User login
Timeliness of Lung Cancer Diagnosis and Treatment (FULL)
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
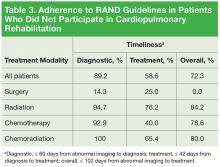
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
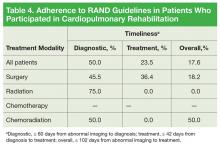
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
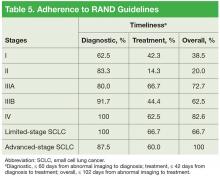
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
Letters to the Editor
We believe medications can safely be prescribed to most patients who leave against medical advice (AMA), and that follow‐up should be offered to most if not all such patients. Why should we do this? Consider a wheezing asthma patient who leaves AMA. She or he is probably more likely to return to the emergency department (somewhere) or be readmitted (somewhere) and cost more money (to the system) than if given an inhaler and steroid taper.
Dr. Querques et al. suggest that doctors should potentially not prescribe and should not offer follow‐up to certain patients who want to leave AMA, particularly those who show disinterest in heeding the doctor's advice and have already demonstrated a lack of adherence. How should doctors make those judgments? Patients leave AMA for a variety of reasons: for example to avoid cost, because they feel better, or poor communication. Certainly, not all patients who want to leave AMA are categorically nonadherent. Conversely, up to 50% of all continuity patients are not fully adherent to the lifestyle changes and medications their physicians prescribe,[1] yet they would rarely if ever threaten AMA. Is withholding treatments that are likely to be effective and have minimal risk worth the potential benefit of increasing a patient's priority on their own healthcare? As emphasized by Berger (2008),[2] interventions with low risk and high potential for efficacy (assistance with establishing a follow‐up) should be pursued, and those with potential risks (starting new long‐term medications) should be avoided. At minimum, considering these options is an ethical requirement in the care of patients. We maintain that this reasoning should be explained and documented, which often is not being done in healthcare today.
How many AMAs are avoided by truly collaborative relationships with patients (nonevents), and how many are fueled by a more paternalistic relationship? For example, if a patient truly has a sick family member or child to take care of or has financial problems or no insurance, then it seems reasonable, perhaps even responsible, to leave the hospital even if maximal benefits of care have not been reached. In a collaborative relationship, providers may then tailor treatment to the patient's circumstances, even if this means the patient is not getting the best possible care.
- , . Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314.
- . Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403–408.
We believe medications can safely be prescribed to most patients who leave against medical advice (AMA), and that follow‐up should be offered to most if not all such patients. Why should we do this? Consider a wheezing asthma patient who leaves AMA. She or he is probably more likely to return to the emergency department (somewhere) or be readmitted (somewhere) and cost more money (to the system) than if given an inhaler and steroid taper.
Dr. Querques et al. suggest that doctors should potentially not prescribe and should not offer follow‐up to certain patients who want to leave AMA, particularly those who show disinterest in heeding the doctor's advice and have already demonstrated a lack of adherence. How should doctors make those judgments? Patients leave AMA for a variety of reasons: for example to avoid cost, because they feel better, or poor communication. Certainly, not all patients who want to leave AMA are categorically nonadherent. Conversely, up to 50% of all continuity patients are not fully adherent to the lifestyle changes and medications their physicians prescribe,[1] yet they would rarely if ever threaten AMA. Is withholding treatments that are likely to be effective and have minimal risk worth the potential benefit of increasing a patient's priority on their own healthcare? As emphasized by Berger (2008),[2] interventions with low risk and high potential for efficacy (assistance with establishing a follow‐up) should be pursued, and those with potential risks (starting new long‐term medications) should be avoided. At minimum, considering these options is an ethical requirement in the care of patients. We maintain that this reasoning should be explained and documented, which often is not being done in healthcare today.
How many AMAs are avoided by truly collaborative relationships with patients (nonevents), and how many are fueled by a more paternalistic relationship? For example, if a patient truly has a sick family member or child to take care of or has financial problems or no insurance, then it seems reasonable, perhaps even responsible, to leave the hospital even if maximal benefits of care have not been reached. In a collaborative relationship, providers may then tailor treatment to the patient's circumstances, even if this means the patient is not getting the best possible care.
We believe medications can safely be prescribed to most patients who leave against medical advice (AMA), and that follow‐up should be offered to most if not all such patients. Why should we do this? Consider a wheezing asthma patient who leaves AMA. She or he is probably more likely to return to the emergency department (somewhere) or be readmitted (somewhere) and cost more money (to the system) than if given an inhaler and steroid taper.
Dr. Querques et al. suggest that doctors should potentially not prescribe and should not offer follow‐up to certain patients who want to leave AMA, particularly those who show disinterest in heeding the doctor's advice and have already demonstrated a lack of adherence. How should doctors make those judgments? Patients leave AMA for a variety of reasons: for example to avoid cost, because they feel better, or poor communication. Certainly, not all patients who want to leave AMA are categorically nonadherent. Conversely, up to 50% of all continuity patients are not fully adherent to the lifestyle changes and medications their physicians prescribe,[1] yet they would rarely if ever threaten AMA. Is withholding treatments that are likely to be effective and have minimal risk worth the potential benefit of increasing a patient's priority on their own healthcare? As emphasized by Berger (2008),[2] interventions with low risk and high potential for efficacy (assistance with establishing a follow‐up) should be pursued, and those with potential risks (starting new long‐term medications) should be avoided. At minimum, considering these options is an ethical requirement in the care of patients. We maintain that this reasoning should be explained and documented, which often is not being done in healthcare today.
