
Study participants were biologic-naive adults with active PsA and no prior methotrexate treatment for their disease. They had a mean age of 48.4 years, most were white, and median disease duration was 0.6 years.
They were randomized to receive either 50 mg subcutaneous injections of etanercept plus oral placebo weekly, 50 mg subcutaneous etanercept plus 20 mg oral methotrexate weekly, or 20 mg oral methotrexate plus placebo injections weekly; the groups were well balanced with respect to baseline characteristics, the investigators said.
Rescue therapy of etanercept plus methotrexate was given after 24 weeks in patients with less than 20% improvement in tender joint counts and swollen joint counts from baseline.
“Agents used to treat PsA include disease-modifying antirheumatic drugs such as methotrexate and tumor necrosis factor inhibitors, but how to optimally use these agents to treat PsA is unknown,” they wrote, explaining that while methotrexate is widely used in this setting, little clinical evidence exists to guide its use, and that while tumor necrosis factor inhibitors have shown efficacy in PsA, the benefit of adding methotrexate remains unclear.
The current findings, however, demonstrate that adding methotrexate does not appear to increase the efficacy of etanercept monotherapy for most outcomes.
An exception was with combination therapy for some skin-related outcomes, including percent improvement in psoriasis-affected body surface area and percentage of patients with “status clear or almost clear,” they said.
Further, methotrexate monotherapy in this study appeared to have some “meaningful efficacy for both articular and nonarticular PsA symptoms,” the investigators noted.
“These results provide information of practical value for clinical practice when considering treatment option for PsA,” they concluded.
The study was supported by Amgen. Dr. Mease reported receiving research grants, speaker fees, and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, and UCB.
SOURCE: Mease PJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L11.
CHICAGO – Etanercept monotherapy showed greater efficacy, compared with methotrexate monotherapy for the treatment of psoriatic arthritis, and combining the two agents provided no benefit over etanercept alone for most outcomes in the randomized, controlled, international, phase 3 SEAM-PsA study.
A 20% improvement in American College of Rheumatology criteria at week 24 – the primary endpoint of the study – was significantly greater in 284 patients treated with etanercept monotherapy and in 283 patients treated with combination etanercept and methotrexate than in 284 patients treated with methotrexate monotherapy (60.9% and 65.0% vs. 50.7%, respectively), Philip J. Mease, MD, of the Swedish Medical Center and the University of Washington, Seattle, and his colleagues reported in a late-breaking poster on the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study at the annual meeting of the American College of Rheumatology.
The key secondary endpoint of minimal disease activity response at week 24 also was significantly greater in the etanercept monotherapy and combination groups than in the methotrexate monotherapy group (35.9% and 35.7% vs. 22.9%, respectively), the investigators noted.
Additionally, at week 48, the etanercept monotherapy group and combination group both showed less radiographic progression than did the methotrexate monotherapy arm (mean change in modified total Sharp score from baseline, –0.04 and –0.01 vs. 0.08).
Overall, the etanercept monotherapy group and combination therapy group had similar results, with some differences in skin outcomes. Treatment was well tolerated, and except for more nausea occurring with methotrexate, adverse event rates were similar in the three study arms. No new safety signals were observed.
“The most common serious adverse events were infections and infestations, which occurred in 1.1% of patients in the methotrexate monotherapy arm, 2.8% of patients in the etanercept monotherapy arm, and 2.5% of patients in the combination therapy arm,” they wrote.
Study participants were biologic-naive adults with active PsA and no prior methotrexate treatment for their disease. They had a mean age of 48.4 years, most were white, and median disease duration was 0.6 years.
They were randomized to receive either 50 mg subcutaneous injections of etanercept plus oral placebo weekly, 50 mg subcutaneous etanercept plus 20 mg oral methotrexate weekly, or 20 mg oral methotrexate plus placebo injections weekly; the groups were well balanced with respect to baseline characteristics, the investigators said.
Rescue therapy of etanercept plus methotrexate was given after 24 weeks in patients with less than 20% improvement in tender joint counts and swollen joint counts from baseline.
“Agents used to treat PsA include disease-modifying antirheumatic drugs such as methotrexate and tumor necrosis factor inhibitors, but how to optimally use these agents to treat PsA is unknown,” they wrote, explaining that while methotrexate is widely used in this setting, little clinical evidence exists to guide its use, and that while tumor necrosis factor inhibitors have shown efficacy in PsA, the benefit of adding methotrexate remains unclear.
The current findings, however, demonstrate that adding methotrexate does not appear to increase the efficacy of etanercept monotherapy for most outcomes.
An exception was with combination therapy for some skin-related outcomes, including percent improvement in psoriasis-affected body surface area and percentage of patients with “status clear or almost clear,” they said.
Further, methotrexate monotherapy in this study appeared to have some “meaningful efficacy for both articular and nonarticular PsA symptoms,” the investigators noted.
“These results provide information of practical value for clinical practice when considering treatment option for PsA,” they concluded.
The study was supported by Amgen. Dr. Mease reported receiving research grants, speaker fees, and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, and UCB.
SOURCE: Mease PJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L11.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Etanercept monotherapy shows greater efficacy versus methotrexate monotherapy for psoriatic arthritis.
Major finding: A total of 60.9% achieved a 20% improvement in American College of Rheumatology criteria with etanercept monotherapy, compared with 65.0% on combination therapy and 50.7% on methotrexate monotherapy.
Study details: A randomized, controlled, phase 3 study of 851 patients.
Disclosures: The study was supported by Amgen. Dr. Mease reported receiving research grants, speaker fees, and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, and UCB.
Source: Mease PJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L11.
Vascular ultrasound reasonable for first-line imaging of large-vessel GCA
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Vascular ultrasound is reasonable for first-line maging of suspected LV-GCA.
Major finding: Vascular ultrasound had 100% specificity and 80% sensitivity.
Study details: A prospective study of 86 patients.
Disclosures: Dr. Nielsen disclosed a relationship with Roche.
Source: Nielsen BD et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
Leflunomide-hydroxychloroquine combo shows promise in primary Sjögren’s pilot study
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: but larger randomized, controlled trials are needed to confirm the observed effects.
Major finding: Combined treatment was associated with a decline in EULAR Sjögren’s syndrome disease activity index score from about 10 at baseline to about 6 at 24 weeks.
Study details: A randomized, placebo-controlled pilot study of 29 patients.
Disclosures: This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
Source: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
Cervical cancer survival higher with open surgery in LACC trial
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.
The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Cervical cancer recurrence and survival rates were worse with minimally invasive vs. open surgery in a prospective study.
Major finding: Disease-free survival at 4.5 years was 86% with minimally invasive vs. 96.5% with open surgery.
Study details: The phase 3 LACC trial of more than 600 women with cervical cancer, and a population based study of nearly 2,500 women with cervical cancer.
Disclosures: Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani is a member of the OB.GYN. News editorial board, but reported having no other relevant disclosures.
Source: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
Collaboration is key to bridging the AYA cancer care divide
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
Endoscopy-related infections found higher than expected, prophylaxis overused
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Infection risk is about 1 per 1,000 with colonoscopy; 3 per 1,000 with upper gastrointestinal endoscopy; and 4 per 1,000 with cystoscopy.
Study details: Endoscopic procedures performed at ASCs in 2014 all-payer claims data from 6 U.S. states.
Disclosures: Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
Source: Ofstead C et al. ICEID 2018 Presentation.