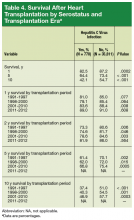
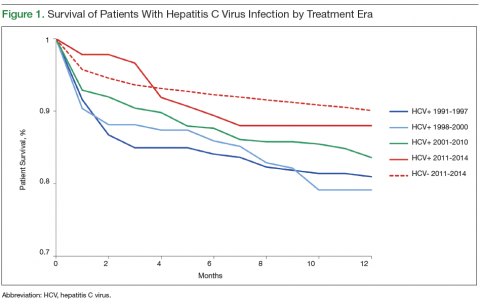
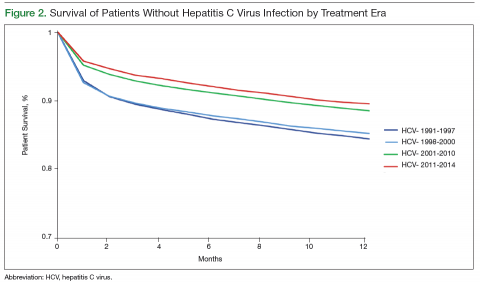
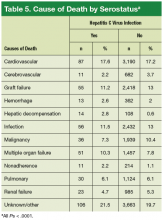
User login
Expanded approval for daratumumab in multiple myeloma
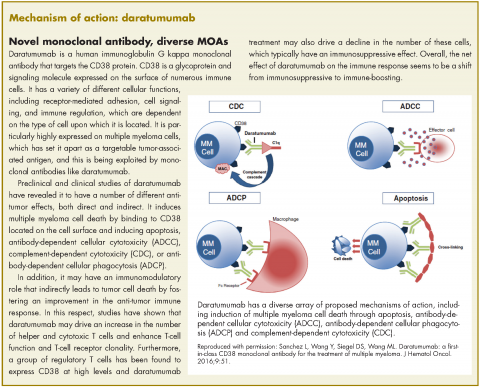
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
Multifaceted pharmacist intervention may reduce postdischarge ED visits and readmissions
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Balanced fluid resuscitation vs. saline does not decrease hospital-free days
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Ketamine Plus Memantine-Based Multimodality Treatment of Chronic Refractory Migraine
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Myeloproliferative neoplasms increase risk for arterial and venous thrombosis
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Steroids do not reduce mortality in patients with septic shock
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Prompting during rounds decreases lab utilization in patients nearing discharge
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Strategies to Improve Hepatocellular Carcinoma Surveillance in Veterans With Hepatitis B Infection (FULL)
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
Sharpen your ax
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Heart Transplantation Outcomes in Patients With Hepatitis C Virus Infection: Potential Impact of Newer Antiviral Treatments After Transplantation (FULL)
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.