User login
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung
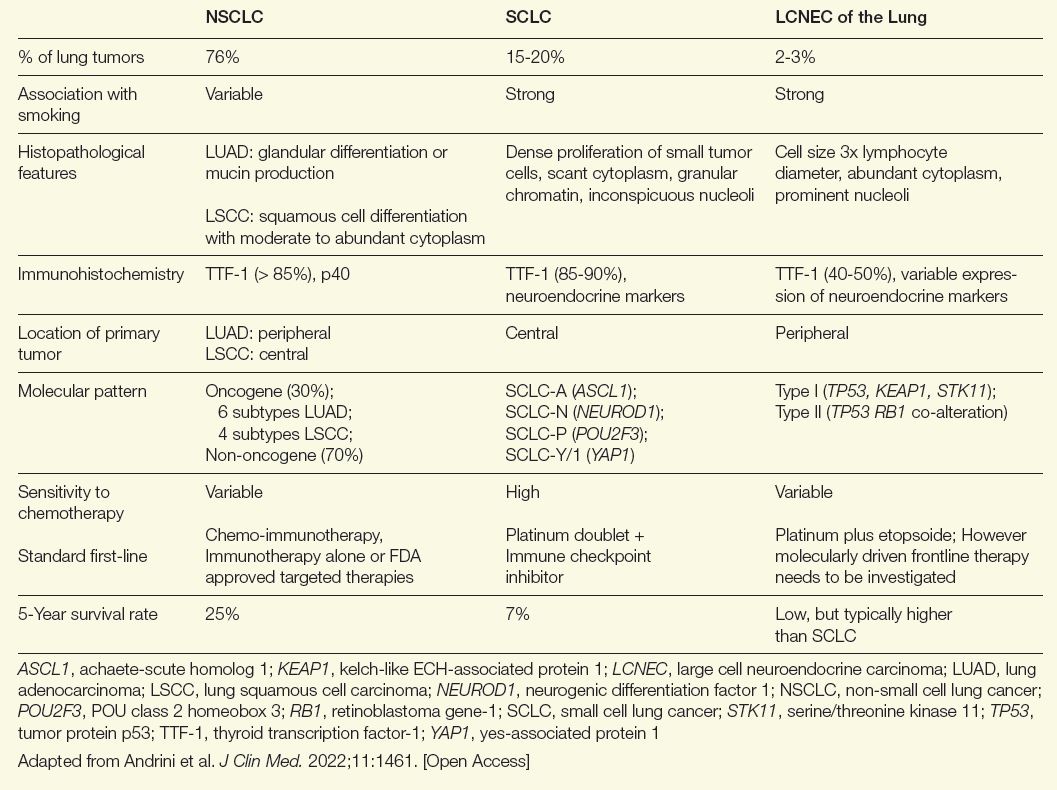
Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung

Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung

Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung

