User login
Improving Functional Outcomes in Patients with Intermittent Claudication
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
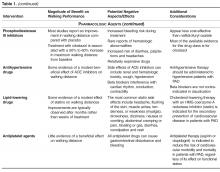
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.