User login
Anal sphincter injury at childbirth
There is a crisis of confidence in vaginal delivery. Women are aware of the potential for devastating consequences, and many ask for elective cesarean solely to avoid any possibility of incontinence or other problems linked to vaginal delivery.
Many obstetricians also have misgivings, though they are well aware that a cesarean is far more likely to cause maternal morbidity.1 In a survey of female obstetricians, 31% chose elective cesarean as their preferred mode of delivery—80% of whom gave fear of perineal trauma as their reason.2
We cannot dispute the risks. The incidence of anal incontinence following recognized obstetric anal sphincter injury (OASI) is estimated at over 60%,3 and the true incidence may be much higher,4 particularly when injury goes unrecognized at the time of delivery.
OASI—any 3rd- or 4th-degree perineal tear—causes far more morbidity than episiotomy alone or 1st- or 2nd-degree tears ( FIGURE 1). It is the most common cause of postpartum anal incontinence. Anal incontinence is defined by the International Continence Society as involuntary loss of flatus or feces that becomes a social or hygienic problem.5 What’s more, incontinence due to OASI causes very high cumulative health service costs.13
Lack of uniform classification, insufficient training, and limited evidence from randomized controlled trials all contribute to the notoriously poor outcomes of obstetric anal sphincter injury.
To improve the outcome and reestablish confidence in vaginal delivery, more training is needed, as is more research directed toward identifying how to prevent, identify, and manage anal sphincter injury following vaginal delivery.
Taboos, embarrassment, and mistaken thinking
Even though anal incontinence may be both physically and psychologically devastating, many women do not seek medical attention due to embarrassment.6-10 One study, for instance, found that only a third of women with fecal incontinence had ever discussed the problem with a physician.11
Wood et al10 reported that most women with anal sphincter injury were either unaware that they had the injury, or felt they did not receive an adequate explanation of their injury.
Some women chose not to speak with their doctors because they believed that anal incontinence was a normal consequence of childbirth.6,12
The scope of life-disrupting morbidities
Perineal pain and dyspareunia may persist for years
Perineal pain can be so distressing for the new mother that it may interfere with her ability to breast feed and cope with the daily tasks of motherhood.14 Short-term perineal pain is associated with reactionary edema, bruising, tight sutures, infection, and wound dehiscence. Persistent pain and discomfort from perineal trauma may also cause urinary retention and defecation problems.
Perineal pain and dyspareunia, which greatly impair sexual and social life, may last for many years after childbirth.6,15-17 Wheeless,18 for instance, reported that some women refrained from sexual intercourse for up to 14 years because of dyspareunia following sphincter injury.
Abscess formation, wound breakdown, rectovaginal fistulae
Following primary repair of OASI, Venkatesh et al19 noted a 10% wound disruption rate.
Price of missed injury could be colostomy. Most rectovaginal fistulae occur when the physician fails to recognize the true extent of sphincter injury at the time of repair, resulting in inadequate sphincter reconstruction and wound breakdown.17 Once rectovaginal fistulae have occurred, treatment is difficult and may ultimately require permanent colostomy.17,20
6 Risk factors for perineal trauma
1. Nulliparity
Because nulliparous women have a relatively inelastic perineum,21 time for perineal stretching during the second stage of labor is often inadequate, and perineal trauma is therefore more likely. Further, compared to the multipara, nulliparous women undergo more episiotomies to prevent perineal trauma, and are more likely to have instrumental delivery. This combination of factors increases their risk of OASI.
2. Macrosomia
Birth weight of more than 4 kg imposes risk of perineal injury, especially 3rd- and 4th-degree tears,8,22,23 due to larger head circumference, prolonged labor, and difficult delivery, especially if instrumental delivery is used. Even after safe delivery of the head, shoulder dystocia—more common in macrosomic infants—may contribute to perineal and anal sphincter trauma. A large baby is also likely to disrupt the fascial supports of the pelvic floor and cause a stretch injury to the pelvic and pudendal nerves.
3. Malposition, malpresentation
Occipito-posterior position incurs increased incidence of sphincter injury, for these reasons:8,22,24
- Incomplete flexion of fetal head increases the presenting diameter.
- Prolonged second stage of labor results in persistent pressure on the perineum, leading to edematous and friable tissues, which are more vulnerable to laceration, than during occipito-anterior labor.
- Instrumental delivery is more likely than with occipito-anterior position.
Malpresentations such as face and brow presentations are also reported as risk factors for anal sphincter injury.22
Breech delivery does not appear to increase risk, but this may be due to stringent selection criteria and a low threshold for cesarean section during labor.
4. Precipitate labor
Cervical, perineal, labial, and urethral injury, all notable complications of precipitate labor, are largely due to inadequate time for maternal tissues to adjust to delivery forces. And delivery in unfavorable circumstances such as in transit to the hospital or in a standing position, without experienced assistance, allows no opportunity for management.
5. Prolonged second stage
Several studies have reported that a second stage of more than 60 minutes increases the incidence of anal sphincter injury.22,25,26 Evidence suggests that a prolonged active second stage causes pudendal nerve damage; however, if damage occurs in the first stage, as one report indicates, then a cesarean performed after onset of labor during which the cervix dilates more than 8 cm would not avert pudendal nerve damage.27
Routine versus restrictive
A Cochrane review38 recommends restrictive use of episiotomy, based on an analysis of 6 randomized controlled trials, which concluded that there was no difference, in terms of severe vaginal or perineal trauma, between routine and restrictive episiotomy groups.
Compared to routine use, restrictive episiotomy had a lower incidence of posterior perineal trauma (relative risk 0.88; 95% confidence interval, 0.84-0.92), but a higher incidence of anterior perineal trauma (relative risk 1.02; 95% confidence interval, 0.90-1.16).
Mediolateral versus median
The reviewers also concluded that results for mediolateral versus median episiotomy were similar to the overall comparison, and recommended that, until further research is available, obstetricians should choose the technique with which they are most familiar.
Other data, however, have implied that mediolateral is superior to midline episiotomy. A retrospective study by Bodner-Adler and colleagues,25 for instance, reported a 6-fold increase in anal sphincter injury with midline episiotomy compared to mediolateral episiotomy. And a prospective nonrandomized controlled study by Combs et al21 reported an adjusted odds ratio of 5.92 for anal sphincter injury with midline episiotomy compared to mediolateral episiotomy.
As the Cochrane review noted, “There is a pressing need to evaluate which episiotomy technique (mediolateral or midline) provides the best outcome.”
We still don’t know Anal sphincter following vaginal delivery is a major cause of maternal morbidity worldwide, yet at present its management is based on limited evidence and expert opinion. Future research directed towards prevention and management of obstetric anal sphincter injury, and management of subsequent delivery, is needed.
It has been suggested that a passive second stage, particularly with an epidural, should be accelerated with oxytocics, rather than resorting to instrumental delivery, which itself may cause trauma.
6. Operative delivery
Though operative delivery is integral to obstetrics and reduces the cesarean rate, maternal morbidity is more likely, compared to unassisted delivery. Injuries caused by instrumental delivery include cervical laceration, as well as anal sphincter injury.
Forceps delivery. The operator needs to be skilled in use of both forceps and vacuum extraction, since some circumstances preclude use of the vacuum extractor (prematurity, face presentation, potential fetal bleeding tendency, delivery of the aftercoming head at breech presentation, lift out at cesarean section, and equipment failure). However, it is well established that maternal injury is more likely with forceps than vacuum extraction. The reasons:
- The forceps occupy almost 10% more space in the pelvis.
- The shanks of the forceps stretch the perineum and can cause injury. The anal sphincter is particularly vulnerable when the physician pulls in the posterolateral direction to encourage flexion of the head.
