User login
Defining disease activity and damage in patients with small-vessel vasculitis
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).
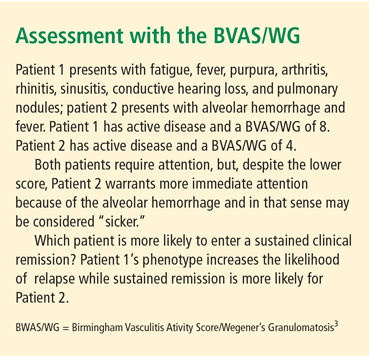
Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).

Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).

Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
