User login
Oozing puncture wound on foot
A 49-year-old, unkempt-looking Indian man came into our emergency department in Singapore complaining of increasing right foot swelling and worsening pain that he’d had for a month after having stepped on a fish bone while walking on the beach. Our patient pulled the bone out himself and did not seek immediate medical attention.
Our patient said that he had occasional fever with chills and rigors. His medical history was unremarkable, and there was no history of previous injury or surgery to the right foot. He worked as an “oiler” refueling ships, and said that he did not drink alcohol or smoke.
He was dehydrated, and there were no other sources of infection or sores on his body. Our patient was febrile (38.3°C [100.9°F]), his blood pressure was 112/74 mm Hg, and he was tachycardic at 117 beats per minute.
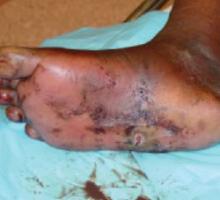
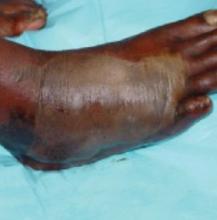
His right foot was boggy, swollen, and tender with significant crepitus. Brownish discharge was oozing from the sole of his foot (FIGURE 1). There were large infected subcutaneous bullae on the dorsum of his foot (FIGURE 2). We could feel a popliteal pulse in both legs, but could not feel a right dorsalis pedis pulse.
What is your diagnosis?
FIGURE 1
Swollen, dusky foot
FIGURE 2
Dark bullae
Diagnosis: Necrotizing fasciitis
Necrotizing fasciitis infections are characterized by fulminant destruction of tissue, systemic signs of toxicity, and high rates of mortality. The incidence of necrotizing fasciitis in adults is 0.40 cases per 100,000 population, while the incidence in children is 0.08 cases per 100,000 population. Mortality rates as high as 73% have been reported.1 Early clinical suspicion, early surgical intervention (surgical debridement), and systemic antibiotics are of the utmost importance.
Necrotizing fasciitis is caused by gram positive, gram negative, and/or anaerobic bacteria. Local tissue hypoxia from trauma, surgery, or a medically compromised state creates an ideal opportunistic environment for bacterial proliferation.
Necrotizing fasciitis can be broadly classified into 2 main types:
Type 1 necrotizing fasciitis is a polymicrobial infection caused by facultative bacteria along with anaerobes. Polymicrobial infections with anaerobes are common (up to 74%) in infected puncture wounds in patients with diabetes.2
Type 2 necrotizing fasciitis is caused by group A streptococci alone, but sometimes in association with Staphylococcus aureus. Vibrio vulnificus infection has also been reported in fish bone piercing injuries leading to necrotizing fasciitis.3
Typical early signs and symptoms include severe pain, rapidly progressing erythema, dusky or purplish skin discoloration, and systemic signs of septic toxicity such as fever, tachycardia, a generalized unwell feeling, and even hypotension. (A lack of classic tissue inflammatory signs may mask an ongoing necrotizing fasciitis beneath the skin.) The involved region may become numb due to the necrosis of the innervating nerve fibers. Discharge or crepitus may also be noted.
Late clinical signs of necrotizing fasciitis include cellulitis, skin discoloration, discharge of “dishwater” fluid, blistering, and hemorrhagic bullae. Findings of crepitus and soft tissue air on plain radiographs are seen in 37% and 57% of patients, respectively.4 Our patient’s X-ray findings revealed extensive gas pockets within soft tissue and osteomyelitis changes of the 5th metatarsal head.
The differential diagnosis for necrotizing fasciitis includes:
- pyogenic soft tissue cellulitis
- clostridial cellulitis (which may also present with soft tissue crepitus)
- nonclostridial anaerobic cellulitis
- acute febrile neutrophilic dermatosis
- acute hemorrhagic edema of infancy
- erythema induratum (nodular vasculitis).
Diagnosis is often made on clinical evaluation
The definitive diagnosis of necrotizing fasciitis is made by histological examination of the debrided specimen or deep skin tissue biopsy. Fascial necrosis with thrombosed blood vessels and a dense infiltrate of inflammatory cells is seen on histological evaluation.
However, diagnosis is often reached on clinical evaluation. Rapidly deteriorating local signs and symptoms together with systemic toxicity should prompt a working diagnosis of necrotizing fasciitis.