- Unlike the vacuum extractor, which can detach, the forceps has no fail-safe mechanism, and therefore excessive force can be applied, particularly under epidural anaesthesia.
- Forceps delivery always requires an episiotomy, but it is not an absolute necessity with the vacuum extractor.
Vacuum delivery. A Cochrane review28 of 10 trials concluded that vacuum-assisted vaginal delivery had significantly less maternal trauma (odds ratio [OR] 0.41; 95% confidence interval [CI], 0.33 to 0.50) and less general and regional anesthesia than forceps delivery.
A reduction in cephalhematoma and retinal hemorrhages with forceps might be considered a compensatory benefit; however, a 5-year follow-up of a randomized controlled trial comparing forceps with vacuum extraction found no significant differences in visual problems or child development.
Which cup for which position? Metal cups appear to be more suitable for occipitoposterior, transverse, and difficult occipitoanterior position deliveries.28
Soft cups seem appropriate for straightforward deliveries, as they are significantly more likely to fail to achieve vaginal delivery (OR 1.65; 95% CI, 1.19 to 2.29). Though scalp injury was less likely with soft cups (OR 0.45; 95% CI, 0.15 to 0.60), the 2 groups did not differ in maternal injury.
Let mother choose position—it’s not critical
Women should be encouraged to deliver in whichever position is most comfortable. Though some evidence suggests that perineal injury is more likely with a standing position delivery, a Cochrane review found that, with the possible exception of increased blood loss, there were no deleterious effects to the mother or fetus.29
The current evidence on various delivery positions is inconclusive.
Tactics for management of anal sphincter injury
Recognition and proper classification. Examination of perineal injury under adequate analgesia and light, and a combined vaginal and rectal examination are essential to assess the degree of anal sphincter injury.
If any doubt exists about the extent of the injury, a second opinion must be sought. It has been reported that the presence of an experienced person at the time of perineal assessment has increased the detection rate of anal sphincter injury.
Immediate repair of the perineal injury is advisable compared to delayed repair, as the immediate repair will reduce the bleeding and pain associated with the injury, which may in turn affect early breastfeeding and bonding. Immediate repair also prevents the development of edema (which may hinder subsequent recognition of structures involved) and reduces the possibility of infection.
Careful examination of the labia, clitoris, and urethra is essential to identify any injury. These structures need repair prior to the perineal repair.
Only a doctor experienced in anal sphincter repair or a trainee under supervision should perform a repair.
I prefer to repair the injury in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions.
General or regional (spinal, epidural, caudal) anesthesia is an important prerequisite—particularly for overlap repair, as the inherent tone in the sphincter muscle can cause the torn muscle ends to retract within the sheath. Muscle relaxation is necessary to retrieve the ends and overlap without tension.
The woman is placed in the lithotomy position and the full extent of the injury is evaluated by careful vaginal and rectal examination.
In the presence of a 4th-degree tear, the torn anal epithelium is repaired with interrupted 3/0 polyglactin (Vicryl, Ethicon, Somerville, NJ) sutures, with the knots tied in the anal lumen. Another option: A subcuticular repair of the anal epithelium using 3/0 polyglactin via the transvaginal approach has been used with equal success.
The sphincter muscles are repaired with 3/0 polydioxanone sulphate (PDS) clear sutures. Compared to a braided suture, these monofilamentous sutures are less likely to precipitate infection.
The internal anal sphincter should be identified and any tear repaired separately from the external sphincter, with interrupted 3/0 PDS. I advocate primary surgical repair of the internal sphincter, which has been shown to be beneficial in patients with established anal incontinence.
The external anal sphincter should be repaired with 3/0 PDS sutures, with either end-to-end or overlapping technique. No published randomized studies at present suggest that primary overlap technique is better than primary end-to-end technique. However the secondary overlapping techniques carried out by coloproctologists have shown better continence rates compared to secondary end-to-end technique.
Extra attention should be directed to reconstructing the perineal muscles, to provide support to the sphincter repair and maintain the vaginoanal distance. This may offer some protection in subsequent vaginal delivery and may prevent suture migration.
A vaginal and rectal examination must be performed and swabs and needles should be checked.
Intravenous antibiotics should be commenced intraoperatively and continued orally for 1 week.
A stool softener (lactulose 10 mL, 3 times daily) and a bulking agent should be prescribed for at least 2 weeks post-operatively, as passage of a large bolus of hard stool may disrupt the repair.
A comprehensive record should be documented, together with a diagram to demonstrate the injury.
The woman should be informed of the injury and the possible sequelae.
It is usual to ensure that a bowel action has occurred prior to discharge.
A hospital follow-up by an experienced doctor is essential.
Obstetric anal sphincter injury by the numbers
| 0.5%–5% | Incidence in centers performing mediolateral episiotomy15,34 |
| Up to 50% | Incidence for forceps delivery with midline episiotomy35 |
| At least 1 in 20 | Number of women with anal incontinence up to 1 year after childbirth36,37 |
| Over 60% | Incidence of anal incontinence following recognized anal sphincter injury3 |
| One third | Number of women with anal incontinence who have discussed the problem with a doctor11 |
Future pregnancies: Set course by symptoms
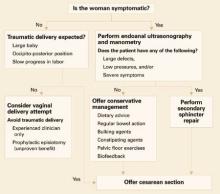
Consider subsequent vaginal delivery only under these circumstances (FIGURE 2):
- The woman is asymptomatic.
- She has no evidence of anal sphincter defects detected by endoanal scan or low pressures on manometry.
- Delivery will be carried out by an experienced midwife or doctor.
Since no evidence suggests that an elective prophylactic episiotomy will prevent another tear, perform episiotomy only if clinically indicated (ie, if the perineum is thick and inelastic, and an episiotomy will prevent multiple radial tears).
Asymptomatic women with low squeeze pressures and a defect greater than 1 quadrant are at increased risk of developing anal incontinence following another vaginal delivery; therefore, counseling should include the option of cesarean section.
Symptomatic women with severe injuries. Offer a secondary sphincter repair, and deliver future pregnancies by cesarean.
Women with mild symptoms can be managed conservatively with:
- dietary advice to avoid gas-producing foods,
- regulation of bowel action,
- bulking agents,
- constipating agents such as loperamide and codeine phosphate,
- pelvic floor exercises, and
- biofeedback.
This group of women is at risk of deterioration with a subsequent vaginal delivery, and should therefore be offered cesarean section. The risk of developing a repeat 3rd-degree tear is low, but no randomized studies have been performed to evaluate the benefit of routine cesarean section.
The author reports no financial relationships relevant to this article.
FIGURE 2 Pregnancy after sphincter injury: How to manage delivery30
1. Sultan AH, Stanton SL. Preserving the pelvic floor and perineum during childbirth—elective CS?. Br J Obstet Gynaecol. 1996;103:731-734.
2. Al-Mufti R, McCarthy A, Fisk NM. Obstetricians’ personal choice and mode of delivery. Lancet. 1996;347:544.-
3. Nazir M, Stein R, Carlsen E, Jacobsen AF, Nesheim B. Early evaluation of bowel symptoms after primary repair of obstetric perineal rupture is misleading—an observational cohort study. Dis Colon Rectum. 2003;46:1245-1250.
4. Goffeng AR, Andersch B, Andersson M, Berndtsson I, Hulten I, Oresland T. Objective methods cannot predict anal incontinence after primary repair of extensive anal tears. Acta Obstet Gynecol Scand. 1998;77:439-443.
5. Sultan AH, Kamm MA. Faecal incontinence after childbirth. Br J Obstet Gynaecol. 1997;104:972-982.
6. Haadam K, Ohrlander S, Lingman G. Long term ailments due ASR caused by delivery—a hidden problem. Eur J Obste Gynecol Reprod Biol. 1988;27:27-32.
7. Browning GG, Motson RW. Results of Parks operation for faecal incontinence after anal sphincter repair. BMJ. 1983;286:1873-1875.
8. Sultan AH, Kamm MA, Hudson CN, Bartrum CI. 3rd degree obstetric anal sphincter tears: risk factors & outcome of primary repair. BMJ. 1994;308:887-891.
9. Gjessing H, Backe B, Sahlin Y. Third degree obstetric tears; outcome after primary repair. Acta Obstet Gyaecol Scand. 1998;77:736-740.
10. Wood J, Amos L, Rieger N. Third degree anal sphincter tears—risk factors and outcome. Aust NZ J Obstet Gynaecol. 1998;38:3:414-417.
11. Johanson JF, Lafferty J. Epidemiology of faecal incontinence: the silent affliction. Am J Gastroenterol. January 1996;91:33-36.
12. Walsh CJ, Mooney EF, Upton GJ, Motson RW. Incidence of third degree perineal tears in labour and outcome after primary repair. Br J Surg. 1996;83:218-221.
13. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of faecal incontinence secondary to obstetric injuries. Dis Colon Rectum. 1999;42:857-867.
14. Sleep J. Perineal care: a series of five randomized controlled trials. In: Robinson S, Thomson A, eds. Midwives, Research and Childbirth. Vol. 2. 1st ed. London, England: Chapman and Hall; 1991;199-251.
15. Sorensen SM, Bondesen H, Istre O, Vilmann P. Perineal rupture following vaginal delivery. Acta Obstet Gynecol Scand. 1988;67:315-318.
16. Sultan AH, Kamm MA, Bartrum CI, Hudson CN. Perienal damage at delivery. Contemp Review Obstet Gynaecol. 1994;6:18-24.
17. Giebel GD, Mennigen R, Chalabi K. Secondary anal reconstruction after obstetric injury. Coloproctology. 1993;1:55-58.
18. Wheeless CR, Jr. Ten steps to avoid FI secondary to 4th-degree obstetrical tear [Guest Editorial]. Obstet Gynecol Surv. March 1998;53:131-132.
19. Venkatesh KS, Ramanujam PS, Larson DM, Haywood MA. Anorectal complications of vaginal delivery. Dis Colon Rectum. 1989;32:1039-1041.
20. Pezim ME, Spencer RJ, Stanhope CR, Beart RW, Jr, Ready RL, Ilstrup DM. Sphincter repair for faecal incontinence after obstetrical or iatrogenic injury. Dis Colon Rectum. 1987;30:521-525.
21. Combs CA, Robertson PA, Laros RK. Risk factors in 3rd-and 4th-degree perineal lacerations in forceps and vacuum deliveries. Am J Obstet Gynecol. 1990;163:100-104.
22. de Leeuw JW, Sruijk PC, Vierhout ME, Wallenburg HCS. Risk factors for third-degree perineal ruptures during delivery. Br J Obstet Gynaecol. 2001;108:383-387.
23. Green JR, Soohoo SL. Factors associated with rectal injury in spontaneous delivery. Obstet Gynecol. 1989;73:732-738.
24. Pearl ML, Roberts JM, Laros RK, Hurd WW. Vaginal delivery from persistent occipito posterior position. Influence on maternal and neonatal morbidity. J Reprod Med. 1993;38:955-961.
25. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third degree perineal tears in vaginal delivery with an analysis of episiotomy types. J Reprod Med. 2001;46:752-756.
26. McLeod NL, Gilmour DT, Joseph KS, Farrell SA, Luther ER. Trends in major risk factors for anal sphincter lacerations: a 10 year study. J Obstet Gynaecol Can. 2003;25:586-593.
27. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. Br J Obstet Gynaecol. 1994;101:22-28.
28. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
29. Gupta JK, Hofmeyr GJ. Position for women during second stage of labour. Cochrane Database Syst Rev. 2004;(1):CD002006.-
30. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynecol. February 2002;16:99-115.
31. Sultan AH. Obsteric perineal injury and anal incontinence [editorial]. Clin Risk. 1999;5:193-196.
32. Adams EJ, Fernando RJ. Royal College of Obstetrics and Gynecology Green Top Guidelines. Guideline #29: Management of third- and fourth-degree perineal tears following vaginal delivery. RCOG; 2001.
33. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. Biomed Cent Health Serv Res. 2002;2:9.-
34. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol. 2001;98:225-230.
35. Kammerer-Doak DN, Wesol AB, Rogers RG, Dominguez CE, Dorin MH. A prospective cohort study of women after primary repair of obstetric anal sphincter laceration. Am J Obstet Gynecol. 1999;181:1317-1322.
36. Macarthur C, Lewis M, Knox EG. Health after childbirth: an investigation of long-term health problems beginning after childbirth in 11,701 women. London, England: HMSO; 1991;83-103.
37. Glazener CMA, Abdalla M, Stroud P, Naji S, Templeton A, Russell IT. Postnatal maternal morbidity: extent, causes, prevention and treatment. Br J Obstet Gynaecol. 1995;102:282-287.
38. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2000;(2):CD000081.-
There is a crisis of confidence in vaginal delivery. Women are aware of the potential for devastating consequences, and many ask for elective cesarean solely to avoid any possibility of incontinence or other problems linked to vaginal delivery.
Many obstetricians also have misgivings, though they are well aware that a cesarean is far more likely to cause maternal morbidity.1 In a survey of female obstetricians, 31% chose elective cesarean as their preferred mode of delivery—80% of whom gave fear of perineal trauma as their reason.2
We cannot dispute the risks. The incidence of anal incontinence following recognized obstetric anal sphincter injury (OASI) is estimated at over 60%,3 and the true incidence may be much higher,4 particularly when injury goes unrecognized at the time of delivery.
OASI—any 3rd- or 4th-degree perineal tear—causes far more morbidity than episiotomy alone or 1st- or 2nd-degree tears ( FIGURE 1). It is the most common cause of postpartum anal incontinence. Anal incontinence is defined by the International Continence Society as involuntary loss of flatus or feces that becomes a social or hygienic problem.5 What’s more, incontinence due to OASI causes very high cumulative health service costs.13
Lack of uniform classification, insufficient training, and limited evidence from randomized controlled trials all contribute to the notoriously poor outcomes of obstetric anal sphincter injury.
To improve the outcome and reestablish confidence in vaginal delivery, more training is needed, as is more research directed toward identifying how to prevent, identify, and manage anal sphincter injury following vaginal delivery.
Taboos, embarrassment, and mistaken thinking
Even though anal incontinence may be both physically and psychologically devastating, many women do not seek medical attention due to embarrassment.6-10 One study, for instance, found that only a third of women with fecal incontinence had ever discussed the problem with a physician.11
Wood et al10 reported that most women with anal sphincter injury were either unaware that they had the injury, or felt they did not receive an adequate explanation of their injury.
Some women chose not to speak with their doctors because they believed that anal incontinence was a normal consequence of childbirth.6,12
The scope of life-disrupting morbidities
Perineal pain and dyspareunia may persist for years
Perineal pain can be so distressing for the new mother that it may interfere with her ability to breast feed and cope with the daily tasks of motherhood.14 Short-term perineal pain is associated with reactionary edema, bruising, tight sutures, infection, and wound dehiscence. Persistent pain and discomfort from perineal trauma may also cause urinary retention and defecation problems.
Perineal pain and dyspareunia, which greatly impair sexual and social life, may last for many years after childbirth.6,15-17 Wheeless,18 for instance, reported that some women refrained from sexual intercourse for up to 14 years because of dyspareunia following sphincter injury.
Abscess formation, wound breakdown, rectovaginal fistulae
Following primary repair of OASI, Venkatesh et al19 noted a 10% wound disruption rate.
Price of missed injury could be colostomy. Most rectovaginal fistulae occur when the physician fails to recognize the true extent of sphincter injury at the time of repair, resulting in inadequate sphincter reconstruction and wound breakdown.17 Once rectovaginal fistulae have occurred, treatment is difficult and may ultimately require permanent colostomy.17,20
6 Risk factors for perineal trauma
1. Nulliparity
Because nulliparous women have a relatively inelastic perineum,21 time for perineal stretching during the second stage of labor is often inadequate, and perineal trauma is therefore more likely. Further, compared to the multipara, nulliparous women undergo more episiotomies to prevent perineal trauma, and are more likely to have instrumental delivery. This combination of factors increases their risk of OASI.
2. Macrosomia
Birth weight of more than 4 kg imposes risk of perineal injury, especially 3rd- and 4th-degree tears,8,22,23 due to larger head circumference, prolonged labor, and difficult delivery, especially if instrumental delivery is used. Even after safe delivery of the head, shoulder dystocia—more common in macrosomic infants—may contribute to perineal and anal sphincter trauma. A large baby is also likely to disrupt the fascial supports of the pelvic floor and cause a stretch injury to the pelvic and pudendal nerves.
3. Malposition, malpresentation
Occipito-posterior position incurs increased incidence of sphincter injury, for these reasons:8,22,24
- Incomplete flexion of fetal head increases the presenting diameter.
- Prolonged second stage of labor results in persistent pressure on the perineum, leading to edematous and friable tissues, which are more vulnerable to laceration, than during occipito-anterior labor.
- Instrumental delivery is more likely than with occipito-anterior position.
Malpresentations such as face and brow presentations are also reported as risk factors for anal sphincter injury.22
Breech delivery does not appear to increase risk, but this may be due to stringent selection criteria and a low threshold for cesarean section during labor.
4. Precipitate labor
Cervical, perineal, labial, and urethral injury, all notable complications of precipitate labor, are largely due to inadequate time for maternal tissues to adjust to delivery forces. And delivery in unfavorable circumstances such as in transit to the hospital or in a standing position, without experienced assistance, allows no opportunity for management.
5. Prolonged second stage
Several studies have reported that a second stage of more than 60 minutes increases the incidence of anal sphincter injury.22,25,26 Evidence suggests that a prolonged active second stage causes pudendal nerve damage; however, if damage occurs in the first stage, as one report indicates, then a cesarean performed after onset of labor during which the cervix dilates more than 8 cm would not avert pudendal nerve damage.27
Routine versus restrictive
A Cochrane review38 recommends restrictive use of episiotomy, based on an analysis of 6 randomized controlled trials, which concluded that there was no difference, in terms of severe vaginal or perineal trauma, between routine and restrictive episiotomy groups.
Compared to routine use, restrictive episiotomy had a lower incidence of posterior perineal trauma (relative risk 0.88; 95% confidence interval, 0.84-0.92), but a higher incidence of anterior perineal trauma (relative risk 1.02; 95% confidence interval, 0.90-1.16).
Mediolateral versus median
The reviewers also concluded that results for mediolateral versus median episiotomy were similar to the overall comparison, and recommended that, until further research is available, obstetricians should choose the technique with which they are most familiar.
Other data, however, have implied that mediolateral is superior to midline episiotomy. A retrospective study by Bodner-Adler and colleagues,25 for instance, reported a 6-fold increase in anal sphincter injury with midline episiotomy compared to mediolateral episiotomy. And a prospective nonrandomized controlled study by Combs et al21 reported an adjusted odds ratio of 5.92 for anal sphincter injury with midline episiotomy compared to mediolateral episiotomy.
As the Cochrane review noted, “There is a pressing need to evaluate which episiotomy technique (mediolateral or midline) provides the best outcome.”
We still don’t know Anal sphincter following vaginal delivery is a major cause of maternal morbidity worldwide, yet at present its management is based on limited evidence and expert opinion. Future research directed towards prevention and management of obstetric anal sphincter injury, and management of subsequent delivery, is needed.
It has been suggested that a passive second stage, particularly with an epidural, should be accelerated with oxytocics, rather than resorting to instrumental delivery, which itself may cause trauma.
6. Operative delivery
Though operative delivery is integral to obstetrics and reduces the cesarean rate, maternal morbidity is more likely, compared to unassisted delivery. Injuries caused by instrumental delivery include cervical laceration, as well as anal sphincter injury.
Forceps delivery. The operator needs to be skilled in use of both forceps and vacuum extraction, since some circumstances preclude use of the vacuum extractor (prematurity, face presentation, potential fetal bleeding tendency, delivery of the aftercoming head at breech presentation, lift out at cesarean section, and equipment failure). However, it is well established that maternal injury is more likely with forceps than vacuum extraction. The reasons:
- The forceps occupy almost 10% more space in the pelvis.
- The shanks of the forceps stretch the perineum and can cause injury. The anal sphincter is particularly vulnerable when the physician pulls in the posterolateral direction to encourage flexion of the head.
- Unlike the vacuum extractor, which can detach, the forceps has no fail-safe mechanism, and therefore excessive force can be applied, particularly under epidural anaesthesia.
- Forceps delivery always requires an episiotomy, but it is not an absolute necessity with the vacuum extractor.
Vacuum delivery. A Cochrane review28 of 10 trials concluded that vacuum-assisted vaginal delivery had significantly less maternal trauma (odds ratio [OR] 0.41; 95% confidence interval [CI], 0.33 to 0.50) and less general and regional anesthesia than forceps delivery.
A reduction in cephalhematoma and retinal hemorrhages with forceps might be considered a compensatory benefit; however, a 5-year follow-up of a randomized controlled trial comparing forceps with vacuum extraction found no significant differences in visual problems or child development.
Which cup for which position? Metal cups appear to be more suitable for occipitoposterior, transverse, and difficult occipitoanterior position deliveries.28
Soft cups seem appropriate for straightforward deliveries, as they are significantly more likely to fail to achieve vaginal delivery (OR 1.65; 95% CI, 1.19 to 2.29). Though scalp injury was less likely with soft cups (OR 0.45; 95% CI, 0.15 to 0.60), the 2 groups did not differ in maternal injury.
Let mother choose position—it’s not critical
Women should be encouraged to deliver in whichever position is most comfortable. Though some evidence suggests that perineal injury is more likely with a standing position delivery, a Cochrane review found that, with the possible exception of increased blood loss, there were no deleterious effects to the mother or fetus.29
The current evidence on various delivery positions is inconclusive.
Tactics for management of anal sphincter injury
Recognition and proper classification. Examination of perineal injury under adequate analgesia and light, and a combined vaginal and rectal examination are essential to assess the degree of anal sphincter injury.
If any doubt exists about the extent of the injury, a second opinion must be sought. It has been reported that the presence of an experienced person at the time of perineal assessment has increased the detection rate of anal sphincter injury.
Immediate repair of the perineal injury is advisable compared to delayed repair, as the immediate repair will reduce the bleeding and pain associated with the injury, which may in turn affect early breastfeeding and bonding. Immediate repair also prevents the development of edema (which may hinder subsequent recognition of structures involved) and reduces the possibility of infection.
Careful examination of the labia, clitoris, and urethra is essential to identify any injury. These structures need repair prior to the perineal repair.
Only a doctor experienced in anal sphincter repair or a trainee under supervision should perform a repair.
I prefer to repair the injury in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions.
General or regional (spinal, epidural, caudal) anesthesia is an important prerequisite—particularly for overlap repair, as the inherent tone in the sphincter muscle can cause the torn muscle ends to retract within the sheath. Muscle relaxation is necessary to retrieve the ends and overlap without tension.
The woman is placed in the lithotomy position and the full extent of the injury is evaluated by careful vaginal and rectal examination.
In the presence of a 4th-degree tear, the torn anal epithelium is repaired with interrupted 3/0 polyglactin (Vicryl, Ethicon, Somerville, NJ) sutures, with the knots tied in the anal lumen. Another option: A subcuticular repair of the anal epithelium using 3/0 polyglactin via the transvaginal approach has been used with equal success.
The sphincter muscles are repaired with 3/0 polydioxanone sulphate (PDS) clear sutures. Compared to a braided suture, these monofilamentous sutures are less likely to precipitate infection.
The internal anal sphincter should be identified and any tear repaired separately from the external sphincter, with interrupted 3/0 PDS. I advocate primary surgical repair of the internal sphincter, which has been shown to be beneficial in patients with established anal incontinence.
The external anal sphincter should be repaired with 3/0 PDS sutures, with either end-to-end or overlapping technique. No published randomized studies at present suggest that primary overlap technique is better than primary end-to-end technique. However the secondary overlapping techniques carried out by coloproctologists have shown better continence rates compared to secondary end-to-end technique.
Extra attention should be directed to reconstructing the perineal muscles, to provide support to the sphincter repair and maintain the vaginoanal distance. This may offer some protection in subsequent vaginal delivery and may prevent suture migration.
A vaginal and rectal examination must be performed and swabs and needles should be checked.
Intravenous antibiotics should be commenced intraoperatively and continued orally for 1 week.
A stool softener (lactulose 10 mL, 3 times daily) and a bulking agent should be prescribed for at least 2 weeks post-operatively, as passage of a large bolus of hard stool may disrupt the repair.
A comprehensive record should be documented, together with a diagram to demonstrate the injury.
The woman should be informed of the injury and the possible sequelae.
It is usual to ensure that a bowel action has occurred prior to discharge.
A hospital follow-up by an experienced doctor is essential.
Obstetric anal sphincter injury by the numbers
| 0.5%–5% | Incidence in centers performing mediolateral episiotomy15,34 |
| Up to 50% | Incidence for forceps delivery with midline episiotomy35 |
| At least 1 in 20 | Number of women with anal incontinence up to 1 year after childbirth36,37 |
| Over 60% | Incidence of anal incontinence following recognized anal sphincter injury3 |
| One third | Number of women with anal incontinence who have discussed the problem with a doctor11 |
Future pregnancies: Set course by symptoms
Consider subsequent vaginal delivery only under these circumstances (FIGURE 2):
- The woman is asymptomatic.
- She has no evidence of anal sphincter defects detected by endoanal scan or low pressures on manometry.
- Delivery will be carried out by an experienced midwife or doctor.
Since no evidence suggests that an elective prophylactic episiotomy will prevent another tear, perform episiotomy only if clinically indicated (ie, if the perineum is thick and inelastic, and an episiotomy will prevent multiple radial tears).
Asymptomatic women with low squeeze pressures and a defect greater than 1 quadrant are at increased risk of developing anal incontinence following another vaginal delivery; therefore, counseling should include the option of cesarean section.
Symptomatic women with severe injuries. Offer a secondary sphincter repair, and deliver future pregnancies by cesarean.
Women with mild symptoms can be managed conservatively with:
- dietary advice to avoid gas-producing foods,
- regulation of bowel action,
- bulking agents,
- constipating agents such as loperamide and codeine phosphate,
- pelvic floor exercises, and
- biofeedback.
This group of women is at risk of deterioration with a subsequent vaginal delivery, and should therefore be offered cesarean section. The risk of developing a repeat 3rd-degree tear is low, but no randomized studies have been performed to evaluate the benefit of routine cesarean section.
The author reports no financial relationships relevant to this article.
FIGURE 2 Pregnancy after sphincter injury: How to manage delivery30
There is a crisis of confidence in vaginal delivery. Women are aware of the potential for devastating consequences, and many ask for elective cesarean solely to avoid any possibility of incontinence or other problems linked to vaginal delivery.
Many obstetricians also have misgivings, though they are well aware that a cesarean is far more likely to cause maternal morbidity.1 In a survey of female obstetricians, 31% chose elective cesarean as their preferred mode of delivery—80% of whom gave fear of perineal trauma as their reason.2
We cannot dispute the risks. The incidence of anal incontinence following recognized obstetric anal sphincter injury (OASI) is estimated at over 60%,3 and the true incidence may be much higher,4 particularly when injury goes unrecognized at the time of delivery.
OASI—any 3rd- or 4th-degree perineal tear—causes far more morbidity than episiotomy alone or 1st- or 2nd-degree tears ( FIGURE 1). It is the most common cause of postpartum anal incontinence. Anal incontinence is defined by the International Continence Society as involuntary loss of flatus or feces that becomes a social or hygienic problem.5 What’s more, incontinence due to OASI causes very high cumulative health service costs.13
Lack of uniform classification, insufficient training, and limited evidence from randomized controlled trials all contribute to the notoriously poor outcomes of obstetric anal sphincter injury.
To improve the outcome and reestablish confidence in vaginal delivery, more training is needed, as is more research directed toward identifying how to prevent, identify, and manage anal sphincter injury following vaginal delivery.
Taboos, embarrassment, and mistaken thinking
Even though anal incontinence may be both physically and psychologically devastating, many women do not seek medical attention due to embarrassment.6-10 One study, for instance, found that only a third of women with fecal incontinence had ever discussed the problem with a physician.11
Wood et al10 reported that most women with anal sphincter injury were either unaware that they had the injury, or felt they did not receive an adequate explanation of their injury.
Some women chose not to speak with their doctors because they believed that anal incontinence was a normal consequence of childbirth.6,12
The scope of life-disrupting morbidities
Perineal pain and dyspareunia may persist for years
Perineal pain can be so distressing for the new mother that it may interfere with her ability to breast feed and cope with the daily tasks of motherhood.14 Short-term perineal pain is associated with reactionary edema, bruising, tight sutures, infection, and wound dehiscence. Persistent pain and discomfort from perineal trauma may also cause urinary retention and defecation problems.
Perineal pain and dyspareunia, which greatly impair sexual and social life, may last for many years after childbirth.6,15-17 Wheeless,18 for instance, reported that some women refrained from sexual intercourse for up to 14 years because of dyspareunia following sphincter injury.
Abscess formation, wound breakdown, rectovaginal fistulae
Following primary repair of OASI, Venkatesh et al19 noted a 10% wound disruption rate.
Price of missed injury could be colostomy. Most rectovaginal fistulae occur when the physician fails to recognize the true extent of sphincter injury at the time of repair, resulting in inadequate sphincter reconstruction and wound breakdown.17 Once rectovaginal fistulae have occurred, treatment is difficult and may ultimately require permanent colostomy.17,20
6 Risk factors for perineal trauma
1. Nulliparity
Because nulliparous women have a relatively inelastic perineum,21 time for perineal stretching during the second stage of labor is often inadequate, and perineal trauma is therefore more likely. Further, compared to the multipara, nulliparous women undergo more episiotomies to prevent perineal trauma, and are more likely to have instrumental delivery. This combination of factors increases their risk of OASI.
2. Macrosomia
Birth weight of more than 4 kg imposes risk of perineal injury, especially 3rd- and 4th-degree tears,8,22,23 due to larger head circumference, prolonged labor, and difficult delivery, especially if instrumental delivery is used. Even after safe delivery of the head, shoulder dystocia—more common in macrosomic infants—may contribute to perineal and anal sphincter trauma. A large baby is also likely to disrupt the fascial supports of the pelvic floor and cause a stretch injury to the pelvic and pudendal nerves.
3. Malposition, malpresentation
Occipito-posterior position incurs increased incidence of sphincter injury, for these reasons:8,22,24
- Incomplete flexion of fetal head increases the presenting diameter.
- Prolonged second stage of labor results in persistent pressure on the perineum, leading to edematous and friable tissues, which are more vulnerable to laceration, than during occipito-anterior labor.
- Instrumental delivery is more likely than with occipito-anterior position.
Malpresentations such as face and brow presentations are also reported as risk factors for anal sphincter injury.22
Breech delivery does not appear to increase risk, but this may be due to stringent selection criteria and a low threshold for cesarean section during labor.
4. Precipitate labor
Cervical, perineal, labial, and urethral injury, all notable complications of precipitate labor, are largely due to inadequate time for maternal tissues to adjust to delivery forces. And delivery in unfavorable circumstances such as in transit to the hospital or in a standing position, without experienced assistance, allows no opportunity for management.
5. Prolonged second stage
Several studies have reported that a second stage of more than 60 minutes increases the incidence of anal sphincter injury.22,25,26 Evidence suggests that a prolonged active second stage causes pudendal nerve damage; however, if damage occurs in the first stage, as one report indicates, then a cesarean performed after onset of labor during which the cervix dilates more than 8 cm would not avert pudendal nerve damage.27
Routine versus restrictive
A Cochrane review38 recommends restrictive use of episiotomy, based on an analysis of 6 randomized controlled trials, which concluded that there was no difference, in terms of severe vaginal or perineal trauma, between routine and restrictive episiotomy groups.
Compared to routine use, restrictive episiotomy had a lower incidence of posterior perineal trauma (relative risk 0.88; 95% confidence interval, 0.84-0.92), but a higher incidence of anterior perineal trauma (relative risk 1.02; 95% confidence interval, 0.90-1.16).
Mediolateral versus median
The reviewers also concluded that results for mediolateral versus median episiotomy were similar to the overall comparison, and recommended that, until further research is available, obstetricians should choose the technique with which they are most familiar.
Other data, however, have implied that mediolateral is superior to midline episiotomy. A retrospective study by Bodner-Adler and colleagues,25 for instance, reported a 6-fold increase in anal sphincter injury with midline episiotomy compared to mediolateral episiotomy. And a prospective nonrandomized controlled study by Combs et al21 reported an adjusted odds ratio of 5.92 for anal sphincter injury with midline episiotomy compared to mediolateral episiotomy.
As the Cochrane review noted, “There is a pressing need to evaluate which episiotomy technique (mediolateral or midline) provides the best outcome.”
We still don’t know Anal sphincter following vaginal delivery is a major cause of maternal morbidity worldwide, yet at present its management is based on limited evidence and expert opinion. Future research directed towards prevention and management of obstetric anal sphincter injury, and management of subsequent delivery, is needed.
It has been suggested that a passive second stage, particularly with an epidural, should be accelerated with oxytocics, rather than resorting to instrumental delivery, which itself may cause trauma.
6. Operative delivery
Though operative delivery is integral to obstetrics and reduces the cesarean rate, maternal morbidity is more likely, compared to unassisted delivery. Injuries caused by instrumental delivery include cervical laceration, as well as anal sphincter injury.
Forceps delivery. The operator needs to be skilled in use of both forceps and vacuum extraction, since some circumstances preclude use of the vacuum extractor (prematurity, face presentation, potential fetal bleeding tendency, delivery of the aftercoming head at breech presentation, lift out at cesarean section, and equipment failure). However, it is well established that maternal injury is more likely with forceps than vacuum extraction. The reasons:
- The forceps occupy almost 10% more space in the pelvis.
- The shanks of the forceps stretch the perineum and can cause injury. The anal sphincter is particularly vulnerable when the physician pulls in the posterolateral direction to encourage flexion of the head.
- Unlike the vacuum extractor, which can detach, the forceps has no fail-safe mechanism, and therefore excessive force can be applied, particularly under epidural anaesthesia.
- Forceps delivery always requires an episiotomy, but it is not an absolute necessity with the vacuum extractor.
Vacuum delivery. A Cochrane review28 of 10 trials concluded that vacuum-assisted vaginal delivery had significantly less maternal trauma (odds ratio [OR] 0.41; 95% confidence interval [CI], 0.33 to 0.50) and less general and regional anesthesia than forceps delivery.
A reduction in cephalhematoma and retinal hemorrhages with forceps might be considered a compensatory benefit; however, a 5-year follow-up of a randomized controlled trial comparing forceps with vacuum extraction found no significant differences in visual problems or child development.
Which cup for which position? Metal cups appear to be more suitable for occipitoposterior, transverse, and difficult occipitoanterior position deliveries.28
Soft cups seem appropriate for straightforward deliveries, as they are significantly more likely to fail to achieve vaginal delivery (OR 1.65; 95% CI, 1.19 to 2.29). Though scalp injury was less likely with soft cups (OR 0.45; 95% CI, 0.15 to 0.60), the 2 groups did not differ in maternal injury.
Let mother choose position—it’s not critical
Women should be encouraged to deliver in whichever position is most comfortable. Though some evidence suggests that perineal injury is more likely with a standing position delivery, a Cochrane review found that, with the possible exception of increased blood loss, there were no deleterious effects to the mother or fetus.29
The current evidence on various delivery positions is inconclusive.
Tactics for management of anal sphincter injury
Recognition and proper classification. Examination of perineal injury under adequate analgesia and light, and a combined vaginal and rectal examination are essential to assess the degree of anal sphincter injury.
If any doubt exists about the extent of the injury, a second opinion must be sought. It has been reported that the presence of an experienced person at the time of perineal assessment has increased the detection rate of anal sphincter injury.
Immediate repair of the perineal injury is advisable compared to delayed repair, as the immediate repair will reduce the bleeding and pain associated with the injury, which may in turn affect early breastfeeding and bonding. Immediate repair also prevents the development of edema (which may hinder subsequent recognition of structures involved) and reduces the possibility of infection.
Careful examination of the labia, clitoris, and urethra is essential to identify any injury. These structures need repair prior to the perineal repair.
Only a doctor experienced in anal sphincter repair or a trainee under supervision should perform a repair.
I prefer to repair the injury in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions.
General or regional (spinal, epidural, caudal) anesthesia is an important prerequisite—particularly for overlap repair, as the inherent tone in the sphincter muscle can cause the torn muscle ends to retract within the sheath. Muscle relaxation is necessary to retrieve the ends and overlap without tension.
The woman is placed in the lithotomy position and the full extent of the injury is evaluated by careful vaginal and rectal examination.
In the presence of a 4th-degree tear, the torn anal epithelium is repaired with interrupted 3/0 polyglactin (Vicryl, Ethicon, Somerville, NJ) sutures, with the knots tied in the anal lumen. Another option: A subcuticular repair of the anal epithelium using 3/0 polyglactin via the transvaginal approach has been used with equal success.
The sphincter muscles are repaired with 3/0 polydioxanone sulphate (PDS) clear sutures. Compared to a braided suture, these monofilamentous sutures are less likely to precipitate infection.
The internal anal sphincter should be identified and any tear repaired separately from the external sphincter, with interrupted 3/0 PDS. I advocate primary surgical repair of the internal sphincter, which has been shown to be beneficial in patients with established anal incontinence.
The external anal sphincter should be repaired with 3/0 PDS sutures, with either end-to-end or overlapping technique. No published randomized studies at present suggest that primary overlap technique is better than primary end-to-end technique. However the secondary overlapping techniques carried out by coloproctologists have shown better continence rates compared to secondary end-to-end technique.
Extra attention should be directed to reconstructing the perineal muscles, to provide support to the sphincter repair and maintain the vaginoanal distance. This may offer some protection in subsequent vaginal delivery and may prevent suture migration.
A vaginal and rectal examination must be performed and swabs and needles should be checked.
Intravenous antibiotics should be commenced intraoperatively and continued orally for 1 week.
A stool softener (lactulose 10 mL, 3 times daily) and a bulking agent should be prescribed for at least 2 weeks post-operatively, as passage of a large bolus of hard stool may disrupt the repair.
A comprehensive record should be documented, together with a diagram to demonstrate the injury.
The woman should be informed of the injury and the possible sequelae.
It is usual to ensure that a bowel action has occurred prior to discharge.
A hospital follow-up by an experienced doctor is essential.
Obstetric anal sphincter injury by the numbers
| 0.5%–5% | Incidence in centers performing mediolateral episiotomy15,34 |
| Up to 50% | Incidence for forceps delivery with midline episiotomy35 |
| At least 1 in 20 | Number of women with anal incontinence up to 1 year after childbirth36,37 |
| Over 60% | Incidence of anal incontinence following recognized anal sphincter injury3 |
| One third | Number of women with anal incontinence who have discussed the problem with a doctor11 |
Future pregnancies: Set course by symptoms
Consider subsequent vaginal delivery only under these circumstances (FIGURE 2):
- The woman is asymptomatic.
- She has no evidence of anal sphincter defects detected by endoanal scan or low pressures on manometry.
- Delivery will be carried out by an experienced midwife or doctor.
Since no evidence suggests that an elective prophylactic episiotomy will prevent another tear, perform episiotomy only if clinically indicated (ie, if the perineum is thick and inelastic, and an episiotomy will prevent multiple radial tears).
Asymptomatic women with low squeeze pressures and a defect greater than 1 quadrant are at increased risk of developing anal incontinence following another vaginal delivery; therefore, counseling should include the option of cesarean section.
Symptomatic women with severe injuries. Offer a secondary sphincter repair, and deliver future pregnancies by cesarean.
Women with mild symptoms can be managed conservatively with:
- dietary advice to avoid gas-producing foods,
- regulation of bowel action,
- bulking agents,
- constipating agents such as loperamide and codeine phosphate,
- pelvic floor exercises, and
- biofeedback.
This group of women is at risk of deterioration with a subsequent vaginal delivery, and should therefore be offered cesarean section. The risk of developing a repeat 3rd-degree tear is low, but no randomized studies have been performed to evaluate the benefit of routine cesarean section.
The author reports no financial relationships relevant to this article.
FIGURE 2 Pregnancy after sphincter injury: How to manage delivery30
1. Sultan AH, Stanton SL. Preserving the pelvic floor and perineum during childbirth—elective CS?. Br J Obstet Gynaecol. 1996;103:731-734.
2. Al-Mufti R, McCarthy A, Fisk NM. Obstetricians’ personal choice and mode of delivery. Lancet. 1996;347:544.-
3. Nazir M, Stein R, Carlsen E, Jacobsen AF, Nesheim B. Early evaluation of bowel symptoms after primary repair of obstetric perineal rupture is misleading—an observational cohort study. Dis Colon Rectum. 2003;46:1245-1250.
4. Goffeng AR, Andersch B, Andersson M, Berndtsson I, Hulten I, Oresland T. Objective methods cannot predict anal incontinence after primary repair of extensive anal tears. Acta Obstet Gynecol Scand. 1998;77:439-443.
5. Sultan AH, Kamm MA. Faecal incontinence after childbirth. Br J Obstet Gynaecol. 1997;104:972-982.
6. Haadam K, Ohrlander S, Lingman G. Long term ailments due ASR caused by delivery—a hidden problem. Eur J Obste Gynecol Reprod Biol. 1988;27:27-32.
7. Browning GG, Motson RW. Results of Parks operation for faecal incontinence after anal sphincter repair. BMJ. 1983;286:1873-1875.
8. Sultan AH, Kamm MA, Hudson CN, Bartrum CI. 3rd degree obstetric anal sphincter tears: risk factors & outcome of primary repair. BMJ. 1994;308:887-891.
9. Gjessing H, Backe B, Sahlin Y. Third degree obstetric tears; outcome after primary repair. Acta Obstet Gyaecol Scand. 1998;77:736-740.
10. Wood J, Amos L, Rieger N. Third degree anal sphincter tears—risk factors and outcome. Aust NZ J Obstet Gynaecol. 1998;38:3:414-417.
11. Johanson JF, Lafferty J. Epidemiology of faecal incontinence: the silent affliction. Am J Gastroenterol. January 1996;91:33-36.
12. Walsh CJ, Mooney EF, Upton GJ, Motson RW. Incidence of third degree perineal tears in labour and outcome after primary repair. Br J Surg. 1996;83:218-221.
13. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of faecal incontinence secondary to obstetric injuries. Dis Colon Rectum. 1999;42:857-867.
14. Sleep J. Perineal care: a series of five randomized controlled trials. In: Robinson S, Thomson A, eds. Midwives, Research and Childbirth. Vol. 2. 1st ed. London, England: Chapman and Hall; 1991;199-251.
15. Sorensen SM, Bondesen H, Istre O, Vilmann P. Perineal rupture following vaginal delivery. Acta Obstet Gynecol Scand. 1988;67:315-318.
16. Sultan AH, Kamm MA, Bartrum CI, Hudson CN. Perienal damage at delivery. Contemp Review Obstet Gynaecol. 1994;6:18-24.
17. Giebel GD, Mennigen R, Chalabi K. Secondary anal reconstruction after obstetric injury. Coloproctology. 1993;1:55-58.
18. Wheeless CR, Jr. Ten steps to avoid FI secondary to 4th-degree obstetrical tear [Guest Editorial]. Obstet Gynecol Surv. March 1998;53:131-132.
19. Venkatesh KS, Ramanujam PS, Larson DM, Haywood MA. Anorectal complications of vaginal delivery. Dis Colon Rectum. 1989;32:1039-1041.
20. Pezim ME, Spencer RJ, Stanhope CR, Beart RW, Jr, Ready RL, Ilstrup DM. Sphincter repair for faecal incontinence after obstetrical or iatrogenic injury. Dis Colon Rectum. 1987;30:521-525.
21. Combs CA, Robertson PA, Laros RK. Risk factors in 3rd-and 4th-degree perineal lacerations in forceps and vacuum deliveries. Am J Obstet Gynecol. 1990;163:100-104.
22. de Leeuw JW, Sruijk PC, Vierhout ME, Wallenburg HCS. Risk factors for third-degree perineal ruptures during delivery. Br J Obstet Gynaecol. 2001;108:383-387.
23. Green JR, Soohoo SL. Factors associated with rectal injury in spontaneous delivery. Obstet Gynecol. 1989;73:732-738.
24. Pearl ML, Roberts JM, Laros RK, Hurd WW. Vaginal delivery from persistent occipito posterior position. Influence on maternal and neonatal morbidity. J Reprod Med. 1993;38:955-961.
25. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third degree perineal tears in vaginal delivery with an analysis of episiotomy types. J Reprod Med. 2001;46:752-756.
26. McLeod NL, Gilmour DT, Joseph KS, Farrell SA, Luther ER. Trends in major risk factors for anal sphincter lacerations: a 10 year study. J Obstet Gynaecol Can. 2003;25:586-593.
27. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. Br J Obstet Gynaecol. 1994;101:22-28.
28. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
29. Gupta JK, Hofmeyr GJ. Position for women during second stage of labour. Cochrane Database Syst Rev. 2004;(1):CD002006.-
30. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynecol. February 2002;16:99-115.
31. Sultan AH. Obsteric perineal injury and anal incontinence [editorial]. Clin Risk. 1999;5:193-196.
32. Adams EJ, Fernando RJ. Royal College of Obstetrics and Gynecology Green Top Guidelines. Guideline #29: Management of third- and fourth-degree perineal tears following vaginal delivery. RCOG; 2001.
33. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. Biomed Cent Health Serv Res. 2002;2:9.-
34. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol. 2001;98:225-230.
35. Kammerer-Doak DN, Wesol AB, Rogers RG, Dominguez CE, Dorin MH. A prospective cohort study of women after primary repair of obstetric anal sphincter laceration. Am J Obstet Gynecol. 1999;181:1317-1322.
36. Macarthur C, Lewis M, Knox EG. Health after childbirth: an investigation of long-term health problems beginning after childbirth in 11,701 women. London, England: HMSO; 1991;83-103.
37. Glazener CMA, Abdalla M, Stroud P, Naji S, Templeton A, Russell IT. Postnatal maternal morbidity: extent, causes, prevention and treatment. Br J Obstet Gynaecol. 1995;102:282-287.
38. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2000;(2):CD000081.-
1. Sultan AH, Stanton SL. Preserving the pelvic floor and perineum during childbirth—elective CS?. Br J Obstet Gynaecol. 1996;103:731-734.
2. Al-Mufti R, McCarthy A, Fisk NM. Obstetricians’ personal choice and mode of delivery. Lancet. 1996;347:544.-
3. Nazir M, Stein R, Carlsen E, Jacobsen AF, Nesheim B. Early evaluation of bowel symptoms after primary repair of obstetric perineal rupture is misleading—an observational cohort study. Dis Colon Rectum. 2003;46:1245-1250.
4. Goffeng AR, Andersch B, Andersson M, Berndtsson I, Hulten I, Oresland T. Objective methods cannot predict anal incontinence after primary repair of extensive anal tears. Acta Obstet Gynecol Scand. 1998;77:439-443.
5. Sultan AH, Kamm MA. Faecal incontinence after childbirth. Br J Obstet Gynaecol. 1997;104:972-982.
6. Haadam K, Ohrlander S, Lingman G. Long term ailments due ASR caused by delivery—a hidden problem. Eur J Obste Gynecol Reprod Biol. 1988;27:27-32.
7. Browning GG, Motson RW. Results of Parks operation for faecal incontinence after anal sphincter repair. BMJ. 1983;286:1873-1875.
8. Sultan AH, Kamm MA, Hudson CN, Bartrum CI. 3rd degree obstetric anal sphincter tears: risk factors & outcome of primary repair. BMJ. 1994;308:887-891.
9. Gjessing H, Backe B, Sahlin Y. Third degree obstetric tears; outcome after primary repair. Acta Obstet Gyaecol Scand. 1998;77:736-740.
10. Wood J, Amos L, Rieger N. Third degree anal sphincter tears—risk factors and outcome. Aust NZ J Obstet Gynaecol. 1998;38:3:414-417.
11. Johanson JF, Lafferty J. Epidemiology of faecal incontinence: the silent affliction. Am J Gastroenterol. January 1996;91:33-36.
12. Walsh CJ, Mooney EF, Upton GJ, Motson RW. Incidence of third degree perineal tears in labour and outcome after primary repair. Br J Surg. 1996;83:218-221.
13. Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of faecal incontinence secondary to obstetric injuries. Dis Colon Rectum. 1999;42:857-867.
14. Sleep J. Perineal care: a series of five randomized controlled trials. In: Robinson S, Thomson A, eds. Midwives, Research and Childbirth. Vol. 2. 1st ed. London, England: Chapman and Hall; 1991;199-251.
15. Sorensen SM, Bondesen H, Istre O, Vilmann P. Perineal rupture following vaginal delivery. Acta Obstet Gynecol Scand. 1988;67:315-318.
16. Sultan AH, Kamm MA, Bartrum CI, Hudson CN. Perienal damage at delivery. Contemp Review Obstet Gynaecol. 1994;6:18-24.
17. Giebel GD, Mennigen R, Chalabi K. Secondary anal reconstruction after obstetric injury. Coloproctology. 1993;1:55-58.
18. Wheeless CR, Jr. Ten steps to avoid FI secondary to 4th-degree obstetrical tear [Guest Editorial]. Obstet Gynecol Surv. March 1998;53:131-132.
19. Venkatesh KS, Ramanujam PS, Larson DM, Haywood MA. Anorectal complications of vaginal delivery. Dis Colon Rectum. 1989;32:1039-1041.
20. Pezim ME, Spencer RJ, Stanhope CR, Beart RW, Jr, Ready RL, Ilstrup DM. Sphincter repair for faecal incontinence after obstetrical or iatrogenic injury. Dis Colon Rectum. 1987;30:521-525.
21. Combs CA, Robertson PA, Laros RK. Risk factors in 3rd-and 4th-degree perineal lacerations in forceps and vacuum deliveries. Am J Obstet Gynecol. 1990;163:100-104.
22. de Leeuw JW, Sruijk PC, Vierhout ME, Wallenburg HCS. Risk factors for third-degree perineal ruptures during delivery. Br J Obstet Gynaecol. 2001;108:383-387.
23. Green JR, Soohoo SL. Factors associated with rectal injury in spontaneous delivery. Obstet Gynecol. 1989;73:732-738.
24. Pearl ML, Roberts JM, Laros RK, Hurd WW. Vaginal delivery from persistent occipito posterior position. Influence on maternal and neonatal morbidity. J Reprod Med. 1993;38:955-961.
25. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third degree perineal tears in vaginal delivery with an analysis of episiotomy types. J Reprod Med. 2001;46:752-756.
26. McLeod NL, Gilmour DT, Joseph KS, Farrell SA, Luther ER. Trends in major risk factors for anal sphincter lacerations: a 10 year study. J Obstet Gynaecol Can. 2003;25:586-593.
27. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. Br J Obstet Gynaecol. 1994;101:22-28.
28. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
29. Gupta JK, Hofmeyr GJ. Position for women during second stage of labour. Cochrane Database Syst Rev. 2004;(1):CD002006.-
30. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynecol. February 2002;16:99-115.
31. Sultan AH. Obsteric perineal injury and anal incontinence [editorial]. Clin Risk. 1999;5:193-196.
32. Adams EJ, Fernando RJ. Royal College of Obstetrics and Gynecology Green Top Guidelines. Guideline #29: Management of third- and fourth-degree perineal tears following vaginal delivery. RCOG; 2001.
33. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. Biomed Cent Health Serv Res. 2002;2:9.-
34. Handa VL, Danielsen BH, Gilbert WM. Obstetric anal sphincter lacerations. Obstet Gynecol. 2001;98:225-230.
35. Kammerer-Doak DN, Wesol AB, Rogers RG, Dominguez CE, Dorin MH. A prospective cohort study of women after primary repair of obstetric anal sphincter laceration. Am J Obstet Gynecol. 1999;181:1317-1322.
36. Macarthur C, Lewis M, Knox EG. Health after childbirth: an investigation of long-term health problems beginning after childbirth in 11,701 women. London, England: HMSO; 1991;83-103.
37. Glazener CMA, Abdalla M, Stroud P, Naji S, Templeton A, Russell IT. Postnatal maternal morbidity: extent, causes, prevention and treatment. Br J Obstet Gynaecol. 1995;102:282-287.
38. Carroli G, Belizan J. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2000;(2):CD000081.-