User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
The 2019 novel coronavirus: Case review IDs clinical characteristics
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
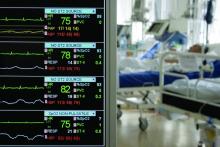
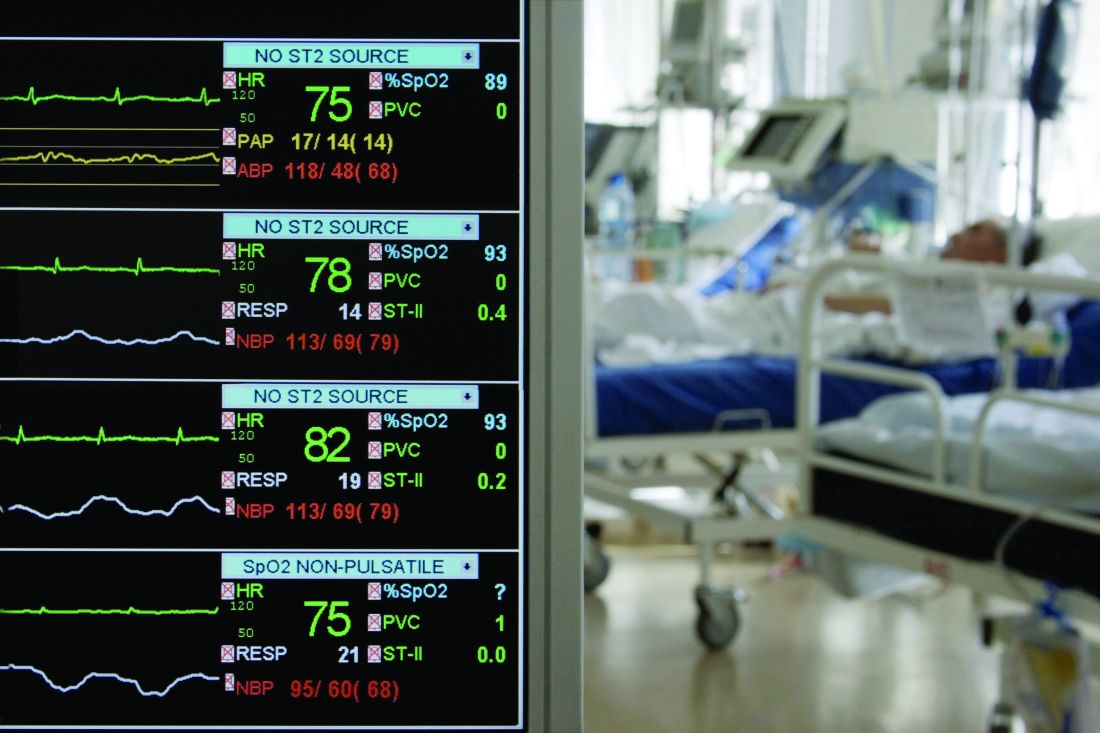
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
FROM THE LANCET
Deferiprone noninferior to deferoxamine for iron overload in SCD, rare anemias
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
REPORTING FROM ASH 2019
Psoriasis: A look back over the past 50 years, and forward to next steps
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
REPORTING FROM ACR 2019
Phase 2 study shows regimen benefit with dasatinib in Ph+ALL therapy
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
REPORTING FROM ASH 2019
Sevuparin failed for acute VOC in sickle cell, but may have preventive potential
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
REPORTING FROM ASH 2019
Liver fibrosis scores predict CV event risk associated with NAFLD
Patients with nonalcoholic fatty liver disease (NAFLD) in a prospective observational study had a twofold increase in cardiovascular events, .
At median follow-up of 41.4 months representing 3,044.4 person-years of observation in the Progression of Liver Damage and Cardiometabolic Disorders in NAFLD (PLINIO) study, 58 cardiovascular events (CVEs) were reported in 898 consecutive outpatients who were screened for liver steatosis by ultrasound. The annual rate of CVEs among 643 patients with NAFLD was 2.1%, compared with 1.0% among 255 patients without NAFLD, according to Francesco Baratta, MD, of Clinica Medica, Sapienza University of Rome, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
The difference did not meet a priori thresholds for statistical significance (P = .066), but became significant after exclusion of new-onset atrial fibrillation events (annual CVE rates of 1.9% vs. 0.7%; P = .034), and on multivariate analysis, age, male sex, and NAFLD were found to be independently associated with CVE occurrence (hazard ratios, 1.07, 3.20, and 2.73, respectively), the investigators found.
