User login
Detecting and managing device leads inadvertently placed in the left ventricle
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
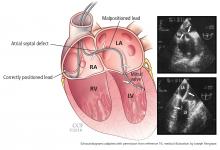
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
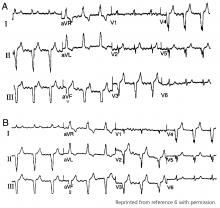
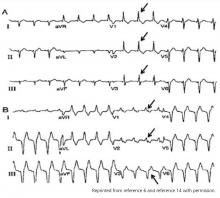
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
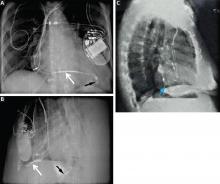
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
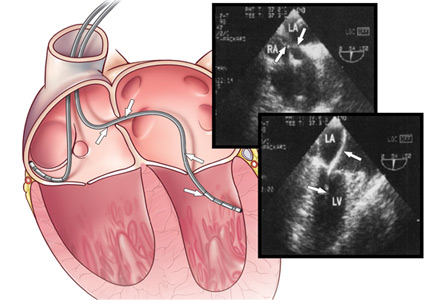
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
KEY POINTS
- During device implantation, fluoroscopy in progressively lateral left anterior oblique views should be used to ensure correct lead position.
- After implantation, malposition can almost always be detected promptly by examining a 12-lead electrocardiogram for the paced QRS morphology and by lateral chest radiography.
- Echocardiography and computed tomography may enhance diagnostic accuracy and clarify equivocal findings.
- Late surgical correction of a malpositioned lead is best done when a patient is undergoing cardiac surgery for other reasons.
- Long-term warfarin therapy is recommended to prevent thromboembolism if malpositioning cannot be corrected.