How many AMAs are avoided by truly collaborative relationships with patients (nonevents), and how many are fueled by a more paternalistic relationship? For example, if a patient truly has a sick family member or child to take care of or has financial problems or no insurance, then it seems reasonable, perhaps even responsible, to leave the hospital even if maximal benefits of care have not been reached. In a collaborative relationship, providers may then tailor treatment to the patient's circumstances, even if this means the patient is not getting the best possible care.
- , . Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314.
- . Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403–408.
- , . Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314.
- . Discharge against medical advice: ethical considerations and professional obligations. J Hosp Med. 2008;3(5):403–408.
AMA Documentation Analysis
Approximately 1% to 2% of inpatient stays result in discharges against medical advice (AMA).[1] Though relatively infrequent, AMA discharges warrant attention as they are associated with higher morbidity, increased risk of readmission, and greater 30‐day mortality.[2] A recent study found a 30‐day readmission rate among AMA patients of 24.5%, nearly twice that of matched non‐AMA patients, and a 30‐day mortality rate of 1.3%, also nearly double that of planned discharges.[3] Discharges AMA may be expected to decrease index length of stay, yet accounting for 30‐day readmissions they are estimated to increase costs 56% higher than expected from an initial hospitalization.[4] Patients note several possible reasons for leaving AMA including family emergencies, dissatisfaction with care, financial concerns, or simply feeling better, among others.[5, 6, 7] Risk factors for AMA discharges include previous AMA discharge, having no primary care physician, younger age, lack of insurance, male sex, substance abuse, and lower socioeconomic status.[4, 6, 7, 8]
A number of prior studies have assessed risk factors for AMA discharges, the long‐ and short‐term outcomes, patient reasons for leaving, and physician perceptions of why patients leave AMA.[3, 5, 7, 9] However, there is limited information about opportunities for discharge transition interventions in this potentially more vulnerable population. Because of the increased short‐term and long‐term risks to these patients, treatment and follow‐up plans at the time of discharge may carry even greater importance than follow‐up plans with standard discharges. This study analyzed AMA documentation and what interventions were carried out at the time of discharge.
METHODS
We reviewed the records of all adult patients, ages 18 years and older, admitted to a university‐affiliated tertiary care hospital in Dayton, Ohio (a 520‐bed hospital with approximately 17,000 adult patient encounters per year) over a 2‐year period, and who subsequently left AMA. A hospital database identified 351 adult AMA cases (1.0% of adult admissions). A single reviewer performed an in‐depth review of the 291 patient admissions to the general medical service between January 1, 2009 and December 31, 2010, and manually reviewed and abstracted the data of interest. The Wright State University institutional review board approved the study.
Documentation review focused on the presence of a specified AMA note, the presence of documentation addressing informed consent, patient decision‐making capacity, patient health literacy, follow‐up plans, whether or not medications were prescribed, and whether or not any warning indicators of impending AMA were apparent. These items represented key elements of the discharge policy and procedure in place at our institution during the period of study. We speculated that nurses may be more immediately available at the time of AMA discharge and thus might carry out AMA documentation more often than physicians. To assess this we recorded the role (nurse vs provider) of the writer of AMA notes. We also assessed patient gender, length of stay, prior AMA, 30‐day emergency department (ED) re‐encounters, and 30‐day hospital readmission after AMA discharge.
Informed consent was deemed present if patients signed the hospital's standardized AMA form. Decision‐making capacity was assessed as present if there was specific mention of the patient's capacity on the day of discharge. Any mention of health literacy or the patient's stated understanding of his medical condition at any time during the hospitalization was considered positive documentation of healthcare literacy. Follow‐up plans included any mention of where and when the patient would return. Discharge medications included prescribed medication or indication that no medications were warranted. Warning indicators included specific mention of the patient's desire to leave AMA. For example, patients who left the unit without informing staff were considered to have given no warning of AMA. Alternatively, when documentation was present stating that the patient had verbally expressed a desire to leave AMA, this was considered advanced warning of AMA.
Statistical Analysis
Continuous variables were reported as means and standard deviations. Categorical variables were reported as counts and percents. The independent samples t test was used for comparisons involving 2 groups and a second variable measured on a continuous scale. The 2 test was used to compare 2 categorical variables. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons.
RESULTS
Mean age and gender distribution were similar to those reported in other AMA studies (Table 1).[3] Thirty‐day ED revisit and 30‐day hospital readmission frequencies for medical service patients were 121 (41.6%) and 88 (30.2%), respectively, also similar to those reported in other AMA studies.[3]
| Study Population, Mean SD or Count (%) | Hospital Population, Mean or Count (%) | |
|---|---|---|
| ||
| Age, y | 45.3 15.9 | 62.8 18.2 |
| Sex | ||
| Male | 168 (57.7) | 14,965 (43.6) |
| Female | 123 (42.3) | 19,333 (56.4) |
| Length of stay, d | 2.46 2.82 | 4.72 4.74 |
| 30‐day ED re‐encounter rate | 121 (41.6) | |
| 30‐day hospital readmission rate | 88 (30.2) | 4424 (12.9) |
| Prior AMA discharge | 49 (16.8) | |
Although our intent was to conduct a quantitative assessment of discharge interventions, we found stated reasons for leaving similar to those previously reported. In our study, AMA patients tended to be younger, more likely male, and at increased risk for AMA discharge if they had prior AMA discharges (Table 1). The most common reasons found in the medical record for leaving AMA were caring for sick family members, financial concerns, feeling better, and occasionally dissatisfaction with care, reasons similar to those reported in previous studies.[5, 7, 9, 10]
AMA notes were present in 276 (94.8%) charts. AMA notes were written by physicians in 163 (59.1%) and nurses in 110 (37.8%) encounters. The informed consent form was present in 88 (30.2%) charts, mentioned in the note but not present in the electronic medical record in 111 (38.1%), and not signed in 92 (31.6%) charts. Decision‐making capacity and health literacy were documented in 108 (37.1%) and 75 (25.8%) records, respectively. Warning of impending AMA was present in 217 (74.6%) charts. Medications prescribed and follow‐up plans were only documented in 71 (24.4%) and 91 (31.3%) charts, respectively (Table 2).
| Count (%) | |
|---|---|
| |
| AMA note present | 276 (94.8) |
| Primary AMA note author | |
| Physician | 163 (56) |
| Nurse, without physician note | 110 (37.8) |
| Other (ie, social worker) | 3 (1.0) |
| Warning of impending AMA | 217 (74.6) |
| Informed consent signed | |
| Yes | 88 (30.2) |
| No | 92 (31.6) |
| Absenta | 111 (38.1) |
| Documentation of decision‐making capacity | 108 (37.1) |
| Documentation of health literacy | 75 (25.8) |
| Documentation of follow‐up plan | 91 (31.3) |
| Documentation of medications at discharge | 71 (24.4) |
Patients with documentation of medications given did not have decreased 30‐day ED revisits (33.8% vs 44.3%, P = 0.12) or 30‐day hospital readmission (23.9% vs 32.4%, P = 0.18). Similarly, there was no relationship between documentation of follow‐up plans and 30‐day ED revisits (37.4% vs 43.7%, P = 0.31) or 30‐day hospital readmission (29.7% vs 30.7%, P = 0.87). Finally, there was no relationship between physician versus nurse authorship of AMA notes and 30‐day ED revisits (37.4% vs 46.4%, P = 0.14) or 30‐day hospital readmission (28.2% vs 31.8%, P = 0.52) (Table 3).
| Yes, % | No, % | P Value | |
|---|---|---|---|
| |||
| Documentation of discharge medicationsa | |||
| 30‐day ED revisit | 33.8 | 44.3 | 0.12 |
| 30‐day rehospitalization | 23.9 | 32.4 | 0.18 |
| Documentation of follow‐up | |||
| 30‐day ED revisit | 37.4 | 43.7 | 0.31 |
| 30‐day rehospitalization | 29.7 | 30.7 | 0.87 |
| Physician author of AMA note | |||
| 30‐day ED revisit | 37.4 | 46.4 | 0.14 |
| 30‐day rehospitalization | 28.2 | 31.8 | 0.52 |
| Documentation of medications | 36.2 | 10.0 | 0.001 |
| Documentation of follow‐up plan | 43.6 | 16.4 | 0.001 |
| Warning of AMA | |||
| Documentation of medications | 30.4 | 6.8 | 0.001 |
| Documentation of follow‐up plan | 37.3 | 13.5 | 0.001 |
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed (36.2% vs 10.0%, P 0.001) and with an increased finding of documented follow‐up plans (43.6% vs 16.4%, P 0.001). A documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) (Table 3).
DISCUSSION
To gain insights into opportunities for discharge transition interventions in this potentially more vulnerable population,[1, 5] we analyzed AMA documentation and what interventions were carried out at the time of discharge. Our intent was a quantitative assessment of discharge interventions, but we also found stated reasons for leaving AMA that were similar to those previously reported.[5, 6, 10]
We identified several opportunities for improved documentation as well as targeted discharge intervention among AMA patients. Documentation in the charts of AMA patients was often suboptimal. In our study, a physician's AMA note was present only half of the time. Mention of the patient's mental status or health literacy was present in only one‐fourth of cases. Protection from litigation in AMA cases is enhanced when these elements and others, like informed consent, are present in the medical record.[11]
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed and with an increased finding of documented follow‐up plans. This association might be confounded by the fact that physicians can prescribe whereas most nurses cannot. The findings that a documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) suggest opportunities for improvement through early inquiry about potential for AMA as well as early responses when patients threaten to leave AMA.
An important focus of our study was on documentation of discharge medications and follow‐up plans. These elements were documented in 31% and 25% of charts, respectively. A warning of impending AMA was present in 74.6% of encounters, yet medications and follow‐up plans were documented at a much lower rate. This represents an area where caregivers have the possibility to intervene, but are not documenting that they are doing so. We found no relationship between the documentation of giving prescriptions and giving explicit follow‐up plans with decreased rates of return to the ED or readmission, but that possibility may still warrant future prospective study.
Our study did not attempt to explain why only a minority of AMA discharges include medication prescription or follow‐up plans, but a number of potential explanations are possible. Some AMA discharges may occur unannounced with a patient simply walking off the ward giving little or no advance notice. It is also possible that provider perceptions and attitudes toward AMA patients may influence potential interventions.[12] An AMA discharge is against the caregiver's preferred advice for the patient, and it may seem illogical to offer patients second‐best advice. Perhaps some providers have the misconception that medications cannot or must not be prescribed for an AMA discharge. However, second‐best therapy may be better than no therapy, and some follow‐up better than no follow‐up plan.
