User login
Aortic Dissection
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
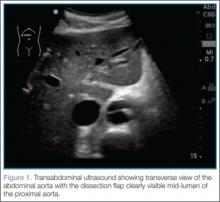
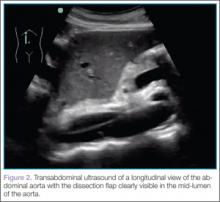
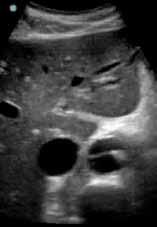
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
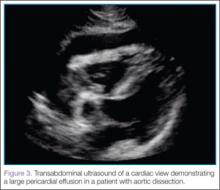
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
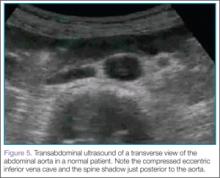
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
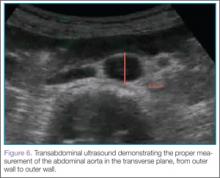
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
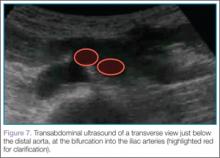
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
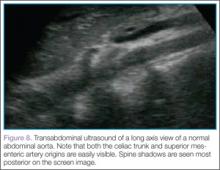
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
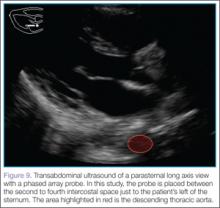
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.