Laboratory tests (white blood cell count, blood urea nitrogen level, sodium levels, creatinine levels, erythrocyte sedimentation rates, C-reactive protein levels) and radiographic evaluation (X-rays, computed tomography [CT], and magnetic resonance imaging [MRI]) are useful adjuncts in reaching the diagnosis.
Prompt treatment is the name of the game
Antibiotic therapy is guided by gram stain and bacterial culture results. (When the clinical suspicion of necrotizing fasciitis is reached, empirical antibiotics should be started right away.) Broad-spectrum antibiotics covering gram positive, gram negative, and anaerobic bacteria should be used. The patient’s age, weight, and liver and renal function should also be considered before starting antibiotics.
Choices of antibiotics include penicillin for gram positive cover and an aminoglycoside or third-generation cephalosporin for gram negative counteraction. Metronidazole (Flagyl) may be considered for anaerobic superimposed infections. Adjunctive clindamycin is also recommended—especially in group A streptococcal infections—because it suppresses toxin production, inhibits M-protein synthesis, and facilitates phagocytosis.5 In addition, hyperbaric oxygen therapy and intravenous immunoglobulin are increasingly being utilized in the management of necrotizing fasciitis.6 Their efficacy has yet to be conclusively established.
Surgical debridement. The importance of prompt and thorough surgical debridement cannot be stressed enough. Large soft-tissue defects created by surgery can be treated with vacuum-assisted closure dressings, local or free soft-tissue flaps, and/or skin grafts. In extreme cases, limb amputation may be necessary.
Aggressive steps were needed for our patient
In the emergency department, we treated our patient with intravenous cloxacillin, gentamicin, and metronidazole. He later underwent surgical debridement and had pus drained from deep abscesses in his foot. Intraoperative findings indicated that there was necrotic tissue involving 80% of the dorsum of the foot and the necrosis extended proximally to the ankle and distally to the toes.
Wound cultures grew group B Streptococcus (Peptostreptococcus species) and Bacteroides. He was treated with intravenous amoxicillin and clavulanic acid (IV Augmentin, available in Singapore) and oral metronidazole. An endocrinologist evaluated him and determined that he had diabetes. He was started on insulin.
Postoperatively, our patient remained septic. Five days later, he underwent a below-the-knee amputation; the surgeons noted that his foot was gangrenous.
Our patient stayed in the hospital for 7 more days and was discharged about 2 weeks after his arrival at the ER.
Correspondence
Ramesh Subramaniam, MBBS, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074
1. Trent JT, Kirsner RS. Necrotizing fasciitis. Wounds. 2002;14:284-292.
2. Lavery LA, Harkless LB, Felder-Johnson K, et al. Bacterial pathogens in infected puncture wounds in adults with diabetes. J Foot Ankle Surg. 1994;33:91-97.
3. Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect. 2005;133:383-391.
4. Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672-683.
5. Stevens DL. Streptococcal toxic shock syndrome: spectrum of disease, pathogenesis and new concepts in treatment. Emerg Infect Dis. 1995;1:69.
6. Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther. 2005;3:279-294.
A 49-year-old, unkempt-looking Indian man came into our emergency department in Singapore complaining of increasing right foot swelling and worsening pain that he’d had for a month after having stepped on a fish bone while walking on the beach. Our patient pulled the bone out himself and did not seek immediate medical attention.
Our patient said that he had occasional fever with chills and rigors. His medical history was unremarkable, and there was no history of previous injury or surgery to the right foot. He worked as an “oiler” refueling ships, and said that he did not drink alcohol or smoke.
He was dehydrated, and there were no other sources of infection or sores on his body. Our patient was febrile (38.3°C [100.9°F]), his blood pressure was 112/74 mm Hg, and he was tachycardic at 117 beats per minute.
His right foot was boggy, swollen, and tender with significant crepitus. Brownish discharge was oozing from the sole of his foot (FIGURE 1). There were large infected subcutaneous bullae on the dorsum of his foot (FIGURE 2). We could feel a popliteal pulse in both legs, but could not feel a right dorsalis pedis pulse.
What is your diagnosis?
