User login
Does primary nocturnal enuresis affect childrens’ self-esteem?
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Evidence-based answers from the Family Physicians Inquiries Network
Is red-yeast rice a safe and effective alternative to statins?
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does any antidepressant besides bupropion help smokers quit?
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
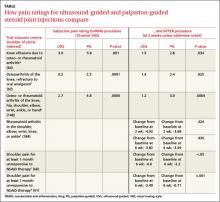
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
EVIDENCE-BASED ANSWER:
Yes, nortriptyline approximately doubles smoking cessation rates, an effect comparable to bupropion. Adding nortriptyline to nicotine replacement therapy (NRT) doesn’t improve rates further (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram), venlafaxine, monoamine oxidase inhibitors (MAOIs; moclobemide, selegiline), doxepin, and St. John’s wort don’t improve smoking cessation rates (SOR: A, systematic reviews and RCTs).
EVIDENCE SUMMARY
Bupropion is the only Food and Drug Administration (FDA)-approved antidepressant recommended as a first-line pharmacologic agent to assist with smoking cessation, based in part on a meta-analysis of 44 placebo-controlled RCTs (13,728 patients), which found that bupropion had a relative risk (RR) of 1.62 for smoking cessation compared with placebo (95% confidence interval [CI], 1.49-1.76). Bupropion produced quit rates that were approximately double those of placebo rates (18% [range 4%-43%] for bupropion vs 9% [range 0%-18%] for placebo).1
Nortriptyline is also effective, other antidepressants not so much
A Cochrane systematic review of 10 antidepressants used for smoking cessation included 64 placebo-controlled trials, measuring at least 6-month abstinence rates as primary outcomes, and monitoring biochemical markers (such as breath carbon monoxide and urinary cotinine) to verify abstinence. Some trials included participants with previous depressive episodes, but most didn’t enroll patients with active major depression.1 The TABLE1 gives an overview of the studies and outcomes.

Nortriptyline, which was evaluated in 6 trials, was the only antidepressant besides bupropion that was superior to placebo.1 Two of the nortriptyline trials included participants with active depression and the other trials had participants with a history of depression. One trial found no difference in quit rates for patients taking nortriptyline with or without a history of major depression, although the subgroups were small. Two trials measured quit rates for 12 months whereas the other 4 trials used 6-month quit rates.
Four additional RCTs with 1644 patients that combined nortriptyline with NRT found no improvement in quit rates compared with NRT alone (RR=1.21; 95% CI, 0.94-1.55).1 Three RCTs with 417 patients compared bupropion with nortriptyline and found no difference (RR=1.3; 95% CI, 0.93-1.8).1
SSRIs. None of the 4 SSRIs investigated in the trials (fluoxetine, paroxetine, sertraline, citalopram) improved smoking cessation rates more than placebo.1 The 5 RCTs that studied the drugs followed participants for as long as a year. None of the participants were depressed at the time of the studies, although some had a history of depression.
The sertraline RCT used individual counseling sessions in conjunction with either sertraline or placebo. All participants had a history of major depression.
The paroxetine trial used NRT in all patients randomized to either paroxetine or placebo.
Venlafaxine. The serotonin-norepinephrine reuptake inhibitor venlafaxine didn’t improve smoking cessation rates over 12 months.1
MAOIs. Neither of the 2 MAOIs increased smoking cessation rates.1 The moclobemide RCT followed participants for 12 months; the 5 selegiline RCTs followed participants for as long as 6 months.
Other antidepressants. An RCT with 19 participants found that doxepin didn’t improve smoking cessation at 2 months.1 One RCT and one open, randomized trial of St. John’s wort found no benefit for smoking cessation.1,2
RECOMMENDATIONS
The United States Public Health Service (USPHS) and the University of Michigan Health System (UMHS) guidelines recommend the following FDA-approved pharmacotherapies as first-line agents for smoking cessation: sustained-release bupropion, NRT (gum, inhaler, lozenge, nasal spray, or patch), and varenicline.3,4 They say that clonidine and nortriptyline are also effective but recommend them as second-line agents because these drugs lack FDA approval for this purpose.
The USPHS also recommends combinations of NRT and bupropion for long-term use. Because of additional cost and limited benefit, UMHS recommends reserving NRT-bupropion combination therapy for highly addicted tobacco users who have several failed quit attempts.
The United States Preventive Services Task Force guideline emphasizes counseling and interventions to prevent tobacco use; it doesn’t provide recommendations for pharmacotherapy.5 It does cite the same agents recommended by USPHS and UMHS as effective.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
1. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
2. Sood A, Ebbert JO, Prasad K, et al. A randomized clinical trial of St. John’s wort for smoking cessation. J Altern Complement Med. 2010;16:761-767.
3. Agency for Healthcare Research and Quality. Treating tobacco use and dependence: 2008 update. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 9, 2014.
4. University of Michigan Health System. Tobacco treatment. University of Michigan Health System Web site. Available at: http://www.med.umich.edu/1info/fhp/practiceguides/smoking/smoking.pdf. Accessed October 9, 2014.
5. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551-555.
Evidence-based answers from the Family Physicians Inquiries Network
Do oral contraceptives put women with a family history of breast cancer at increased risk?
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
No. Modern combined oral contraceptive pills (OCPs) don’t increase breast cancer risk in women with a family history (strength of recommendation [SOR]: B, systematic review of cohort, case-control studies). However, older, higher-dose OCPs (in use before 1975) did increase breast cancer risk in these women (SOR: C, case-control study).
Similarly, modern OCPs don’t raise breast cancer risk in women with BRCA1/2 mutations, although higher-dose, pre-1975 OCPs did (SOR: B and C, a meta-analysis of cohort and case-control studies).
EVIDENCE SUMMARY
A systematic review of the effect of combined OCPs on women with a family history of breast cancer found no additional increase in risk.1 Investigators identified 3 retrospective cohort studies (N=66,500, with 8500 cases) and 7 case-control studies (total 10,500 cases) from the past 40 years, most including women from the United States and Canada, but one including women from 5 continents.
In most trials, women of reproductive age using combined OCPs had 1 or more first-degree female relatives with breast cancer, although a few trials also included second-degree relatives. Women ranged in age from 20 to 79 years at diagnosis, and most trials controlled for age, parity, menstrual and menopausal history, duration of OCP exposure, and age at first use. Follow-up intervals for the retrospective cohort studies ranged from 5 to 16 years. Investigators were unable to combine results because of heterogenous populations.
Three of the cohort studies found no significant difference in breast cancer risk between OCP users and nonusers, regardless of age or duration of use. One cohort study found an increased risk in women taking older, higher-dose OCPs from before 1975 (relative risk [RR]=3.3; 95% confidence interval [CI], 1.5-7.2). All of the case-control studies found no significant difference in breast cancer risk for any age of starting, duration of OCP use, or degree of relative with breast cancer.
A meta-analysis of 54 case-control studies (6757 cases), comprising approximately 90% of the epidemiologic information on this topic, also found no significant difference in breast cancer risk related to OCP use among women with one or more first-degree relatives with breast cancer.2 Investigators found that neither recent OCP use (<10 years, RR=0.77; 95% CI, 0.54-1.11) nor past OCP use (>10 years, RR=1.01; 95% CI, 0.80-1.28) affected risk of developing breast cancer.
