User login
Osteofibrous Dysplasia–like Adamantinoma of the Tibia in a 15-Year-Old Girl
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
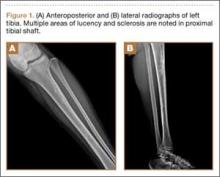
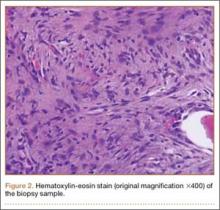
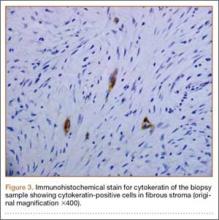
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.