User login
Nonopioid Alternatives to Addressing Pain Intensity: A Retrospective Look at 2 Noninvasive Pain Treatment Devices
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
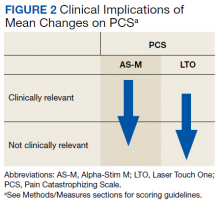
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
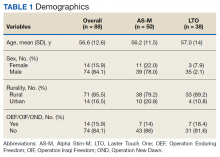
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
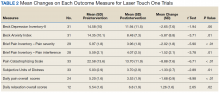
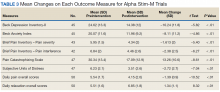
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
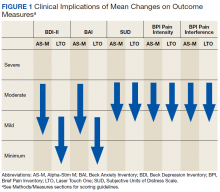
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.