Three additional case-control studies involving women with a family history of breast cancer also found no significant association for breast cancer incidence among OCP users compared with nonusers.3-5
Modern combined OCPs don’t raise risk in women with BRCA1/2 mutations
A meta-analysis of 5 studies (one retrospective cohort, 4 case-control, with a total of 2855 breast cancer cases and 2944 controls) evaluated whether combined OCPs increased the risk of breast cancer in women, all of whom were carrying BRCA1/2 mutations.6
Using modern combined OCPs didn’t raise the risk of breast cancer in BRCA1/2 carriers overall (RR=1.13; 95% CI, 0.88-1.45) or separately in BRCA1 carriers (5 studies, RR=1.09; 95% CI, 0.77-1.54) or BRCA2 carriers (3 studies, RR=1.15; 95% CI, 0.88-1.45).
However, pre-1975 (higher dose) combined OCPs produced significantly increased risk (RR=1.47; 95% CI, 1.06-2.04). Similarly, women who had used combined OCPs >10 years before the study (older women, likely to have been using pre-1975 OCPs) also had significantly increased risk (RR=1.46; 95% CI, 1.07-2.07).
A bit of good news: Combined OCPs reduce ovarian cancer risk
The analysis also determined that combined OCPs significantly reduced the risk of ovarian cancer in women carrying BRCA1/2 mutations (RR=0.50; 95% CI, 0.33-0.75), with an additional linear decrease in risk for each 10 years of OCP use (RR=0.64; 95% CI, 0.53-0.78).
RECOMMENDATIONS
The World Health Organization guidelines outlining criteria for contraceptive use state that OCPs don’t alter the risk of breast cancer among women with either a family history of breast cancer or breast cancer susceptibility genes.7
The American College of Obstetricians and Gynecologists (ACOG) says that a positive family history of breast cancer shouldn’t be regarded as a contraindication to OCP use.8 ACOG also says that women with the BRCA1 mutation have an increased risk of breast cancer if they used OCPs for longer than 5 years before age 30, but this risk may be more than balanced by the benefit of a greatly reduced risk of ovarian cancer.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
1. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
2. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative re-analysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
3. Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312-2320.
4. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Human Reprod Update. 2010;16: 631-650.
5. Long-term oral contraceptive use and the risk of breast cancer. The Centers for Disease Control Cancer and Steroid Hormone Study. JAMA. 1983;249:1591-1595.
6. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
7. World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed September 24, 2013.
8. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
Evidence-based answers from the Family Physicians Inquiries Network
Are topical nitrates safe and effective for upper extremity tendinopathies?
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
Topical nitrates provide short-term relief with some side effects, especially headache. Topical nitroglycerin (NTG) patches improve subjective pain scores by about 30% and range of motion over 3 days in patients with acute shoulder tendinopathy (strength of recommendation [SOR]: C, small randomized controlled trial [RCT] with no methodologic flaws).
NTG patches, when combined with tendon rehabilitation, improve subjective pain ratings by about 30% and shoulder strength by about 10% in patients with chronic shoulder tendinopathy over 3 to 6 months, but not in the long term (SOR: C, RCTs with methodologic flaws). They improve pain and strength 15% to 50% for chronic extensor tendinosis of the elbow over a 6-month period (SOR: C, small RCT with methodologic flaws).
NTG patches used without tendon rehabilitation don’t improve pain or strength in chronic lateral epicondylitis over 8 weeks (SOR: C, RCT).
Topical NTG patches commonly produce headaches and rashes (SOR: B, multiple RCTs).
EVIDENCE SUMMARY
A small RCT found that NTG therapy improved short-term pain and joint mobility in patients with acute supraspinatus tendinitis.1 Investigators randomized 10 men and 10 women with acute shoulder tendonitis (fewer than 7 days’ duration) to use either 5-mg NTG patches or placebo patches daily for 3 days. Patients rated pain on a 10-point scale, and investigators measured joint mobility on a 4-point scale.
After 48 hours of treatment, NTG patches significantly reduced pain ratings from baseline (from 7 to 2 points; P<.001), whereas placebo didn’t (6 vs 6 points; P not significant). NTG patches also improved joint mobility from baseline (from 2 points “moderately restricted” to .1 points “not restricted”; P<.001), but placebo didn’t (1.2 points “mildly restricted” vs 1.2 points; P not significant). The placebo group had less pain and joint restriction than the NTG group at the start of the study. Two patients reported headache 24 hours after starting treatment.
NTG plus rehabilitation improves chronic shoulder pain, range of motion
A double-blind RCT evaluating NTG patches for 53 patients (57 shoulders) with chronic supraspinatus tendinopathy (shoulder pain lasting longer than 3 months) found that they improved pain, strength, and range of motion at 3 to 6 months.2 Investigators randomized patients to receive one-quarter of a 5-mg 24-hour NTG patch or placebo patch daily and enrolled all patients in a rehabilitation program. They assessed subjective pain (at night and with activity), strength, and external rotation at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved nighttime pain about 30% (at 12 and 24 weeks), pain with activity about 60% (at 24 weeks), strength about 10% (at 12 and 24 weeks), and range of motion about 20% (at 24 weeks; P<.05 for all comparisons). The placebo group initially had more pain, less strength, and less mobility than the NTG group. Investigators reported no adverse effects.
NTG and rehab improve elbow pain, but with side effects
Another RCT comparing topical NTG patches in patients with chronic extensor tendinosis of the elbow found that they improved most parameters.3 Investigators randomized 86 patients with elbow tendonitis (longer than 3 months) to NTG patches (one-quarter of a 5-mg 24-hour patch) or placebo patches and enrolled all patients in a tendon rehabilitation program. They assessed subjective pain, extensor tendon tenderness, and muscle strength at baseline and at 2, 6, 12, and 24 weeks.
NTG patches improved subjective pain, tendon tenderness, and strength significantly more than placebo at all follow-up points, by 15% to 50% (P<.05 for all comparisons). The study was flawed because the control group started with more pain, tenderness, and weakness than the NTG group. Five patients discontinued NTG because of adverse effects (headache, dermatitis, and facial flushing).
A follow-up study done 5 years after discontinuation of therapy found equal outcomes with NTG and placebo.4 Investigators evaluated, by phone or in person, 58 of the 86 patients in the original study. NTG and placebo therapy produced equivalent reductions in subjective 0 to 4 elbow pain scores over baseline (average pain 2.5 initially, 1.5 at 12 weeks, and 1.0 at 5 years; P<.01 for all comparisons with baseline, no significant difference between nitrates and placebo).
NTG without rehab works no better than placebo
Another RCT that evaluated 3 different doses of NTG patches for 8 weeks in 154 patients with chronic lateral epicondylosis found NTG treatment was no better than placebo for pain or strength.5 Investigators randomized patients with more than 3 months of symptoms to 3 NTG patch doses (.72 mg/24 h, 1.44 mg/ 24 h, or 3.6 mg/24 h) compared with placebo and evaluated subjective pain (at rest, with activity, and at night), grip strength, and force, at baseline and 8 weeks.
The study lacked a formal wrist strengthening rehabilitation program. Patients in the placebo group had lower baseline pain scores than the NTG groups. Seven patients dropped out of the study because of headaches.
RECOMMENDATIONS
We found no authoritative recommendations regarding the use of topical nitrates for upper extremity tendinopathies.
An online reference text doesn’t make a recommendation, but references the studies described previously.6 The authors state that headache is the most common adverse effect of topical nitrates, but it becomes less severe over the course of treatment. They recommend caution in patients with hypotension, pregnancy, or migraines, and those who take diuretics. The authors also note that nitrates are relatively contraindicated in patients with ischemic heart disease, anemia, phosphodiesterase inhibitor therapy (such as sildenafil), angle-closure glaucoma, and allergy to nitrates.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
1. Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996;66:63-67.
2. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
3. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920.
4. McCallum SD, Paoloni JA, Murrell GA, et al. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
5. Paolini JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med. 2009;43:299-302.
6. Simons SM, Kruse D. Rotator cuff tendinopathy. UpToDate Web site. Available at: www.uptodate.com/contents/rotator-cuff-tendinopathy. Accessed February 19, 2014.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best imaging method for patients with a presumed acute stroke?
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
It depends on whether the stroke is schemic or hemorrhagic. For early detection of ischemic stroke, magnetic resonance imaging (MRI) using diffusion-weighted imaging (DWI) is highly sensitive and specific, whereas computed tomography (CT) is less sensitive but about as specific (strength of recommendation [SOR]: B, a meta-analysis of lower quality RCTs). MRI using DWI and CT are probably comparable for detecting acute hemorrhagic stroke (SOR: B, a cohort study).
When thrombolysis is being considered and hemorrhage must be ruled out rapidly, either test is acceptable if it can be performed and interpreted within 45 minutes of patient arrival, although MRI typically costs about twice as much as CT (SOR: C, expert opinion).
EVIDENCE SUMMARY
Clinical A Cochrane review identified 7 studies that compared MRI with CT for detecting ischemic stroke in a total of 226 patients, average age 65 years, with stroke-like symptoms.1 Investigators performed imaging within 12 hours of symptom onset in all patients, including those whose final diagnosis was transient ischemic attack (TIA). They identified 161 patients with ischemic stroke based on a combination of imaging and clinical examination. MRI with DWI was more sensitive than CT (0.99; 95% confidence interval [CI], 0.23-1.00 vs 0.39; 95% CI, 0.16-0.69); both techniques had comparable specificity (0.92; 95% CI, 0.83-0.97 and 1.00; 95% CI, 0.94-1.00, respectively).
Many issues could have affected the ischemic stroke analysis: All studies included some retrospective data collection; in all but one study, the MRI was performed a mean of one hour after the CT; and in 4 studies, the physicians reading the scans weren’t blinded to the clinical outcome. The Cochrane authors also found evidence of “prescreening” that appeared to select for patients with middle-cerebral artery infarcts. They concluded that the reliability and generalizability of the results “were questionable.”
MRI and CT have similar sensitivity and specificity for hemorrhagic stroke
A prospective cohort study of 27 patients (mean age 76 years) who had an acute hemorrhagic stroke that was imaged using both MRI with DWI and CT within 3 hours of symptom onset found that both imaging studies had comparable sensitivity (0.81; 95% CI, 0.61-0.93 vs 0.89; 95% CI, 0.70-0.97, respectively) and specificity (1.0; 95% CI, 0.98-1.0 for both).2
A retrospective case-control study evaluated the ability of DWI to detect hemorrhagic stroke in 86 patients who presented with symptoms consistent with acute stroke.3 Investigators compared the sensitivity and specificity of DWI against the pooled results of 5 different MRI sequences. Both case and control imaging was performed within 6 hours of symptom onset. Half of the patients in the study had hemorrhagic strokes (43); the rest had ischemic strokes (41) or a TIA and postictal deficit (2). The sensitivity and specificity of DWI for hemorrhagic stroke were both 1.0. However, there was no independent reference standard.
MRI costs more than CT
Although costs vary widely, one textbook put the national average charge for a head CT at about $1000.4 MRI neuroimaging charges ranged from $1000 to $4700, with an average of about $2300. Medicare reimbursements were significantly less, although the cost of MRIs was still about double that of CTs.
Recommendations
The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology says that DWI is more useful than noncontrast CT for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.5 The subcommittee made no recommendation for imaging hemorrhagic stroke.
American Heart Association and American Stroke Association guidelines for early management of adults with ischemic stroke recommend neuroimaging with either DWI or CT within 45 minutes of arrival in candidates for tissue plasminogen activator.6 They also recommend neuroimaging with either CT or MRI to distinguish ischemic from hemorrhagic stroke.7 The guidelines state that other imaging methods (including CT angiography, contrast-enhanced MRI, and magnetic resonance angiography) “may be considered” to evaluate for clinically suspected underlying structural lesions, including vascular malformations and tumors.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
1. Brazeli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;(4):CD007424.
2. Chelela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
3. Oppenheim C, Touzé E, Hernalsteen D, et al. Comparison of five MR sequences for the detection of acute intracranial hemorrhage. Cerebrovasc Dis. 2005;20:388-394.
4. Broder J, Preston R. Imaging the head and brain. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, Pa: Elsevier/Saunders; 2011:26-27.
5. Shellinger PD, Bryan RN, Caplan LR, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177-185.
6. Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
7. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
Evidence-based answers from the Family Physicians Inquiries Network
Does ultrasound guidance improve outcomes for steroid joint injections?
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
A Patients yes, at least in the short term. Ultrasound-guided (USG) injections of triamcinolone into the shoulder improve function more than palpation-guided (PG) steroid injections over 6 weeks (strength of recommendation [SOR]: B, 2 small randomized, controlled trials [RCTs]).
USG steroid injections are also less painful than PG injections (SOR: A, multiple RCTs). They reduce pain more than PG injections in arthritic joints (shoulder, elbow, wrist, hand, hip, knee, or ankle) over 2 weeks (SOR: B, lower quality RCTs with some inconsistent results) but possibly not at 6 weeks (SOR: B, multiple RCTs with conflicting results).
EVIDENCE SUMMARY
A prospective RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with shoulder pain unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs).1 Investigators randomized 60 patients (mean age 52.5 years) to either USG or PG injections of triamcinolone 40 mg given by a rheumatologist. They used a 10-point visual analog scale (VAS) to assess pain and evaluated joint function at 6 weeks using a validated 100-point scale for shoulder function,2 with high scores indicating better function.
The USG group showed greater improvement from baseline in pain (TABLE)1,3-7 and function scores than the PG group (32 vs 12 points; P<.05).1 Investigators didn’t control for a possible placebo effect from ultrasound in this trial (or any trial described here). Another RCT found that USG steroid joint injections improved shoulder function more than PG injections in patients with rheumatoid arthritis and at least one month of shoulder pain unresponsive to NSAIDs.3 Investigators randomized 41 rheumatology clinic patients (mean age 52.4 years) to USG or PG injections of 20 mg triamcinolone.
They assessed function at 6 weeks with a validated 70-point shoulder function assessment tool designed for patients with rheumatoid arthritis,8 which evaluates pain with motion, range of motion, and activities of daily living (higher scores indicate better shoulder function), and used a 100-point VAS to assess pain.3 Function scores showed greater improvement from baseline in the USG group than the PG group (15 vs 6 points; P=.012), as did pain scores (TABLE).
Ultrasound injections hurt less than palpation-guided injections
Three RCTs, all using triamcinolone, found that USG joint injections were less painful than PG joint injections (TABLE).4-6 Three of 4 studies found that USG injections also were associated with lower pain scores 2 weeks after injection, as measured with a standardized VAS.1,3-7 A common weakness of the 3 studies demonstrating a difference at 2 weeks was that they compared end scores rather than the magnitude of change from baseline between groups.
Two of 3 RCTs found that USG injections produced a greater reduction in the VAS pain score at 6 weeks, although the negative study was larger than the other 2 combined—184 patients, compared with a total of 285 patients for all 3 studies.1,3,7
Recommendations
The American College of Radiology’s practice guidelines for musculoskeletal ultrasound examination recommend using ultrasound to guide interventional procedures.9 However, no consensus statements comment on the use of ultrasound as opposed to palpation for guiding steroid joint injections.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
1. Ucuncu F, Capkin E, Karkucak M, et al. A comparison of the effectiveness of landmark-guided injections and ultrasonography-guided injections for shoulder pain. Clin J Pain. 2009;25:786-789.
2. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
3. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308-314.
4. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41:66-72.
5. Sibbitt W Jr, Band PA, Kettwich LG, et al. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409-415.
6. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
7. Cunnington J, Marshall N, Hide G, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joints of patients with inflammatory arthritis. Arthritis Rheum. 2010;62;1862-1869.
8. van Den Ende CH, Rozing PM, Dijkmans BA, et al. Assessment of shoulder function in rheumatoid arthritis. J Rheumatol. 1996;23:2043-2048.
9. ACR-AIUM-SPR-SRU practice guideline for the performance of the musculoskeletal ultrasound examination. Updated 2012. American College of Radiology; 2007, updated 2012. Available at: http://amclc.acr.org/LinkClick.aspx?fileticket=z6ih9CEE6_w%3D&tabid=61. Accessed August 3, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
How best to treat agitation in patients with irreversible dementia?
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
Atypical antipsychotics modestly reduce agitation compared with placebo but have significant adverse effects (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Haloperidol doesn’t reduce symptoms and has serious adverse effects (SOR: A, systematic reviews of RCTs).
Selective serotonin reuptake inhibitors (SSRIs) and melatonin—although well tolerated—don’t reduce agitation (SOR: B, extrapolated data from systematic reviews of RCTs).
Evidence summary
A meta-analysis by the Agency for Healthcare Research and Quality of 37 RCTs examined off-label use of atypical antipsychotics in a total of 5364 patients.1 Pooled results from 17 RCTs showed a statistically significant but clinically modest difference between atypical antipsychotics and placebo for agitation; the standard mean difference was 0.22 (95% confidence interval [CI], 0.09-0.35). Investigators found statistically significant but small effect sizes for aripiprazole, olanzapine, and risperidone.
Atypical antipsychotics are associated with serious adverse cerebrovascular events and extrapyramidal symptoms. A meta-analysis of 17 RCTs (N= 5106) demonstrated that patients who received antipsychotics had higher mortality than patients who received placebo (3.5% vs 2.3%).2
Haloperidol has significant adverse effects without significant results
A systematic review of 5 RCTs compared haloperidol with placebo over 3 to 16 weeks in 856 patients ages 72 to 81 years with dementia and agitation.3 When investigators pooled results from 3 RCTs (N=690) using an intention-to-treat analysis and 3 assessment tools, they found that haloperidol produced a statistically significant, but not clinically meaningful, standard mean difference in aggression.
Adverse effects included extrapyramidal symptoms (odds ratio [OR]=2.34; 95% CI, 1.25-4.38; number needed to harm [NNH]=6), somnolence (OR=4.20; 95% CI, 1.78-9.91; NNH=8), and fatigue (OR=5.39; 95% CI, 2.04-14.22; NNH=3). Most studies were underpowered, didn’t document randomization, and had dropout rates as high as 20%.
Antidepressants have no effect
A systematic review of 9 RCTs involving 692 patients with dementia compared antidepressants with placebo, other antidepressants, and antipsychotics using various neuropsychiatric symptom scales.4 Investigators performed meta-analyses for numerous outcomes but found none of clinical or statistical significance.
Pooled analysis of 2 RCTs that examined a total of 250 outpatients with Alzheimer’s disease found that sertraline and fluoxetine produced a statistically, but not clinically, significant difference in the Cohen Mansfield Agitation Inventory total score. One RCT (N=52) demonstrated that citalopram improved the Neurobehavioral Rating Scale total score after adjusting for baseline severity.
Investigators found no difference in withdrawal rates between SSRIs and placebo (relative risk=1.07; 95% CI, 0.55-2.11). All studies had multiple methodological limitations.
Melatonin has no adverse effects, but no benefit either
A systematic review that included 2 RCTs compared melatonin with placebo for agitation in 121 patients ages 77 to 79 years with dementia.5 Investigators prescribed melatonin for periods of 4 to 7 weeks and found reductions in agitation that were statistically significant, but not clinically meaningful. They reported no adverse events. The studies had a low risk of bias.
Recommendations
The American Psychiatric Association (APA) advocates evaluating and treating secondary causes of agitation and using environmental and behavioral measures to reduce agitation.6 The APA advocates using the lowest effective dosages of antipsychotics after considering adverse effect profiles and the risks of not treating.
The APA recommends benzodiazepines to treat prominent anxiety or infrequent agitation, preferably lorazepam and oxazepam rather than diazepam or clonazepam and suggests trazodone or SSRIs as alternative therapy for agitation in patients without psychosis or those who are intolerant to antipsychotics.6
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review Number 43. Executive Summary. Rockville, Md: Agency for Healthcare Research and Quality; 2011.
2. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo controlled trials. JAMA. 2005;295:1934-1943.
3. Lonergan E, Luxenberg J, Colford JM, et al. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
4. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
5. Jansen SL, Forbes D, Duncan V, et al. Melatonin for the treatment of dementia. Cochrane Database Syst Rev. 2006;(1):CD003802.
6. American Psychiatric Association Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. Available at: http://psychiatryonline.org/
pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed April 3, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
Do any topical agents help prevent or reduce stretch marks?
NO TOPICAL AGENT has been proven to prevent or reduce stretch marks. Randomized controlled trials (RCTs) show that cocoa butter doesn’t prevent stretch marks (strength of recommendation [SOR]: A, 2 RCTs); neither does olive oil (SOR: B, 1 small RCT).
A cream containing Centella asiatica extract, vitamin E, and collagen hydrolysates doesn’t prevent new stretch marks but might avoid additional stretch marks in women who had already developed them during puberty. Massage with vitamin E ointment alone may reduce the number of stretch marks (SOR: C, 2 small RCTs with methodologic flaws).
Evidence summary
Two double-blind RCTs that compared cocoa butter with placebo to prevent stretch marks in pregnant women found no difference. In the first, investigators enrolled 300 Afro-Caribbean women at 12 to 15 weeks’ gestation. Women used either 25% cocoa butter cream or a placebo cream comprised of emollients and vitamin E daily. Investigators monitored compliance and assessed stretch marks using a validated scale at 26 weeks, 36 weeks, and after delivery. Cocoa butter cream didn’t reduce stretch marks (44% vs 55% for placebo; P=.09). Three women (1 using cocoa butter, 2 using placebo) discontinued the cream because of mild self-limiting reactions.1
In the second RCT, investigators randomized 210 nulliparous women (mainly with intermediate skin color) to use cocoa butter lotion with vitamin E or placebo. Women applied the lotion daily to their abdomen, breasts, and thighs, starting at 12 to 18 weeks’ gestation. Investigators assessed the severity of stretch marks either at delivery or postpartum using a validated scale. Cocoa butter lotion didn’t prevent stretch marks (45.1% vs 48.8% for placebo; P=.730).2
Save the olive oil for cooking
A nonblinded RCT that compared twice-daily olive oil massage with no treatment in 70 nulliparous women beginning at 18 to 20 weeks’ gestation found no reduction in stretch marks (45.7% vs 62.9% without olive oil massage; P=.115). The investigators didn’t report whether they performed a sample-size analysis to determine if the study was adequately powered to demonstrate no difference.3
Mixed, but mostly negative, results for multi-ingredient cream
A double-blind RCT found that Trofolastin cream containing Centella asiatica (also known as Gotu kola, a member of the parsley family), vitamin E, and collagen hydrolysates didn’t prevent pregnancy-related stretch marks among 80 women who applied the treatment beginning at 12 weeks’ gestation.
When investigators evaluated a subgroup of 18 women who had already developed stretch marks during puberty, they found that fewer of the women acquired additional stretch marks during pregnancy (11% vs 100% with placebo; P=.0001). The investigators didn’t calculate whether the sample was large enough to prove a significant difference.4
Fewer stretch marks with vitamin E in small flawed study
An older systematic review (1996) included a prospective RCT that randomized 50 women at 20 weeks’ gestation to massage their abdomen, thighs, and breasts with vitamin E ointment or perform no massage. The trial found fewer stretch marks with vitamin E ointment massage (odds ratio=0.26; 95% confidence interval, 0.08-0.84). The authors of the review described this RCT as poorly randomized and without blinding; investigators didn’t report whether the sample size was adequate to demonstrate a significant effect.5
Recommendations
The American Congress of Obstetricians and Gynecologists Web site states that although many creams, lotions, and oils on the market claim to prevent stretch marks, no proof exists that these treatments work. Using a heavy moisturizer may help keep skin soft, but it won’t help get rid of stretch marks.6
The American Academy of Dermatology Web site also says that a moisturizer can improve the appearance of stretch marks and reduce itchiness; sunless tanning products can hide the marks.7
1. Buchanan K, Fletcher HM, Reid M. Prevention of striae gravidarum with cocoa butter cream. Int J Gynaecol Obstet. 2010;108:65-68.
2. Osman H, Usta IM, Rubeiz N, et al. Cocoa butter lotion for prevention of stretch marks: a double-blind, randomized and placebo-controlled trial. BJOG. 2008;115:1138-1142.
3. Taavoni S, Soltanipour F, Haghani H, et al. Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract. 2011;17:167-169.
4. Mallol J, Belda MA, Costa D, et al. Prophylaxis of striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci. 1991;13:51-57.
5. Young GL, Jewell D. Creams for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2000;(2):CD00066.-
6. Skin Conditions During Pregnancy. The American College of Obstetricians and Gynecologists. Available at: www.acog.org/~/media/For%20Patients/faq169.pdf?dmc=1&ts=20120314T1222535345. Accessed April 20, 2012.
7. Mom and baby skin care. American Academy of Dermatology. Available at: www.aad.org/media-resources/stats-and-facts/prevention-and-care/mom-and-baby-skin-care. Accessed April 20, 2012.
NO TOPICAL AGENT has been proven to prevent or reduce stretch marks. Randomized controlled trials (RCTs) show that cocoa butter doesn’t prevent stretch marks (strength of recommendation [SOR]: A, 2 RCTs); neither does olive oil (SOR: B, 1 small RCT).
A cream containing Centella asiatica extract, vitamin E, and collagen hydrolysates doesn’t prevent new stretch marks but might avoid additional stretch marks in women who had already developed them during puberty. Massage with vitamin E ointment alone may reduce the number of stretch marks (SOR: C, 2 small RCTs with methodologic flaws).
Evidence summary
Two double-blind RCTs that compared cocoa butter with placebo to prevent stretch marks in pregnant women found no difference. In the first, investigators enrolled 300 Afro-Caribbean women at 12 to 15 weeks’ gestation. Women used either 25% cocoa butter cream or a placebo cream comprised of emollients and vitamin E daily. Investigators monitored compliance and assessed stretch marks using a validated scale at 26 weeks, 36 weeks, and after delivery. Cocoa butter cream didn’t reduce stretch marks (44% vs 55% for placebo; P=.09). Three women (1 using cocoa butter, 2 using placebo) discontinued the cream because of mild self-limiting reactions.1
In the second RCT, investigators randomized 210 nulliparous women (mainly with intermediate skin color) to use cocoa butter lotion with vitamin E or placebo. Women applied the lotion daily to their abdomen, breasts, and thighs, starting at 12 to 18 weeks’ gestation. Investigators assessed the severity of stretch marks either at delivery or postpartum using a validated scale. Cocoa butter lotion didn’t prevent stretch marks (45.1% vs 48.8% for placebo; P=.730).2
Save the olive oil for cooking
A nonblinded RCT that compared twice-daily olive oil massage with no treatment in 70 nulliparous women beginning at 18 to 20 weeks’ gestation found no reduction in stretch marks (45.7% vs 62.9% without olive oil massage; P=.115). The investigators didn’t report whether they performed a sample-size analysis to determine if the study was adequately powered to demonstrate no difference.3
Mixed, but mostly negative, results for multi-ingredient cream
A double-blind RCT found that Trofolastin cream containing Centella asiatica (also known as Gotu kola, a member of the parsley family), vitamin E, and collagen hydrolysates didn’t prevent pregnancy-related stretch marks among 80 women who applied the treatment beginning at 12 weeks’ gestation.
When investigators evaluated a subgroup of 18 women who had already developed stretch marks during puberty, they found that fewer of the women acquired additional stretch marks during pregnancy (11% vs 100% with placebo; P=.0001). The investigators didn’t calculate whether the sample was large enough to prove a significant difference.4
Fewer stretch marks with vitamin E in small flawed study
An older systematic review (1996) included a prospective RCT that randomized 50 women at 20 weeks’ gestation to massage their abdomen, thighs, and breasts with vitamin E ointment or perform no massage. The trial found fewer stretch marks with vitamin E ointment massage (odds ratio=0.26; 95% confidence interval, 0.08-0.84). The authors of the review described this RCT as poorly randomized and without blinding; investigators didn’t report whether the sample size was adequate to demonstrate a significant effect.5
Recommendations
The American Congress of Obstetricians and Gynecologists Web site states that although many creams, lotions, and oils on the market claim to prevent stretch marks, no proof exists that these treatments work. Using a heavy moisturizer may help keep skin soft, but it won’t help get rid of stretch marks.6
The American Academy of Dermatology Web site also says that a moisturizer can improve the appearance of stretch marks and reduce itchiness; sunless tanning products can hide the marks.7
NO TOPICAL AGENT has been proven to prevent or reduce stretch marks. Randomized controlled trials (RCTs) show that cocoa butter doesn’t prevent stretch marks (strength of recommendation [SOR]: A, 2 RCTs); neither does olive oil (SOR: B, 1 small RCT).
A cream containing Centella asiatica extract, vitamin E, and collagen hydrolysates doesn’t prevent new stretch marks but might avoid additional stretch marks in women who had already developed them during puberty. Massage with vitamin E ointment alone may reduce the number of stretch marks (SOR: C, 2 small RCTs with methodologic flaws).