Given the high rates of ED return and 30‐day readmission, the associated increased healthcare costs as well as increased morbidity and mortality associated with AMA dispositions, a continued search for effective intervention strategies and opportunities is warranted. Recently, programs for transition of care/discharge have demonstrated improved outcomes including reduced rates of readmission with standard discharges.[13] At the time of our study, effective programs such as Project BOOST (Better Outcomes for Older adult through Safe Transitions),[14] the Care Transitions Program,[15] and RED (Project Re‐engineered Discharge)[16] were not yet routinely employed, but their common elements may be applicable to the AMA population. In general, these programs focus on elements we investigated (patient understanding, follow‐up plans, medications prescribed) but add a number of additional components. Additional elements include written discharge instructions, patient education, teach‐back process, decision support, emergency plans, caregiver education, telephone follow‐up, and transition coaches to coordinate home and office follow‐up visits. Most potential interventions add significant time (and cost) to the discharge process. Thus, future studies applying these components to AMA discharges should emphasize timely identification of threatened AMA and prioritized interventions. Future studies should focus on which interventions are the most cost‐effective with AMA patients.
Limitations of our study include not being able to access information from area hospitals not in our hospital network, and thus we may not have identified all ED returns and readmissions. Additionally, interventions at the time of discharge (like prescription of medications or provider assessment of decision‐making capacity) may not have been documented and thus not available for our review. Also, our study was a retrospective review at a single institution and included a relatively small population of patients; consequently, our findings may not apply to other healthcare providers in other hospitals or settings. Our study was strengthened by reviewing all consecutive AMA cases over a 2‐year period encompassing a diverse group of healthcare providers.
CONCLUSION
In the majority of cases reviewed, some advance warning of impending AMA is apparent, affording an opportunity for interventions that may improve health outcomes. Despite this advance warning, only a minority of cases result in key interventions such as prescription of medications or development of follow‐up plans. Medical documentation of AMA dispositions is often inadequate, suggesting missed opportunities for potential intervention as well as suboptimal medicolegal scenarios. Future prospective studies examining cost‐effective interventions at the time of AMA discharge and transition of care may provide valuable insight into lowering rates of ED return and rehospitalization.
Disclosures: The views and opinions expressed in this article are those of the author(s) and do not reflect official policy or position of the United States Air Force, Department of Defense, or US government. The authors report no conflicts of interest.
Approximately 1% to 2% of inpatient stays result in discharges against medical advice (AMA).[1] Though relatively infrequent, AMA discharges warrant attention as they are associated with higher morbidity, increased risk of readmission, and greater 30‐day mortality.[2] A recent study found a 30‐day readmission rate among AMA patients of 24.5%, nearly twice that of matched non‐AMA patients, and a 30‐day mortality rate of 1.3%, also nearly double that of planned discharges.[3] Discharges AMA may be expected to decrease index length of stay, yet accounting for 30‐day readmissions they are estimated to increase costs 56% higher than expected from an initial hospitalization.[4] Patients note several possible reasons for leaving AMA including family emergencies, dissatisfaction with care, financial concerns, or simply feeling better, among others.[5, 6, 7] Risk factors for AMA discharges include previous AMA discharge, having no primary care physician, younger age, lack of insurance, male sex, substance abuse, and lower socioeconomic status.[4, 6, 7, 8]
A number of prior studies have assessed risk factors for AMA discharges, the long‐ and short‐term outcomes, patient reasons for leaving, and physician perceptions of why patients leave AMA.[3, 5, 7, 9] However, there is limited information about opportunities for discharge transition interventions in this potentially more vulnerable population. Because of the increased short‐term and long‐term risks to these patients, treatment and follow‐up plans at the time of discharge may carry even greater importance than follow‐up plans with standard discharges. This study analyzed AMA documentation and what interventions were carried out at the time of discharge.
METHODS
We reviewed the records of all adult patients, ages 18 years and older, admitted to a university‐affiliated tertiary care hospital in Dayton, Ohio (a 520‐bed hospital with approximately 17,000 adult patient encounters per year) over a 2‐year period, and who subsequently left AMA. A hospital database identified 351 adult AMA cases (1.0% of adult admissions). A single reviewer performed an in‐depth review of the 291 patient admissions to the general medical service between January 1, 2009 and December 31, 2010, and manually reviewed and abstracted the data of interest. The Wright State University institutional review board approved the study.
Documentation review focused on the presence of a specified AMA note, the presence of documentation addressing informed consent, patient decision‐making capacity, patient health literacy, follow‐up plans, whether or not medications were prescribed, and whether or not any warning indicators of impending AMA were apparent. These items represented key elements of the discharge policy and procedure in place at our institution during the period of study. We speculated that nurses may be more immediately available at the time of AMA discharge and thus might carry out AMA documentation more often than physicians. To assess this we recorded the role (nurse vs provider) of the writer of AMA notes. We also assessed patient gender, length of stay, prior AMA, 30‐day emergency department (ED) re‐encounters, and 30‐day hospital readmission after AMA discharge.
Informed consent was deemed present if patients signed the hospital's standardized AMA form. Decision‐making capacity was assessed as present if there was specific mention of the patient's capacity on the day of discharge. Any mention of health literacy or the patient's stated understanding of his medical condition at any time during the hospitalization was considered positive documentation of healthcare literacy. Follow‐up plans included any mention of where and when the patient would return. Discharge medications included prescribed medication or indication that no medications were warranted. Warning indicators included specific mention of the patient's desire to leave AMA. For example, patients who left the unit without informing staff were considered to have given no warning of AMA. Alternatively, when documentation was present stating that the patient had verbally expressed a desire to leave AMA, this was considered advanced warning of AMA.
Statistical Analysis
Continuous variables were reported as means and standard deviations. Categorical variables were reported as counts and percents. The independent samples t test was used for comparisons involving 2 groups and a second variable measured on a continuous scale. The 2 test was used to compare 2 categorical variables. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons.
RESULTS
Mean age and gender distribution were similar to those reported in other AMA studies (Table 1).[3] Thirty‐day ED revisit and 30‐day hospital readmission frequencies for medical service patients were 121 (41.6%) and 88 (30.2%), respectively, also similar to those reported in other AMA studies.[3]
| Study Population, Mean SD or Count (%) | Hospital Population, Mean or Count (%) | |
|---|---|---|
| ||
| Age, y | 45.3 15.9 | 62.8 18.2 |
| Sex | ||
| Male | 168 (57.7) | 14,965 (43.6) |
| Female | 123 (42.3) | 19,333 (56.4) |
| Length of stay, d | 2.46 2.82 | 4.72 4.74 |
| 30‐day ED re‐encounter rate | 121 (41.6) | |
| 30‐day hospital readmission rate | 88 (30.2) | 4424 (12.9) |
| Prior AMA discharge | 49 (16.8) | |
Although our intent was to conduct a quantitative assessment of discharge interventions, we found stated reasons for leaving similar to those previously reported. In our study, AMA patients tended to be younger, more likely male, and at increased risk for AMA discharge if they had prior AMA discharges (Table 1). The most common reasons found in the medical record for leaving AMA were caring for sick family members, financial concerns, feeling better, and occasionally dissatisfaction with care, reasons similar to those reported in previous studies.[5, 7, 9, 10]
AMA notes were present in 276 (94.8%) charts. AMA notes were written by physicians in 163 (59.1%) and nurses in 110 (37.8%) encounters. The informed consent form was present in 88 (30.2%) charts, mentioned in the note but not present in the electronic medical record in 111 (38.1%), and not signed in 92 (31.6%) charts. Decision‐making capacity and health literacy were documented in 108 (37.1%) and 75 (25.8%) records, respectively. Warning of impending AMA was present in 217 (74.6%) charts. Medications prescribed and follow‐up plans were only documented in 71 (24.4%) and 91 (31.3%) charts, respectively (Table 2).
| Count (%) | |
|---|---|
| |
| AMA note present | 276 (94.8) |
| Primary AMA note author | |
| Physician | 163 (56) |
| Nurse, without physician note | 110 (37.8) |
| Other (ie, social worker) | 3 (1.0) |
| Warning of impending AMA | 217 (74.6) |
| Informed consent signed | |
| Yes | 88 (30.2) |
| No | 92 (31.6) |
| Absenta | 111 (38.1) |
| Documentation of decision‐making capacity | 108 (37.1) |
| Documentation of health literacy | 75 (25.8) |
| Documentation of follow‐up plan | 91 (31.3) |
| Documentation of medications at discharge | 71 (24.4) |
Patients with documentation of medications given did not have decreased 30‐day ED revisits (33.8% vs 44.3%, P = 0.12) or 30‐day hospital readmission (23.9% vs 32.4%, P = 0.18). Similarly, there was no relationship between documentation of follow‐up plans and 30‐day ED revisits (37.4% vs 43.7%, P = 0.31) or 30‐day hospital readmission (29.7% vs 30.7%, P = 0.87). Finally, there was no relationship between physician versus nurse authorship of AMA notes and 30‐day ED revisits (37.4% vs 46.4%, P = 0.14) or 30‐day hospital readmission (28.2% vs 31.8%, P = 0.52) (Table 3).
| Yes, % | No, % | P Value | |
|---|---|---|---|
| |||
| Documentation of discharge medicationsa | |||
| 30‐day ED revisit | 33.8 | 44.3 | 0.12 |
| 30‐day rehospitalization | 23.9 | 32.4 | 0.18 |
| Documentation of follow‐up | |||
| 30‐day ED revisit | 37.4 | 43.7 | 0.31 |
| 30‐day rehospitalization | 29.7 | 30.7 | 0.87 |
| Physician author of AMA note | |||
| 30‐day ED revisit | 37.4 | 46.4 | 0.14 |
| 30‐day rehospitalization | 28.2 | 31.8 | 0.52 |
| Documentation of medications | 36.2 | 10.0 | 0.001 |
| Documentation of follow‐up plan | 43.6 | 16.4 | 0.001 |
| Warning of AMA | |||
| Documentation of medications | 30.4 | 6.8 | 0.001 |
| Documentation of follow‐up plan | 37.3 | 13.5 | 0.001 |
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed (36.2% vs 10.0%, P 0.001) and with an increased finding of documented follow‐up plans (43.6% vs 16.4%, P 0.001). A documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) (Table 3).
DISCUSSION
To gain insights into opportunities for discharge transition interventions in this potentially more vulnerable population,[1, 5] we analyzed AMA documentation and what interventions were carried out at the time of discharge. Our intent was a quantitative assessment of discharge interventions, but we also found stated reasons for leaving AMA that were similar to those previously reported.[5, 6, 10]
We identified several opportunities for improved documentation as well as targeted discharge intervention among AMA patients. Documentation in the charts of AMA patients was often suboptimal. In our study, a physician's AMA note was present only half of the time. Mention of the patient's mental status or health literacy was present in only one‐fourth of cases. Protection from litigation in AMA cases is enhanced when these elements and others, like informed consent, are present in the medical record.[11]
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed and with an increased finding of documented follow‐up plans. This association might be confounded by the fact that physicians can prescribe whereas most nurses cannot. The findings that a documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) suggest opportunities for improvement through early inquiry about potential for AMA as well as early responses when patients threaten to leave AMA.
An important focus of our study was on documentation of discharge medications and follow‐up plans. These elements were documented in 31% and 25% of charts, respectively. A warning of impending AMA was present in 74.6% of encounters, yet medications and follow‐up plans were documented at a much lower rate. This represents an area where caregivers have the possibility to intervene, but are not documenting that they are doing so. We found no relationship between the documentation of giving prescriptions and giving explicit follow‐up plans with decreased rates of return to the ED or readmission, but that possibility may still warrant future prospective study.
Our study did not attempt to explain why only a minority of AMA discharges include medication prescription or follow‐up plans, but a number of potential explanations are possible. Some AMA discharges may occur unannounced with a patient simply walking off the ward giving little or no advance notice. It is also possible that provider perceptions and attitudes toward AMA patients may influence potential interventions.[12] An AMA discharge is against the caregiver's preferred advice for the patient, and it may seem illogical to offer patients second‐best advice. Perhaps some providers have the misconception that medications cannot or must not be prescribed for an AMA discharge. However, second‐best therapy may be better than no therapy, and some follow‐up better than no follow‐up plan.
Given the high rates of ED return and 30‐day readmission, the associated increased healthcare costs as well as increased morbidity and mortality associated with AMA dispositions, a continued search for effective intervention strategies and opportunities is warranted. Recently, programs for transition of care/discharge have demonstrated improved outcomes including reduced rates of readmission with standard discharges.[13] At the time of our study, effective programs such as Project BOOST (Better Outcomes for Older adult through Safe Transitions),[14] the Care Transitions Program,[15] and RED (Project Re‐engineered Discharge)[16] were not yet routinely employed, but their common elements may be applicable to the AMA population. In general, these programs focus on elements we investigated (patient understanding, follow‐up plans, medications prescribed) but add a number of additional components. Additional elements include written discharge instructions, patient education, teach‐back process, decision support, emergency plans, caregiver education, telephone follow‐up, and transition coaches to coordinate home and office follow‐up visits. Most potential interventions add significant time (and cost) to the discharge process. Thus, future studies applying these components to AMA discharges should emphasize timely identification of threatened AMA and prioritized interventions. Future studies should focus on which interventions are the most cost‐effective with AMA patients.
Limitations of our study include not being able to access information from area hospitals not in our hospital network, and thus we may not have identified all ED returns and readmissions. Additionally, interventions at the time of discharge (like prescription of medications or provider assessment of decision‐making capacity) may not have been documented and thus not available for our review. Also, our study was a retrospective review at a single institution and included a relatively small population of patients; consequently, our findings may not apply to other healthcare providers in other hospitals or settings. Our study was strengthened by reviewing all consecutive AMA cases over a 2‐year period encompassing a diverse group of healthcare providers.
CONCLUSION
In the majority of cases reviewed, some advance warning of impending AMA is apparent, affording an opportunity for interventions that may improve health outcomes. Despite this advance warning, only a minority of cases result in key interventions such as prescription of medications or development of follow‐up plans. Medical documentation of AMA dispositions is often inadequate, suggesting missed opportunities for potential intervention as well as suboptimal medicolegal scenarios. Future prospective studies examining cost‐effective interventions at the time of AMA discharge and transition of care may provide valuable insight into lowering rates of ED return and rehospitalization.
Disclosures: The views and opinions expressed in this article are those of the author(s) and do not reflect official policy or position of the United States Air Force, Department of Defense, or US government. The authors report no conflicts of interest.
Approximately 1% to 2% of inpatient stays result in discharges against medical advice (AMA).[1] Though relatively infrequent, AMA discharges warrant attention as they are associated with higher morbidity, increased risk of readmission, and greater 30‐day mortality.[2] A recent study found a 30‐day readmission rate among AMA patients of 24.5%, nearly twice that of matched non‐AMA patients, and a 30‐day mortality rate of 1.3%, also nearly double that of planned discharges.[3] Discharges AMA may be expected to decrease index length of stay, yet accounting for 30‐day readmissions they are estimated to increase costs 56% higher than expected from an initial hospitalization.[4] Patients note several possible reasons for leaving AMA including family emergencies, dissatisfaction with care, financial concerns, or simply feeling better, among others.[5, 6, 7] Risk factors for AMA discharges include previous AMA discharge, having no primary care physician, younger age, lack of insurance, male sex, substance abuse, and lower socioeconomic status.[4, 6, 7, 8]
A number of prior studies have assessed risk factors for AMA discharges, the long‐ and short‐term outcomes, patient reasons for leaving, and physician perceptions of why patients leave AMA.[3, 5, 7, 9] However, there is limited information about opportunities for discharge transition interventions in this potentially more vulnerable population. Because of the increased short‐term and long‐term risks to these patients, treatment and follow‐up plans at the time of discharge may carry even greater importance than follow‐up plans with standard discharges. This study analyzed AMA documentation and what interventions were carried out at the time of discharge.
METHODS
We reviewed the records of all adult patients, ages 18 years and older, admitted to a university‐affiliated tertiary care hospital in Dayton, Ohio (a 520‐bed hospital with approximately 17,000 adult patient encounters per year) over a 2‐year period, and who subsequently left AMA. A hospital database identified 351 adult AMA cases (1.0% of adult admissions). A single reviewer performed an in‐depth review of the 291 patient admissions to the general medical service between January 1, 2009 and December 31, 2010, and manually reviewed and abstracted the data of interest. The Wright State University institutional review board approved the study.
Documentation review focused on the presence of a specified AMA note, the presence of documentation addressing informed consent, patient decision‐making capacity, patient health literacy, follow‐up plans, whether or not medications were prescribed, and whether or not any warning indicators of impending AMA were apparent. These items represented key elements of the discharge policy and procedure in place at our institution during the period of study. We speculated that nurses may be more immediately available at the time of AMA discharge and thus might carry out AMA documentation more often than physicians. To assess this we recorded the role (nurse vs provider) of the writer of AMA notes. We also assessed patient gender, length of stay, prior AMA, 30‐day emergency department (ED) re‐encounters, and 30‐day hospital readmission after AMA discharge.
Informed consent was deemed present if patients signed the hospital's standardized AMA form. Decision‐making capacity was assessed as present if there was specific mention of the patient's capacity on the day of discharge. Any mention of health literacy or the patient's stated understanding of his medical condition at any time during the hospitalization was considered positive documentation of healthcare literacy. Follow‐up plans included any mention of where and when the patient would return. Discharge medications included prescribed medication or indication that no medications were warranted. Warning indicators included specific mention of the patient's desire to leave AMA. For example, patients who left the unit without informing staff were considered to have given no warning of AMA. Alternatively, when documentation was present stating that the patient had verbally expressed a desire to leave AMA, this was considered advanced warning of AMA.
Statistical Analysis
Continuous variables were reported as means and standard deviations. Categorical variables were reported as counts and percents. The independent samples t test was used for comparisons involving 2 groups and a second variable measured on a continuous scale. The 2 test was used to compare 2 categorical variables. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons.
RESULTS
Mean age and gender distribution were similar to those reported in other AMA studies (Table 1).[3] Thirty‐day ED revisit and 30‐day hospital readmission frequencies for medical service patients were 121 (41.6%) and 88 (30.2%), respectively, also similar to those reported in other AMA studies.[3]
| Study Population, Mean SD or Count (%) | Hospital Population, Mean or Count (%) | |
|---|---|---|
| ||
| Age, y | 45.3 15.9 | 62.8 18.2 |
| Sex | ||
| Male | 168 (57.7) | 14,965 (43.6) |
| Female | 123 (42.3) | 19,333 (56.4) |
| Length of stay, d | 2.46 2.82 | 4.72 4.74 |
| 30‐day ED re‐encounter rate | 121 (41.6) | |
| 30‐day hospital readmission rate | 88 (30.2) | 4424 (12.9) |
| Prior AMA discharge | 49 (16.8) | |
Although our intent was to conduct a quantitative assessment of discharge interventions, we found stated reasons for leaving similar to those previously reported. In our study, AMA patients tended to be younger, more likely male, and at increased risk for AMA discharge if they had prior AMA discharges (Table 1). The most common reasons found in the medical record for leaving AMA were caring for sick family members, financial concerns, feeling better, and occasionally dissatisfaction with care, reasons similar to those reported in previous studies.[5, 7, 9, 10]
AMA notes were present in 276 (94.8%) charts. AMA notes were written by physicians in 163 (59.1%) and nurses in 110 (37.8%) encounters. The informed consent form was present in 88 (30.2%) charts, mentioned in the note but not present in the electronic medical record in 111 (38.1%), and not signed in 92 (31.6%) charts. Decision‐making capacity and health literacy were documented in 108 (37.1%) and 75 (25.8%) records, respectively. Warning of impending AMA was present in 217 (74.6%) charts. Medications prescribed and follow‐up plans were only documented in 71 (24.4%) and 91 (31.3%) charts, respectively (Table 2).
| Count (%) | |
|---|---|
| |
| AMA note present | 276 (94.8) |
| Primary AMA note author | |
| Physician | 163 (56) |
| Nurse, without physician note | 110 (37.8) |
| Other (ie, social worker) | 3 (1.0) |
| Warning of impending AMA | 217 (74.6) |
| Informed consent signed | |
| Yes | 88 (30.2) |
| No | 92 (31.6) |
| Absenta | 111 (38.1) |
| Documentation of decision‐making capacity | 108 (37.1) |
| Documentation of health literacy | 75 (25.8) |
| Documentation of follow‐up plan | 91 (31.3) |
| Documentation of medications at discharge | 71 (24.4) |
Patients with documentation of medications given did not have decreased 30‐day ED revisits (33.8% vs 44.3%, P = 0.12) or 30‐day hospital readmission (23.9% vs 32.4%, P = 0.18). Similarly, there was no relationship between documentation of follow‐up plans and 30‐day ED revisits (37.4% vs 43.7%, P = 0.31) or 30‐day hospital readmission (29.7% vs 30.7%, P = 0.87). Finally, there was no relationship between physician versus nurse authorship of AMA notes and 30‐day ED revisits (37.4% vs 46.4%, P = 0.14) or 30‐day hospital readmission (28.2% vs 31.8%, P = 0.52) (Table 3).
| Yes, % | No, % | P Value | |
|---|---|---|---|
| |||
| Documentation of discharge medicationsa | |||
| 30‐day ED revisit | 33.8 | 44.3 | 0.12 |
| 30‐day rehospitalization | 23.9 | 32.4 | 0.18 |
| Documentation of follow‐up | |||
| 30‐day ED revisit | 37.4 | 43.7 | 0.31 |
| 30‐day rehospitalization | 29.7 | 30.7 | 0.87 |
| Physician author of AMA note | |||
| 30‐day ED revisit | 37.4 | 46.4 | 0.14 |
| 30‐day rehospitalization | 28.2 | 31.8 | 0.52 |
| Documentation of medications | 36.2 | 10.0 | 0.001 |
| Documentation of follow‐up plan | 43.6 | 16.4 | 0.001 |
| Warning of AMA | |||
| Documentation of medications | 30.4 | 6.8 | 0.001 |
| Documentation of follow‐up plan | 37.3 | 13.5 | 0.001 |
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed (36.2% vs 10.0%, P 0.001) and with an increased finding of documented follow‐up plans (43.6% vs 16.4%, P 0.001). A documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) (Table 3).
DISCUSSION
To gain insights into opportunities for discharge transition interventions in this potentially more vulnerable population,[1, 5] we analyzed AMA documentation and what interventions were carried out at the time of discharge. Our intent was a quantitative assessment of discharge interventions, but we also found stated reasons for leaving AMA that were similar to those previously reported.[5, 6, 10]
We identified several opportunities for improved documentation as well as targeted discharge intervention among AMA patients. Documentation in the charts of AMA patients was often suboptimal. In our study, a physician's AMA note was present only half of the time. Mention of the patient's mental status or health literacy was present in only one‐fourth of cases. Protection from litigation in AMA cases is enhanced when these elements and others, like informed consent, are present in the medical record.[11]
Physician documentation of the AMA was associated with an increased frequency of discharge medication being prescribed and with an increased finding of documented follow‐up plans. This association might be confounded by the fact that physicians can prescribe whereas most nurses cannot. The findings that a documented warning of impending AMA was associated with an increased frequency of discharge medication being prescribed (30.4% vs 6.8%, P 0.001) and increased frequency of follow‐up plans being documented (37.3% vs 13.5%, P 0.001) suggest opportunities for improvement through early inquiry about potential for AMA as well as early responses when patients threaten to leave AMA.
An important focus of our study was on documentation of discharge medications and follow‐up plans. These elements were documented in 31% and 25% of charts, respectively. A warning of impending AMA was present in 74.6% of encounters, yet medications and follow‐up plans were documented at a much lower rate. This represents an area where caregivers have the possibility to intervene, but are not documenting that they are doing so. We found no relationship between the documentation of giving prescriptions and giving explicit follow‐up plans with decreased rates of return to the ED or readmission, but that possibility may still warrant future prospective study.
Our study did not attempt to explain why only a minority of AMA discharges include medication prescription or follow‐up plans, but a number of potential explanations are possible. Some AMA discharges may occur unannounced with a patient simply walking off the ward giving little or no advance notice. It is also possible that provider perceptions and attitudes toward AMA patients may influence potential interventions.[12] An AMA discharge is against the caregiver's preferred advice for the patient, and it may seem illogical to offer patients second‐best advice. Perhaps some providers have the misconception that medications cannot or must not be prescribed for an AMA discharge. However, second‐best therapy may be better than no therapy, and some follow‐up better than no follow‐up plan.
Given the high rates of ED return and 30‐day readmission, the associated increased healthcare costs as well as increased morbidity and mortality associated with AMA dispositions, a continued search for effective intervention strategies and opportunities is warranted. Recently, programs for transition of care/discharge have demonstrated improved outcomes including reduced rates of readmission with standard discharges.[13] At the time of our study, effective programs such as Project BOOST (Better Outcomes for Older adult through Safe Transitions),[14] the Care Transitions Program,[15] and RED (Project Re‐engineered Discharge)[16] were not yet routinely employed, but their common elements may be applicable to the AMA population. In general, these programs focus on elements we investigated (patient understanding, follow‐up plans, medications prescribed) but add a number of additional components. Additional elements include written discharge instructions, patient education, teach‐back process, decision support, emergency plans, caregiver education, telephone follow‐up, and transition coaches to coordinate home and office follow‐up visits. Most potential interventions add significant time (and cost) to the discharge process. Thus, future studies applying these components to AMA discharges should emphasize timely identification of threatened AMA and prioritized interventions. Future studies should focus on which interventions are the most cost‐effective with AMA patients.
Limitations of our study include not being able to access information from area hospitals not in our hospital network, and thus we may not have identified all ED returns and readmissions. Additionally, interventions at the time of discharge (like prescription of medications or provider assessment of decision‐making capacity) may not have been documented and thus not available for our review. Also, our study was a retrospective review at a single institution and included a relatively small population of patients; consequently, our findings may not apply to other healthcare providers in other hospitals or settings. Our study was strengthened by reviewing all consecutive AMA cases over a 2‐year period encompassing a diverse group of healthcare providers.
CONCLUSION
In the majority of cases reviewed, some advance warning of impending AMA is apparent, affording an opportunity for interventions that may improve health outcomes. Despite this advance warning, only a minority of cases result in key interventions such as prescription of medications or development of follow‐up plans. Medical documentation of AMA dispositions is often inadequate, suggesting missed opportunities for potential intervention as well as suboptimal medicolegal scenarios. Future prospective studies examining cost‐effective interventions at the time of AMA discharge and transition of care may provide valuable insight into lowering rates of ED return and rehospitalization.
Disclosures: The views and opinions expressed in this article are those of the author(s) and do not reflect official policy or position of the United States Air Force, Department of Defense, or US government. The authors report no conflicts of interest.