In NAFLD patients, a NAFLD fibrosis score (NFS) greater than 0.676 was significantly associated with CVEs after adjustment for comorbidities (HR, 2.29), and a Fibrosis-4 (FIB-4) score greater than 2.67, a history of cardiovascular disease, and metabolic syndrome predicted incident CVEs (HRs, 4.57, 2.95, and 2.30, respectively). The findings were similar after exclusion of new-onset atrial fibrillation from the composite endpoint (HRs, 2.42 and 4.00, respectively).
“Furthermore, when we analyzed only patients without CVEs at baseline, we found a similar association between liver fibrosis and CVEs,” the researchers wrote, noting that the adjusted HRs for NFS were 2.50 and 4.28, respectively.
In addition to liver-related complications, patients with NAFLD are known to have an increased CVE risk, and while liver fibrosis severity is used to determine prognosis, not all patients can undergo a liver biopsy to assess fibrosis. Therefore, there is a need to identify and validate noninvasive markers of liver fibrosis, but few data exist with respect to the predictive role of noninvasive scoring on CVEs, they said.
The findings of this study suggest the use of the NFS and FIB-4 score may reduce the need for liver biopsy by identifying NAFLD patients at higher risk of having advanced liver fibrosis. They further suggest that liver fibrosis development in patients with NAFLD “may be the result of a long-term exposure to cardiometabolic risk factors such as diabetes,” and that “the concomitant presence of multiple cardiometabolic conditions may induce a chronic low-grade proinflammatory and pro-oxidant status leading to liver inflammation (i.e., macrophage activation) and collagen deposition.”
The findings may also have clinical implications: “The association between liver fibrosis and cardiovascular risk supports a potential role for statin treatment in patients with NAFLD,” they explained.
The authors reported having no disclosures.
SOURCE: Baratta F et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.026.
Patients with nonalcoholic fatty liver disease (NAFLD) in a prospective observational study had a twofold increase in cardiovascular events, .
At median follow-up of 41.4 months representing 3,044.4 person-years of observation in the Progression of Liver Damage and Cardiometabolic Disorders in NAFLD (PLINIO) study, 58 cardiovascular events (CVEs) were reported in 898 consecutive outpatients who were screened for liver steatosis by ultrasound. The annual rate of CVEs among 643 patients with NAFLD was 2.1%, compared with 1.0% among 255 patients without NAFLD, according to Francesco Baratta, MD, of Clinica Medica, Sapienza University of Rome, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
The difference did not meet a priori thresholds for statistical significance (P = .066), but became significant after exclusion of new-onset atrial fibrillation events (annual CVE rates of 1.9% vs. 0.7%; P = .034), and on multivariate analysis, age, male sex, and NAFLD were found to be independently associated with CVE occurrence (hazard ratios, 1.07, 3.20, and 2.73, respectively), the investigators found.
In NAFLD patients, a NAFLD fibrosis score (NFS) greater than 0.676 was significantly associated with CVEs after adjustment for comorbidities (HR, 2.29), and a Fibrosis-4 (FIB-4) score greater than 2.67, a history of cardiovascular disease, and metabolic syndrome predicted incident CVEs (HRs, 4.57, 2.95, and 2.30, respectively). The findings were similar after exclusion of new-onset atrial fibrillation from the composite endpoint (HRs, 2.42 and 4.00, respectively).
“Furthermore, when we analyzed only patients without CVEs at baseline, we found a similar association between liver fibrosis and CVEs,” the researchers wrote, noting that the adjusted HRs for NFS were 2.50 and 4.28, respectively.
In addition to liver-related complications, patients with NAFLD are known to have an increased CVE risk, and while liver fibrosis severity is used to determine prognosis, not all patients can undergo a liver biopsy to assess fibrosis. Therefore, there is a need to identify and validate noninvasive markers of liver fibrosis, but few data exist with respect to the predictive role of noninvasive scoring on CVEs, they said.