Evidence summary
Two double-blind RCTs that compared cocoa butter with placebo to prevent stretch marks in pregnant women found no difference. In the first, investigators enrolled 300 Afro-Caribbean women at 12 to 15 weeks’ gestation. Women used either 25% cocoa butter cream or a placebo cream comprised of emollients and vitamin E daily. Investigators monitored compliance and assessed stretch marks using a validated scale at 26 weeks, 36 weeks, and after delivery. Cocoa butter cream didn’t reduce stretch marks (44% vs 55% for placebo; P=.09). Three women (1 using cocoa butter, 2 using placebo) discontinued the cream because of mild self-limiting reactions.1
In the second RCT, investigators randomized 210 nulliparous women (mainly with intermediate skin color) to use cocoa butter lotion with vitamin E or placebo. Women applied the lotion daily to their abdomen, breasts, and thighs, starting at 12 to 18 weeks’ gestation. Investigators assessed the severity of stretch marks either at delivery or postpartum using a validated scale. Cocoa butter lotion didn’t prevent stretch marks (45.1% vs 48.8% for placebo; P=.730).2
Save the olive oil for cooking
A nonblinded RCT that compared twice-daily olive oil massage with no treatment in 70 nulliparous women beginning at 18 to 20 weeks’ gestation found no reduction in stretch marks (45.7% vs 62.9% without olive oil massage; P=.115). The investigators didn’t report whether they performed a sample-size analysis to determine if the study was adequately powered to demonstrate no difference.3
Mixed, but mostly negative, results for multi-ingredient cream
A double-blind RCT found that Trofolastin cream containing Centella asiatica (also known as Gotu kola, a member of the parsley family), vitamin E, and collagen hydrolysates didn’t prevent pregnancy-related stretch marks among 80 women who applied the treatment beginning at 12 weeks’ gestation.
When investigators evaluated a subgroup of 18 women who had already developed stretch marks during puberty, they found that fewer of the women acquired additional stretch marks during pregnancy (11% vs 100% with placebo; P=.0001). The investigators didn’t calculate whether the sample was large enough to prove a significant difference.4
Fewer stretch marks with vitamin E in small flawed study
An older systematic review (1996) included a prospective RCT that randomized 50 women at 20 weeks’ gestation to massage their abdomen, thighs, and breasts with vitamin E ointment or perform no massage. The trial found fewer stretch marks with vitamin E ointment massage (odds ratio=0.26; 95% confidence interval, 0.08-0.84). The authors of the review described this RCT as poorly randomized and without blinding; investigators didn’t report whether the sample size was adequate to demonstrate a significant effect.5
Recommendations
The American Congress of Obstetricians and Gynecologists Web site states that although many creams, lotions, and oils on the market claim to prevent stretch marks, no proof exists that these treatments work. Using a heavy moisturizer may help keep skin soft, but it won’t help get rid of stretch marks.6
The American Academy of Dermatology Web site also says that a moisturizer can improve the appearance of stretch marks and reduce itchiness; sunless tanning products can hide the marks.7
1. Buchanan K, Fletcher HM, Reid M. Prevention of striae gravidarum with cocoa butter cream. Int J Gynaecol Obstet. 2010;108:65-68.
2. Osman H, Usta IM, Rubeiz N, et al. Cocoa butter lotion for prevention of stretch marks: a double-blind, randomized and placebo-controlled trial. BJOG. 2008;115:1138-1142.
3. Taavoni S, Soltanipour F, Haghani H, et al. Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract. 2011;17:167-169.
4. Mallol J, Belda MA, Costa D, et al. Prophylaxis of striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci. 1991;13:51-57.
5. Young GL, Jewell D. Creams for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2000;(2):CD00066.-
6. Skin Conditions During Pregnancy. The American College of Obstetricians and Gynecologists. Available at: www.acog.org/~/media/For%20Patients/faq169.pdf?dmc=1&ts=20120314T1222535345. Accessed April 20, 2012.
7. Mom and baby skin care. American Academy of Dermatology. Available at: www.aad.org/media-resources/stats-and-facts/prevention-and-care/mom-and-baby-skin-care. Accessed April 20, 2012.
1. Buchanan K, Fletcher HM, Reid M. Prevention of striae gravidarum with cocoa butter cream. Int J Gynaecol Obstet. 2010;108:65-68.
2. Osman H, Usta IM, Rubeiz N, et al. Cocoa butter lotion for prevention of stretch marks: a double-blind, randomized and placebo-controlled trial. BJOG. 2008;115:1138-1142.
3. Taavoni S, Soltanipour F, Haghani H, et al. Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract. 2011;17:167-169.
4. Mallol J, Belda MA, Costa D, et al. Prophylaxis of striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci. 1991;13:51-57.
5. Young GL, Jewell D. Creams for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2000;(2):CD00066.-
6. Skin Conditions During Pregnancy. The American College of Obstetricians and Gynecologists. Available at: www.acog.org/~/media/For%20Patients/faq169.pdf?dmc=1&ts=20120314T1222535345. Accessed April 20, 2012.
7. Mom and baby skin care. American Academy of Dermatology. Available at: www.aad.org/media-resources/stats-and-facts/prevention-and-care/mom-and-baby-skin-care. Accessed April 20, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
Which treatments relieve painful muscle spasms from a black widow spider bite?
OPIOIDS RELIEVE PAIN and benzodiazepines ease muscle spasms in most patients with latrodectism—widespread, sustained spasms—resulting from envenomation by a black widow spider (strength of recommendation [SOR]: C, case series).
Black widow–specific antivenin appears to shorten duration of symptoms and reduce hospitalization more than symptomatic treatment, but can cause allergic reactions, including anaphylaxis and death from acute and delayed serum reactions (SOR: C, case series).
A similar antivenin against the redback spider, a close relative of the black widow, produces clinical effects that are equivalent whether they’re given intravenously (producing measurable serum levels) or intramuscularly (producing no measurable serum levels) (SOR: B, randomized controlled trial [RCT]), raising the possibility that the antivenin might not be effective at all.
Calcium gluconate appears ineffective for symptom relief (SOR: C, case series).
Evidence summary
A bite by the black widow spider (Latrodectus mactans) is painful but rarely fatal. No deaths have resulted from more than 40,000 reported bites in the United States.1 Envenomation may cause latrodectism, a syndrome characterized by widespread, sustained muscle spasms. Victims also may have significant hypertension, autonomic and central nervous system dysfunction, and abdominal pain severe enough to be mistaken for an acute abdomen.2 Our literature search didn’t find any RCTs comparing the efficacy of general symptomatic treatment with administration of specific antivenin against black widow spider bites.
Relief with opioids, benzodiazepines, but not with calcium gluconate
A retrospective case series that evaluated 163 patients who had been bitten by a black widow spider found that IV opioids and benzodiazepines (most often diazepam) relieved symptoms in most patients. Black widow–specific antivenin improved severe symptoms, albeit at the risk of causing allergic complications (antivenin contains whole immunoglobulin G from horses).3
Patients were 8 months to 88 years old (average age 31.6 years); 99 (61%) were male. Investigators reviewed their medical records and categorized symptom severity as mild (asymptomatic or local pain only, 9%), moderate (muscle or abdominal pain with normal vital signs, 37%), or severe (generalized back, chest, or abdominal pain; nausea, headache, and abnormal vital signs, 54%). Physicians treated moderate or severe symptoms with IV opioids (49 patients), IV opioids in combination with benzodiazepines (44 patients), or IV antivenin (58 patients). (Treatment was not specified for 12 patients.)
Treatment relieved pain in 55% of patients taking opioids alone and 70% using both opioids and benzodiazepines. All 58 patients who received antivenin reported complete symptom resolution after an average of 31±27 minutes. Of 24 patients with moderate or severe symptoms who initially received calcium gluconate (mean dose 1400 mg) alone or with a muscle relaxant, 96% continued to have symptoms requiring further treatment. (Numbers add up to more than 163 because some patients received multiple types of treatment.)
Benefits of antivenin come at a price
In this study, antivenin administration shortened total symptom duration (9±23 hours with antivenin compared with 22±25 hours without; P<.05) and reduced the need for hospitalization (number needed to treat with antivenin=3, no comparative statistics supplied).3 However, antivenin complications triggered 80% of the hospital admissions associated with its use (total complication rate 9%, number needed to harm=11). Antivenin caused 4 cases of generalized urticarial reactions. A patient who had asthma and multiple drug allergies died from severe bronchospasm when physicians gave him undiluted IV antivenin.
