User login
Sulindac-erlotinib as chemoprevention for FAP
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited disorder caused by germline mutations in the APC (adenomatous polyposis coli) gene. The disease is characterized by the formation of hundreds to thousands of adenomatous polyps in the colorectum and a nearly 100% lifetime risk of colorectal cancer, if left untreated.
Six randomized clinical trials explore chemoprevention in FAP, including the use of sulindac, celecoxib, low-dose aspirin, eicosapentaenoic acid, and one ongoing trial using sulindac and difluoromethylornithine. These trials have shown at most a 30% decrease in colorectal adenoma burden over short-term treatment, compared with placebo.
Preclinical studies have shown that inactivation of the APC gene and epidermal growth factor receptor (EGFR) signaling promotes cyclooxygenase (COX)-2 expression and subsequent development of intestinal neoplasia. Our group has previously shown that the combination of COX and EGFR inhibition with sulindac and erlotinib led to a 71% reduction in duodenal polyp burden in patients with FAP.
The hypothesis of this study was that a combination of COX and EGFR inhibition would inhibit colorectal adenoma formation in patient with FAP. We designed a phase 2 double-blind, placebo-controlled randomized trial in FAP patients who received combination therapy with 150 mg sulindac b.i.d. and 75 mg erlotinib daily, or placebo tablets for 6 months and assessed the number of polyps in the colorectum, rectum, or ileal pouch at baseline and at 6 months.
The total colorectal polyp count was significantly different between the placebo and sulindac-erlotinib groups at 6 months in FAP patients with an intact colon (P less than .0001), with a net percentage change of 89.3% between the two groups. Similar reductions were found in the ileal pouch anal anastomosis and ileorectal anastomosis groups.
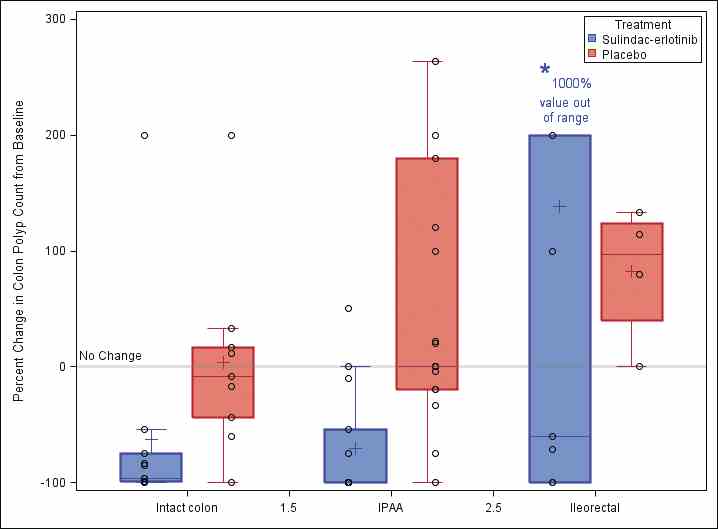
Further research is necessary to evaluate these preliminary findings in a larger study population with longer follow-up to determine whether the observed effects will result in improved long-term clinical outcomes. A new phase 2 multicenter clinical trial (sponsored by the National Cancer Institute) of erlotinib therapy in patients with FAP will be activating this summer across seven U.S. cancer centers and will add further evidence for this chemoprevention strategy.
Dr. Samadder is in the division of gastroenterology and hepatology, Mayo Clinic, Scottsdale, Ariz. His comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited disorder caused by germline mutations in the APC (adenomatous polyposis coli) gene. The disease is characterized by the formation of hundreds to thousands of adenomatous polyps in the colorectum and a nearly 100% lifetime risk of colorectal cancer, if left untreated.
Six randomized clinical trials explore chemoprevention in FAP, including the use of sulindac, celecoxib, low-dose aspirin, eicosapentaenoic acid, and one ongoing trial using sulindac and difluoromethylornithine. These trials have shown at most a 30% decrease in colorectal adenoma burden over short-term treatment, compared with placebo.
Preclinical studies have shown that inactivation of the APC gene and epidermal growth factor receptor (EGFR) signaling promotes cyclooxygenase (COX)-2 expression and subsequent development of intestinal neoplasia. Our group has previously shown that the combination of COX and EGFR inhibition with sulindac and erlotinib led to a 71% reduction in duodenal polyp burden in patients with FAP.
The hypothesis of this study was that a combination of COX and EGFR inhibition would inhibit colorectal adenoma formation in patient with FAP. We designed a phase 2 double-blind, placebo-controlled randomized trial in FAP patients who received combination therapy with 150 mg sulindac b.i.d. and 75 mg erlotinib daily, or placebo tablets for 6 months and assessed the number of polyps in the colorectum, rectum, or ileal pouch at baseline and at 6 months.
The total colorectal polyp count was significantly different between the placebo and sulindac-erlotinib groups at 6 months in FAP patients with an intact colon (P less than .0001), with a net percentage change of 89.3% between the two groups. Similar reductions were found in the ileal pouch anal anastomosis and ileorectal anastomosis groups.

Further research is necessary to evaluate these preliminary findings in a larger study population with longer follow-up to determine whether the observed effects will result in improved long-term clinical outcomes. A new phase 2 multicenter clinical trial (sponsored by the National Cancer Institute) of erlotinib therapy in patients with FAP will be activating this summer across seven U.S. cancer centers and will add further evidence for this chemoprevention strategy.
Dr. Samadder is in the division of gastroenterology and hepatology, Mayo Clinic, Scottsdale, Ariz. His comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited disorder caused by germline mutations in the APC (adenomatous polyposis coli) gene. The disease is characterized by the formation of hundreds to thousands of adenomatous polyps in the colorectum and a nearly 100% lifetime risk of colorectal cancer, if left untreated.
Six randomized clinical trials explore chemoprevention in FAP, including the use of sulindac, celecoxib, low-dose aspirin, eicosapentaenoic acid, and one ongoing trial using sulindac and difluoromethylornithine. These trials have shown at most a 30% decrease in colorectal adenoma burden over short-term treatment, compared with placebo.
Preclinical studies have shown that inactivation of the APC gene and epidermal growth factor receptor (EGFR) signaling promotes cyclooxygenase (COX)-2 expression and subsequent development of intestinal neoplasia. Our group has previously shown that the combination of COX and EGFR inhibition with sulindac and erlotinib led to a 71% reduction in duodenal polyp burden in patients with FAP.
The hypothesis of this study was that a combination of COX and EGFR inhibition would inhibit colorectal adenoma formation in patient with FAP. We designed a phase 2 double-blind, placebo-controlled randomized trial in FAP patients who received combination therapy with 150 mg sulindac b.i.d. and 75 mg erlotinib daily, or placebo tablets for 6 months and assessed the number of polyps in the colorectum, rectum, or ileal pouch at baseline and at 6 months.
The total colorectal polyp count was significantly different between the placebo and sulindac-erlotinib groups at 6 months in FAP patients with an intact colon (P less than .0001), with a net percentage change of 89.3% between the two groups. Similar reductions were found in the ileal pouch anal anastomosis and ileorectal anastomosis groups.

Further research is necessary to evaluate these preliminary findings in a larger study population with longer follow-up to determine whether the observed effects will result in improved long-term clinical outcomes. A new phase 2 multicenter clinical trial (sponsored by the National Cancer Institute) of erlotinib therapy in patients with FAP will be activating this summer across seven U.S. cancer centers and will add further evidence for this chemoprevention strategy.
Dr. Samadder is in the division of gastroenterology and hepatology, Mayo Clinic, Scottsdale, Ariz. His comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.