FIGURE 1
Swollen, dusky foot
FIGURE 2
Dark bullae
Diagnosis: Necrotizing fasciitis
Necrotizing fasciitis infections are characterized by fulminant destruction of tissue, systemic signs of toxicity, and high rates of mortality. The incidence of necrotizing fasciitis in adults is 0.40 cases per 100,000 population, while the incidence in children is 0.08 cases per 100,000 population. Mortality rates as high as 73% have been reported.1 Early clinical suspicion, early surgical intervention (surgical debridement), and systemic antibiotics are of the utmost importance.
Necrotizing fasciitis is caused by gram positive, gram negative, and/or anaerobic bacteria. Local tissue hypoxia from trauma, surgery, or a medically compromised state creates an ideal opportunistic environment for bacterial proliferation.
Necrotizing fasciitis can be broadly classified into 2 main types:
Type 1 necrotizing fasciitis is a polymicrobial infection caused by facultative bacteria along with anaerobes. Polymicrobial infections with anaerobes are common (up to 74%) in infected puncture wounds in patients with diabetes.2
Type 2 necrotizing fasciitis is caused by group A streptococci alone, but sometimes in association with Staphylococcus aureus. Vibrio vulnificus infection has also been reported in fish bone piercing injuries leading to necrotizing fasciitis.3
Typical early signs and symptoms include severe pain, rapidly progressing erythema, dusky or purplish skin discoloration, and systemic signs of septic toxicity such as fever, tachycardia, a generalized unwell feeling, and even hypotension. (A lack of classic tissue inflammatory signs may mask an ongoing necrotizing fasciitis beneath the skin.) The involved region may become numb due to the necrosis of the innervating nerve fibers. Discharge or crepitus may also be noted.
Late clinical signs of necrotizing fasciitis include cellulitis, skin discoloration, discharge of “dishwater” fluid, blistering, and hemorrhagic bullae. Findings of crepitus and soft tissue air on plain radiographs are seen in 37% and 57% of patients, respectively.4 Our patient’s X-ray findings revealed extensive gas pockets within soft tissue and osteomyelitis changes of the 5th metatarsal head.
The differential diagnosis for necrotizing fasciitis includes:
- pyogenic soft tissue cellulitis
- clostridial cellulitis (which may also present with soft tissue crepitus)
- nonclostridial anaerobic cellulitis
- acute febrile neutrophilic dermatosis
- acute hemorrhagic edema of infancy
- erythema induratum (nodular vasculitis).
Diagnosis is often made on clinical evaluation
The definitive diagnosis of necrotizing fasciitis is made by histological examination of the debrided specimen or deep skin tissue biopsy. Fascial necrosis with thrombosed blood vessels and a dense infiltrate of inflammatory cells is seen on histological evaluation.
However, diagnosis is often reached on clinical evaluation. Rapidly deteriorating local signs and symptoms together with systemic toxicity should prompt a working diagnosis of necrotizing fasciitis.
Laboratory tests (white blood cell count, blood urea nitrogen level, sodium levels, creatinine levels, erythrocyte sedimentation rates, C-reactive protein levels) and radiographic evaluation (X-rays, computed tomography [CT], and magnetic resonance imaging [MRI]) are useful adjuncts in reaching the diagnosis.
Prompt treatment is the name of the game
Antibiotic therapy is guided by gram stain and bacterial culture results. (When the clinical suspicion of necrotizing fasciitis is reached, empirical antibiotics should be started right away.) Broad-spectrum antibiotics covering gram positive, gram negative, and anaerobic bacteria should be used. The patient’s age, weight, and liver and renal function should also be considered before starting antibiotics.
Choices of antibiotics include penicillin for gram positive cover and an aminoglycoside or third-generation cephalosporin for gram negative counteraction. Metronidazole (Flagyl) may be considered for anaerobic superimposed infections. Adjunctive clindamycin is also recommended—especially in group A streptococcal infections—because it suppresses toxin production, inhibits M-protein synthesis, and facilitates phagocytosis.5 In addition, hyperbaric oxygen therapy and intravenous immunoglobulin are increasingly being utilized in the management of necrotizing fasciitis.6 Their efficacy has yet to be conclusively established.
Surgical debridement. The importance of prompt and thorough surgical debridement cannot be stressed enough. Large soft-tissue defects created by surgery can be treated with vacuum-assisted closure dressings, local or free soft-tissue flaps, and/or skin grafts. In extreme cases, limb amputation may be necessary.
Aggressive steps were needed for our patient
In the emergency department, we treated our patient with intravenous cloxacillin, gentamicin, and metronidazole. He later underwent surgical debridement and had pus drained from deep abscesses in his foot. Intraoperative findings indicated that there was necrotic tissue involving 80% of the dorsum of the foot and the necrosis extended proximally to the ankle and distally to the toes.
Wound cultures grew group B Streptococcus (Peptostreptococcus species) and Bacteroides. He was treated with intravenous amoxicillin and clavulanic acid (IV Augmentin, available in Singapore) and oral metronidazole. An endocrinologist evaluated him and determined that he had diabetes. He was started on insulin.
Postoperatively, our patient remained septic. Five days later, he underwent a below-the-knee amputation; the surgeons noted that his foot was gangrenous.
Our patient stayed in the hospital for 7 more days and was discharged about 2 weeks after his arrival at the ER.
Correspondence
Ramesh Subramaniam, MBBS, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074
A 49-year-old, unkempt-looking Indian man came into our emergency department in Singapore complaining of increasing right foot swelling and worsening pain that he’d had for a month after having stepped on a fish bone while walking on the beach. Our patient pulled the bone out himself and did not seek immediate medical attention.
Our patient said that he had occasional fever with chills and rigors. His medical history was unremarkable, and there was no history of previous injury or surgery to the right foot. He worked as an “oiler” refueling ships, and said that he did not drink alcohol or smoke.
He was dehydrated, and there were no other sources of infection or sores on his body. Our patient was febrile (38.3°C [100.9°F]), his blood pressure was 112/74 mm Hg, and he was tachycardic at 117 beats per minute.
His right foot was boggy, swollen, and tender with significant crepitus. Brownish discharge was oozing from the sole of his foot (FIGURE 1). There were large infected subcutaneous bullae on the dorsum of his foot (FIGURE 2). We could feel a popliteal pulse in both legs, but could not feel a right dorsalis pedis pulse.
What is your diagnosis?
FIGURE 1
Swollen, dusky foot
FIGURE 2
Dark bullae
Diagnosis: Necrotizing fasciitis
Necrotizing fasciitis infections are characterized by fulminant destruction of tissue, systemic signs of toxicity, and high rates of mortality. The incidence of necrotizing fasciitis in adults is 0.40 cases per 100,000 population, while the incidence in children is 0.08 cases per 100,000 population. Mortality rates as high as 73% have been reported.1 Early clinical suspicion, early surgical intervention (surgical debridement), and systemic antibiotics are of the utmost importance.
Necrotizing fasciitis is caused by gram positive, gram negative, and/or anaerobic bacteria. Local tissue hypoxia from trauma, surgery, or a medically compromised state creates an ideal opportunistic environment for bacterial proliferation.
Necrotizing fasciitis can be broadly classified into 2 main types:
Type 1 necrotizing fasciitis is a polymicrobial infection caused by facultative bacteria along with anaerobes. Polymicrobial infections with anaerobes are common (up to 74%) in infected puncture wounds in patients with diabetes.2
Type 2 necrotizing fasciitis is caused by group A streptococci alone, but sometimes in association with Staphylococcus aureus. Vibrio vulnificus infection has also been reported in fish bone piercing injuries leading to necrotizing fasciitis.3
Typical early signs and symptoms include severe pain, rapidly progressing erythema, dusky or purplish skin discoloration, and systemic signs of septic toxicity such as fever, tachycardia, a generalized unwell feeling, and even hypotension. (A lack of classic tissue inflammatory signs may mask an ongoing necrotizing fasciitis beneath the skin.) The involved region may become numb due to the necrosis of the innervating nerve fibers. Discharge or crepitus may also be noted.
Late clinical signs of necrotizing fasciitis include cellulitis, skin discoloration, discharge of “dishwater” fluid, blistering, and hemorrhagic bullae. Findings of crepitus and soft tissue air on plain radiographs are seen in 37% and 57% of patients, respectively.4 Our patient’s X-ray findings revealed extensive gas pockets within soft tissue and osteomyelitis changes of the 5th metatarsal head.
The differential diagnosis for necrotizing fasciitis includes:
- pyogenic soft tissue cellulitis
- clostridial cellulitis (which may also present with soft tissue crepitus)
- nonclostridial anaerobic cellulitis
- acute febrile neutrophilic dermatosis
- acute hemorrhagic edema of infancy
- erythema induratum (nodular vasculitis).
Diagnosis is often made on clinical evaluation
The definitive diagnosis of necrotizing fasciitis is made by histological examination of the debrided specimen or deep skin tissue biopsy. Fascial necrosis with thrombosed blood vessels and a dense infiltrate of inflammatory cells is seen on histological evaluation.
However, diagnosis is often reached on clinical evaluation. Rapidly deteriorating local signs and symptoms together with systemic toxicity should prompt a working diagnosis of necrotizing fasciitis.
Laboratory tests (white blood cell count, blood urea nitrogen level, sodium levels, creatinine levels, erythrocyte sedimentation rates, C-reactive protein levels) and radiographic evaluation (X-rays, computed tomography [CT], and magnetic resonance imaging [MRI]) are useful adjuncts in reaching the diagnosis.
Prompt treatment is the name of the game
Antibiotic therapy is guided by gram stain and bacterial culture results. (When the clinical suspicion of necrotizing fasciitis is reached, empirical antibiotics should be started right away.) Broad-spectrum antibiotics covering gram positive, gram negative, and anaerobic bacteria should be used. The patient’s age, weight, and liver and renal function should also be considered before starting antibiotics.
Choices of antibiotics include penicillin for gram positive cover and an aminoglycoside or third-generation cephalosporin for gram negative counteraction. Metronidazole (Flagyl) may be considered for anaerobic superimposed infections. Adjunctive clindamycin is also recommended—especially in group A streptococcal infections—because it suppresses toxin production, inhibits M-protein synthesis, and facilitates phagocytosis.5 In addition, hyperbaric oxygen therapy and intravenous immunoglobulin are increasingly being utilized in the management of necrotizing fasciitis.6 Their efficacy has yet to be conclusively established.
Surgical debridement. The importance of prompt and thorough surgical debridement cannot be stressed enough. Large soft-tissue defects created by surgery can be treated with vacuum-assisted closure dressings, local or free soft-tissue flaps, and/or skin grafts. In extreme cases, limb amputation may be necessary.
Aggressive steps were needed for our patient
In the emergency department, we treated our patient with intravenous cloxacillin, gentamicin, and metronidazole. He later underwent surgical debridement and had pus drained from deep abscesses in his foot. Intraoperative findings indicated that there was necrotic tissue involving 80% of the dorsum of the foot and the necrosis extended proximally to the ankle and distally to the toes.
Wound cultures grew group B Streptococcus (Peptostreptococcus species) and Bacteroides. He was treated with intravenous amoxicillin and clavulanic acid (IV Augmentin, available in Singapore) and oral metronidazole. An endocrinologist evaluated him and determined that he had diabetes. He was started on insulin.
Postoperatively, our patient remained septic. Five days later, he underwent a below-the-knee amputation; the surgeons noted that his foot was gangrenous.
Our patient stayed in the hospital for 7 more days and was discharged about 2 weeks after his arrival at the ER.
Correspondence
Ramesh Subramaniam, MBBS, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074
1. Trent JT, Kirsner RS. Necrotizing fasciitis. Wounds. 2002;14:284-292.
2. Lavery LA, Harkless LB, Felder-Johnson K, et al. Bacterial pathogens in infected puncture wounds in adults with diabetes. J Foot Ankle Surg. 1994;33:91-97.
3. Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect. 2005;133:383-391.
4. Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672-683.
5. Stevens DL. Streptococcal toxic shock syndrome: spectrum of disease, pathogenesis and new concepts in treatment. Emerg Infect Dis. 1995;1:69.
6. Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther. 2005;3:279-294.
1. Trent JT, Kirsner RS. Necrotizing fasciitis. Wounds. 2002;14:284-292.
2. Lavery LA, Harkless LB, Felder-Johnson K, et al. Bacterial pathogens in infected puncture wounds in adults with diabetes. J Foot Ankle Surg. 1994;33:91-97.
3. Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect. 2005;133:383-391.
4. Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672-683.
5. Stevens DL. Streptococcal toxic shock syndrome: spectrum of disease, pathogenesis and new concepts in treatment. Emerg Infect Dis. 1995;1:69.
6. Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther. 2005;3:279-294.