Supportive care and antivenin show similar results in a small study
A second retrospective case series found no difference in length of hospitalization or long-term outcomes in 14 patients, 6 of whom were treated with supportive care (methocarbamol and calcium gluconate) and 8 with antivenin.4 The study didn’t include patients treated in the emergency department and didn’t categorize severity of symptoms, however.
Is antivenin ineffective?
Additional information on horse serum antivenin comes from studies of Australian redback spider bites. An RCT of 126 patients treated with either IV or IM antivenin for moderate to severe symptoms of redback latrodectism found statistically equal clinical relief of pain at 2 hours (63% vs 53%, respectively; 95% confidence interval, -8% to 26%).5 However, investigators measured serum antivenin levels in a random sample of 20 patients and found that IV administration of antivenin produced a measurable level, while IM administration did not. In light of the fact that IV and IM administration were associated with equal pain relief and that the IM route didn’t produce a measurable serum level, the investigators raised the possibility that the antivenin might not be an effective treatment.6
A case series in which Australian physicians treated 1972 redback spider bite victims with antivenin reported delayed serum reactions in 1.7% and anaphylaxis in 0.5%.7
Recommendations
A wilderness medicine text recommends admitting all symptomatic children, pregnant women, and patients with hypertension to the hospital after a black widow spider bite.2 The authors commented that severe pain and muscle spasm usually respond to IV narcotics or benzodiazepines.
They noted that Latrodectus antivenin may prevent systemic sequelae and should be used in pregnant women and patients with respiratory arrest, seizures, or uncontrolled hypertension. For patients with less severe symptoms, the authors recommend weighing the value of antivenin against the risks of acute hypersensitivity and delayed serum sickness. They reported that redback antivenin is effective in 94% of patients in Australia and that Australian data show anaphylaxis rates of 0.5% to 1%.
1. Bush SP. Why no antivenom? Ann Emerg Med. 2003;42:431-432.
2. Boyer LV, Binford GJ, Degran JA. Spider bites. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, Pa: Elsevier Mosby; 2011:975–996.
3. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782.-
4. Moss HS, Binder LS. A retrospective review of black widow spider envenomation. Ann Emerg Med. 1992;21:782-787.
5. Isbister GK, Brown SGA, Miller M, et al. A randomized controlled trial of intramuscular vs. intravenous antivenom for latrodectism—the RAVE study. Q J Med. 2008;557:565.-
6. Isbister GK, O’Leary MA, Miller M, et al. A comparison of serum antivenom concentrations after intravenous and intramuscular administration of redback (widow) spider antivenom. Br J Clin Pharmacol. 2008;65:139-143.
7. Sutherland SK, Trinca JC. Survey of 2144 cases of redback spider bites: Australia and New Zealand, 1963-1976. Med J Aust. 1978;2:620.
OPIOIDS RELIEVE PAIN and benzodiazepines ease muscle spasms in most patients with latrodectism—widespread, sustained spasms—resulting from envenomation by a black widow spider (strength of recommendation [SOR]: C, case series).
Black widow–specific antivenin appears to shorten duration of symptoms and reduce hospitalization more than symptomatic treatment, but can cause allergic reactions, including anaphylaxis and death from acute and delayed serum reactions (SOR: C, case series).
A similar antivenin against the redback spider, a close relative of the black widow, produces clinical effects that are equivalent whether they’re given intravenously (producing measurable serum levels) or intramuscularly (producing no measurable serum levels) (SOR: B, randomized controlled trial [RCT]), raising the possibility that the antivenin might not be effective at all.
Calcium gluconate appears ineffective for symptom relief (SOR: C, case series).
Evidence summary
A bite by the black widow spider (Latrodectus mactans) is painful but rarely fatal. No deaths have resulted from more than 40,000 reported bites in the United States.1 Envenomation may cause latrodectism, a syndrome characterized by widespread, sustained muscle spasms. Victims also may have significant hypertension, autonomic and central nervous system dysfunction, and abdominal pain severe enough to be mistaken for an acute abdomen.2 Our literature search didn’t find any RCTs comparing the efficacy of general symptomatic treatment with administration of specific antivenin against black widow spider bites.
Relief with opioids, benzodiazepines, but not with calcium gluconate
A retrospective case series that evaluated 163 patients who had been bitten by a black widow spider found that IV opioids and benzodiazepines (most often diazepam) relieved symptoms in most patients. Black widow–specific antivenin improved severe symptoms, albeit at the risk of causing allergic complications (antivenin contains whole immunoglobulin G from horses).3
Patients were 8 months to 88 years old (average age 31.6 years); 99 (61%) were male. Investigators reviewed their medical records and categorized symptom severity as mild (asymptomatic or local pain only, 9%), moderate (muscle or abdominal pain with normal vital signs, 37%), or severe (generalized back, chest, or abdominal pain; nausea, headache, and abnormal vital signs, 54%). Physicians treated moderate or severe symptoms with IV opioids (49 patients), IV opioids in combination with benzodiazepines (44 patients), or IV antivenin (58 patients). (Treatment was not specified for 12 patients.)
Treatment relieved pain in 55% of patients taking opioids alone and 70% using both opioids and benzodiazepines. All 58 patients who received antivenin reported complete symptom resolution after an average of 31±27 minutes. Of 24 patients with moderate or severe symptoms who initially received calcium gluconate (mean dose 1400 mg) alone or with a muscle relaxant, 96% continued to have symptoms requiring further treatment. (Numbers add up to more than 163 because some patients received multiple types of treatment.)
Benefits of antivenin come at a price
In this study, antivenin administration shortened total symptom duration (9±23 hours with antivenin compared with 22±25 hours without; P<.05) and reduced the need for hospitalization (number needed to treat with antivenin=3, no comparative statistics supplied).3 However, antivenin complications triggered 80% of the hospital admissions associated with its use (total complication rate 9%, number needed to harm=11). Antivenin caused 4 cases of generalized urticarial reactions. A patient who had asthma and multiple drug allergies died from severe bronchospasm when physicians gave him undiluted IV antivenin.
Supportive care and antivenin show similar results in a small study
A second retrospective case series found no difference in length of hospitalization or long-term outcomes in 14 patients, 6 of whom were treated with supportive care (methocarbamol and calcium gluconate) and 8 with antivenin.4 The study didn’t include patients treated in the emergency department and didn’t categorize severity of symptoms, however.
Is antivenin ineffective?
Additional information on horse serum antivenin comes from studies of Australian redback spider bites. An RCT of 126 patients treated with either IV or IM antivenin for moderate to severe symptoms of redback latrodectism found statistically equal clinical relief of pain at 2 hours (63% vs 53%, respectively; 95% confidence interval, -8% to 26%).5 However, investigators measured serum antivenin levels in a random sample of 20 patients and found that IV administration of antivenin produced a measurable level, while IM administration did not. In light of the fact that IV and IM administration were associated with equal pain relief and that the IM route didn’t produce a measurable serum level, the investigators raised the possibility that the antivenin might not be an effective treatment.6
A case series in which Australian physicians treated 1972 redback spider bite victims with antivenin reported delayed serum reactions in 1.7% and anaphylaxis in 0.5%.7
Recommendations
A wilderness medicine text recommends admitting all symptomatic children, pregnant women, and patients with hypertension to the hospital after a black widow spider bite.2 The authors commented that severe pain and muscle spasm usually respond to IV narcotics or benzodiazepines.
They noted that Latrodectus antivenin may prevent systemic sequelae and should be used in pregnant women and patients with respiratory arrest, seizures, or uncontrolled hypertension. For patients with less severe symptoms, the authors recommend weighing the value of antivenin against the risks of acute hypersensitivity and delayed serum sickness. They reported that redback antivenin is effective in 94% of patients in Australia and that Australian data show anaphylaxis rates of 0.5% to 1%.
OPIOIDS RELIEVE PAIN and benzodiazepines ease muscle spasms in most patients with latrodectism—widespread, sustained spasms—resulting from envenomation by a black widow spider (strength of recommendation [SOR]: C, case series).
Black widow–specific antivenin appears to shorten duration of symptoms and reduce hospitalization more than symptomatic treatment, but can cause allergic reactions, including anaphylaxis and death from acute and delayed serum reactions (SOR: C, case series).
A similar antivenin against the redback spider, a close relative of the black widow, produces clinical effects that are equivalent whether they’re given intravenously (producing measurable serum levels) or intramuscularly (producing no measurable serum levels) (SOR: B, randomized controlled trial [RCT]), raising the possibility that the antivenin might not be effective at all.
Calcium gluconate appears ineffective for symptom relief (SOR: C, case series).
Evidence summary
A bite by the black widow spider (Latrodectus mactans) is painful but rarely fatal. No deaths have resulted from more than 40,000 reported bites in the United States.1 Envenomation may cause latrodectism, a syndrome characterized by widespread, sustained muscle spasms. Victims also may have significant hypertension, autonomic and central nervous system dysfunction, and abdominal pain severe enough to be mistaken for an acute abdomen.2 Our literature search didn’t find any RCTs comparing the efficacy of general symptomatic treatment with administration of specific antivenin against black widow spider bites.
Relief with opioids, benzodiazepines, but not with calcium gluconate
A retrospective case series that evaluated 163 patients who had been bitten by a black widow spider found that IV opioids and benzodiazepines (most often diazepam) relieved symptoms in most patients. Black widow–specific antivenin improved severe symptoms, albeit at the risk of causing allergic complications (antivenin contains whole immunoglobulin G from horses).3
Patients were 8 months to 88 years old (average age 31.6 years); 99 (61%) were male. Investigators reviewed their medical records and categorized symptom severity as mild (asymptomatic or local pain only, 9%), moderate (muscle or abdominal pain with normal vital signs, 37%), or severe (generalized back, chest, or abdominal pain; nausea, headache, and abnormal vital signs, 54%). Physicians treated moderate or severe symptoms with IV opioids (49 patients), IV opioids in combination with benzodiazepines (44 patients), or IV antivenin (58 patients). (Treatment was not specified for 12 patients.)
Treatment relieved pain in 55% of patients taking opioids alone and 70% using both opioids and benzodiazepines. All 58 patients who received antivenin reported complete symptom resolution after an average of 31±27 minutes. Of 24 patients with moderate or severe symptoms who initially received calcium gluconate (mean dose 1400 mg) alone or with a muscle relaxant, 96% continued to have symptoms requiring further treatment. (Numbers add up to more than 163 because some patients received multiple types of treatment.)
Benefits of antivenin come at a price
In this study, antivenin administration shortened total symptom duration (9±23 hours with antivenin compared with 22±25 hours without; P<.05) and reduced the need for hospitalization (number needed to treat with antivenin=3, no comparative statistics supplied).3 However, antivenin complications triggered 80% of the hospital admissions associated with its use (total complication rate 9%, number needed to harm=11). Antivenin caused 4 cases of generalized urticarial reactions. A patient who had asthma and multiple drug allergies died from severe bronchospasm when physicians gave him undiluted IV antivenin.
Supportive care and antivenin show similar results in a small study
A second retrospective case series found no difference in length of hospitalization or long-term outcomes in 14 patients, 6 of whom were treated with supportive care (methocarbamol and calcium gluconate) and 8 with antivenin.4 The study didn’t include patients treated in the emergency department and didn’t categorize severity of symptoms, however.
Is antivenin ineffective?
Additional information on horse serum antivenin comes from studies of Australian redback spider bites. An RCT of 126 patients treated with either IV or IM antivenin for moderate to severe symptoms of redback latrodectism found statistically equal clinical relief of pain at 2 hours (63% vs 53%, respectively; 95% confidence interval, -8% to 26%).5 However, investigators measured serum antivenin levels in a random sample of 20 patients and found that IV administration of antivenin produced a measurable level, while IM administration did not. In light of the fact that IV and IM administration were associated with equal pain relief and that the IM route didn’t produce a measurable serum level, the investigators raised the possibility that the antivenin might not be an effective treatment.6
A case series in which Australian physicians treated 1972 redback spider bite victims with antivenin reported delayed serum reactions in 1.7% and anaphylaxis in 0.5%.7
Recommendations
A wilderness medicine text recommends admitting all symptomatic children, pregnant women, and patients with hypertension to the hospital after a black widow spider bite.2 The authors commented that severe pain and muscle spasm usually respond to IV narcotics or benzodiazepines.
They noted that Latrodectus antivenin may prevent systemic sequelae and should be used in pregnant women and patients with respiratory arrest, seizures, or uncontrolled hypertension. For patients with less severe symptoms, the authors recommend weighing the value of antivenin against the risks of acute hypersensitivity and delayed serum sickness. They reported that redback antivenin is effective in 94% of patients in Australia and that Australian data show anaphylaxis rates of 0.5% to 1%.
1. Bush SP. Why no antivenom? Ann Emerg Med. 2003;42:431-432.
2. Boyer LV, Binford GJ, Degran JA. Spider bites. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, Pa: Elsevier Mosby; 2011:975–996.
3. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782.-
4. Moss HS, Binder LS. A retrospective review of black widow spider envenomation. Ann Emerg Med. 1992;21:782-787.
5. Isbister GK, Brown SGA, Miller M, et al. A randomized controlled trial of intramuscular vs. intravenous antivenom for latrodectism—the RAVE study. Q J Med. 2008;557:565.-
6. Isbister GK, O’Leary MA, Miller M, et al. A comparison of serum antivenom concentrations after intravenous and intramuscular administration of redback (widow) spider antivenom. Br J Clin Pharmacol. 2008;65:139-143.
7. Sutherland SK, Trinca JC. Survey of 2144 cases of redback spider bites: Australia and New Zealand, 1963-1976. Med J Aust. 1978;2:620.
1. Bush SP. Why no antivenom? Ann Emerg Med. 2003;42:431-432.
2. Boyer LV, Binford GJ, Degran JA. Spider bites. In: Auerbach PS, ed. Wilderness Medicine. 6th ed. Philadelphia, Pa: Elsevier Mosby; 2011:975–996.
3. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782.-
4. Moss HS, Binder LS. A retrospective review of black widow spider envenomation. Ann Emerg Med. 1992;21:782-787.
5. Isbister GK, Brown SGA, Miller M, et al. A randomized controlled trial of intramuscular vs. intravenous antivenom for latrodectism—the RAVE study. Q J Med. 2008;557:565.-
6. Isbister GK, O’Leary MA, Miller M, et al. A comparison of serum antivenom concentrations after intravenous and intramuscular administration of redback (widow) spider antivenom. Br J Clin Pharmacol. 2008;65:139-143.
7. Sutherland SK, Trinca JC. Survey of 2144 cases of redback spider bites: Australia and New Zealand, 1963-1976. Med J Aust. 1978;2:620.
Evidence-based answers from the Family Physicians Inquiries Network