The findings of this study suggest the use of the NFS and FIB-4 score may reduce the need for liver biopsy by identifying NAFLD patients at higher risk of having advanced liver fibrosis. They further suggest that liver fibrosis development in patients with NAFLD “may be the result of a long-term exposure to cardiometabolic risk factors such as diabetes,” and that “the concomitant presence of multiple cardiometabolic conditions may induce a chronic low-grade proinflammatory and pro-oxidant status leading to liver inflammation (i.e., macrophage activation) and collagen deposition.”
The findings may also have clinical implications: “The association between liver fibrosis and cardiovascular risk supports a potential role for statin treatment in patients with NAFLD,” they explained.
The authors reported having no disclosures.
SOURCE: Baratta F et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.026.
Patients with nonalcoholic fatty liver disease (NAFLD) in a prospective observational study had a twofold increase in cardiovascular events, .
At median follow-up of 41.4 months representing 3,044.4 person-years of observation in the Progression of Liver Damage and Cardiometabolic Disorders in NAFLD (PLINIO) study, 58 cardiovascular events (CVEs) were reported in 898 consecutive outpatients who were screened for liver steatosis by ultrasound. The annual rate of CVEs among 643 patients with NAFLD was 2.1%, compared with 1.0% among 255 patients without NAFLD, according to Francesco Baratta, MD, of Clinica Medica, Sapienza University of Rome, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
The difference did not meet a priori thresholds for statistical significance (P = .066), but became significant after exclusion of new-onset atrial fibrillation events (annual CVE rates of 1.9% vs. 0.7%; P = .034), and on multivariate analysis, age, male sex, and NAFLD were found to be independently associated with CVE occurrence (hazard ratios, 1.07, 3.20, and 2.73, respectively), the investigators found.
In NAFLD patients, a NAFLD fibrosis score (NFS) greater than 0.676 was significantly associated with CVEs after adjustment for comorbidities (HR, 2.29), and a Fibrosis-4 (FIB-4) score greater than 2.67, a history of cardiovascular disease, and metabolic syndrome predicted incident CVEs (HRs, 4.57, 2.95, and 2.30, respectively). The findings were similar after exclusion of new-onset atrial fibrillation from the composite endpoint (HRs, 2.42 and 4.00, respectively).
“Furthermore, when we analyzed only patients without CVEs at baseline, we found a similar association between liver fibrosis and CVEs,” the researchers wrote, noting that the adjusted HRs for NFS were 2.50 and 4.28, respectively.
In addition to liver-related complications, patients with NAFLD are known to have an increased CVE risk, and while liver fibrosis severity is used to determine prognosis, not all patients can undergo a liver biopsy to assess fibrosis. Therefore, there is a need to identify and validate noninvasive markers of liver fibrosis, but few data exist with respect to the predictive role of noninvasive scoring on CVEs, they said.
The findings of this study suggest the use of the NFS and FIB-4 score may reduce the need for liver biopsy by identifying NAFLD patients at higher risk of having advanced liver fibrosis. They further suggest that liver fibrosis development in patients with NAFLD “may be the result of a long-term exposure to cardiometabolic risk factors such as diabetes,” and that “the concomitant presence of multiple cardiometabolic conditions may induce a chronic low-grade proinflammatory and pro-oxidant status leading to liver inflammation (i.e., macrophage activation) and collagen deposition.”
The findings may also have clinical implications: “The association between liver fibrosis and cardiovascular risk supports a potential role for statin treatment in patients with NAFLD,” they explained.
The authors reported having no disclosures.
SOURCE: Baratta F et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.026.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
UKALL14: Rituximab improves EFS in B-ALL, but four doses not enough
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
REPORTING FROM ASH 2019
APPRENTICE registry: Wide variation exists in acute pancreatitis treatment, outcomes
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Share AGA GI Patient Center education to help your patients understand acute versus chronic pancreatitis, testing, treatment, and potential complications at https://www.gastro.org/
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Share AGA GI Patient Center education to help your patients understand acute versus chronic pancreatitis, testing, treatment, and potential complications at https://www.gastro.org/
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Share AGA GI Patient Center education to help your patients understand acute versus chronic pancreatitis, testing, treatment, and potential complications at https://www.gastro.org/
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
APPRENTICE registry: Wide variation exists in acute pancreatitis treatment, outcomes
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY