User login
Homans Lecture: Celebrating the past and looking to the future
NATIONAL HARBOR, MD – “Specialties are like species,” said Frank J. Veith, MD, “they must evolve or go extinct.”
Dr. Veith of the New York University Langone Medical Center made this comparison in his 2016 Homans Lecture on the topic of “The future of vascular surgery,” at this year’s annual meeting hosted by the Society for Vascular Surgery.
Dr. Veith reviewed the history of vascular surgery, touched on its present status, and speculated on its potentially bright future. The vascular specialty has evolved dramatically over the past decades, especially in the area of embracing the endovascular revolution, said Dr. Veith, with that revolution putting vascular surgery at the forefront of research to develop new techniques.
His witnessing such innovations as those developed by Dr. Juan Parodi, and being a part of the early history of endovascular surgery, convinced Dr. Veith of its long-term importance to the development and survival of the specialty.
In his 1996 SVS Presidential Address, he predicted that 40%-70% of the open operations being done then would be replaced by endovascular procedures. “Accordingly, to survive, I recommended that vascular surgeons become endocompetent, learn how to do these procedures, and embrace them.” Dr. Veith added that, although his recommendation was not greeted with open arms by everyone, endovascular techniques moved forward.
In fact, “vascular surgeons often lead in developing many evolving endovascular procedures that are currently the standard of care,” he said.
Dr. Veith pointed out that a wide variety of conditions are now amenable to endovascular treatment, although some, including carotid disease, remain controversial. He listed examples of those conditions that he felt were still best treated with open surgery: thoracic outlet and entrapment syndromes, some ascending aorta and arch lesions, a few rare aneurysms not suited for endovascular treatment, some Takayasu’s lesions, some congenital and genetic aortic and renal artery lesions, some infected arteries and arterial grafts, a rare recurrent or complex lower-extremity lesion, some carotid lesions, and some failed endovascular treatments.
“Our specialty has embraced the endovascular revolution and become endocompetent,” he said. “It is why vascular surgery is doing as well as it is today.” He added. “Vascular surgery is presently an exciting, vibrant specialty in the United States.”
Dr. Veith noted, “Well-trained vascular surgeons are the only ones who can provide the most appropriate, full spectrum of care for patients with vascular disease, outside the head and the heart – whether that treatment be medical, endovascular, or open. There are abundant numbers of patients who require our skills. In addition, we use fascinating technology and have good industry relationships. And finally, many patients regard their vascular surgeon as a key doctor who they see regularly. As a result of these advantages, many bright medical students and general surgery residents are choosing to train as vascular surgeons. Vascular surgery should be flourishing.”
However, despite the fact vascular surgery is an exciting and vibrant specialty, and the best for treating vascular disease outside of the heart and the brain, the vascular specialty has significant problems competing with other specialties, he said.
He blamed in part the size and structure of the specialty, in particular with regard to its competition.
“Vascular surgery competes, as it always has, with general and cardiac surgeons. However, general surgeons have become less competitive, but cardiac surgeons have become more in need of work, and thus more active beyond the heart and thoracic aorta – as their open operations are replaced by coronary stents and transcatheter valves. More importantly, as vascular treatments become increasingly endovascular, vascular surgery will be competing with interventional radiology and, importantly, interventional cardiology.”
He outlined a number of major challenges these other disciplines create, in part, because of the DRG/RVU/dollar orientation of institutions, and the fact that most institutions still consider vascular surgery a subspecialty of general or cardiac surgery, or a subordinate part of a Heart & Vascular Center, with administrative control of these centers rarely in the hands of vascular surgeons. Moreover, when institutional resources – like angiography suites or hybrid operating rooms are distributed, the interests of vascular surgery are often represented by a general or cardiac surgeon – or worse a cardiologist,” he added.
He stated that these conditions limit vascular surgery’s ability to get its fair share of institutional resources.
“The competitive playing field is not level, and vascular surgeons are disadvantaged in the Darwinian struggle to survive,” he stated.
“To survive, vascular surgery needs to unify, recognize this inequity, and fix it. This can only be done if all vascular surgeons engage vigorously in this issue. We need equal administrative status with cardiac and general surgery in our institutions,” Dr. Veith advised.
In discussing the technological future, Dr. Veith said that by 2026, 75%-95% of all vascular cases requiring more than medical therapy will be treated endovascularly, with perhaps 5% in a hybrid fashion (open plus endovascular), and between 5% and 15% being treated fully by open surgery. This shift away from open surgery is and will continue to cause challenges in training and patient access to open treatment.
He asked the question: How should vascular surgery deal with the decreasing numbers of complex open procedures and who should do them?
“One solution is to have centers to which these patients are sent and in which vascular surgeons seeking this skill can get adequate open training,” he answered.
But the technological future he painted was bright. Not only was the future likely to be filled with new advances in medical therapy, but he also highlighted computer-assisted 3-D–device navigational tools to aid endovascular treatment; advances in robotic guidance to decrease radiation exposure and facilitate device placement; computer-enhanced simulation to improve training and, when patient specific, to allow procedure planning and rehearsal; and even 3-D printed modeling of lesions and blood vessels.
He predicted that the endovascular problems of intimal hyperplasia will be overcome by antiproliferative drugs in all vascular beds – once the best way of getting the best drug to the proper location is found – and that computer-enabled remote monitoring of flows within grafts and stents, perhaps using miniaturized piezoelectric sensors, will allow corrective treatment before occlusion occurs.
Dr. Veith stated that, in his view, to take its proper place, vascular surgery should rise above its subspecialty status in the shadow of general surgery and in its competition with cardiology.
This “will help vascular surgery to flourish and be recognized as the main specialty devoted to patients with noncardiac vascular diseases. Vascular surgery can then fulfill its potential for a brighter future. More importantly, patients and society will be the ultimate beneficiaries,” he concluded.
Dr. Veith reported that he had no conflicts to disclose with regard to his remarks.
On Twitter @VascularTweets
NATIONAL HARBOR, MD – “Specialties are like species,” said Frank J. Veith, MD, “they must evolve or go extinct.”
Dr. Veith of the New York University Langone Medical Center made this comparison in his 2016 Homans Lecture on the topic of “The future of vascular surgery,” at this year’s annual meeting hosted by the Society for Vascular Surgery.
Dr. Veith reviewed the history of vascular surgery, touched on its present status, and speculated on its potentially bright future. The vascular specialty has evolved dramatically over the past decades, especially in the area of embracing the endovascular revolution, said Dr. Veith, with that revolution putting vascular surgery at the forefront of research to develop new techniques.
His witnessing such innovations as those developed by Dr. Juan Parodi, and being a part of the early history of endovascular surgery, convinced Dr. Veith of its long-term importance to the development and survival of the specialty.
In his 1996 SVS Presidential Address, he predicted that 40%-70% of the open operations being done then would be replaced by endovascular procedures. “Accordingly, to survive, I recommended that vascular surgeons become endocompetent, learn how to do these procedures, and embrace them.” Dr. Veith added that, although his recommendation was not greeted with open arms by everyone, endovascular techniques moved forward.
In fact, “vascular surgeons often lead in developing many evolving endovascular procedures that are currently the standard of care,” he said.
Dr. Veith pointed out that a wide variety of conditions are now amenable to endovascular treatment, although some, including carotid disease, remain controversial. He listed examples of those conditions that he felt were still best treated with open surgery: thoracic outlet and entrapment syndromes, some ascending aorta and arch lesions, a few rare aneurysms not suited for endovascular treatment, some Takayasu’s lesions, some congenital and genetic aortic and renal artery lesions, some infected arteries and arterial grafts, a rare recurrent or complex lower-extremity lesion, some carotid lesions, and some failed endovascular treatments.
“Our specialty has embraced the endovascular revolution and become endocompetent,” he said. “It is why vascular surgery is doing as well as it is today.” He added. “Vascular surgery is presently an exciting, vibrant specialty in the United States.”
Dr. Veith noted, “Well-trained vascular surgeons are the only ones who can provide the most appropriate, full spectrum of care for patients with vascular disease, outside the head and the heart – whether that treatment be medical, endovascular, or open. There are abundant numbers of patients who require our skills. In addition, we use fascinating technology and have good industry relationships. And finally, many patients regard their vascular surgeon as a key doctor who they see regularly. As a result of these advantages, many bright medical students and general surgery residents are choosing to train as vascular surgeons. Vascular surgery should be flourishing.”
However, despite the fact vascular surgery is an exciting and vibrant specialty, and the best for treating vascular disease outside of the heart and the brain, the vascular specialty has significant problems competing with other specialties, he said.
He blamed in part the size and structure of the specialty, in particular with regard to its competition.
“Vascular surgery competes, as it always has, with general and cardiac surgeons. However, general surgeons have become less competitive, but cardiac surgeons have become more in need of work, and thus more active beyond the heart and thoracic aorta – as their open operations are replaced by coronary stents and transcatheter valves. More importantly, as vascular treatments become increasingly endovascular, vascular surgery will be competing with interventional radiology and, importantly, interventional cardiology.”
He outlined a number of major challenges these other disciplines create, in part, because of the DRG/RVU/dollar orientation of institutions, and the fact that most institutions still consider vascular surgery a subspecialty of general or cardiac surgery, or a subordinate part of a Heart & Vascular Center, with administrative control of these centers rarely in the hands of vascular surgeons. Moreover, when institutional resources – like angiography suites or hybrid operating rooms are distributed, the interests of vascular surgery are often represented by a general or cardiac surgeon – or worse a cardiologist,” he added.
He stated that these conditions limit vascular surgery’s ability to get its fair share of institutional resources.
“The competitive playing field is not level, and vascular surgeons are disadvantaged in the Darwinian struggle to survive,” he stated.
“To survive, vascular surgery needs to unify, recognize this inequity, and fix it. This can only be done if all vascular surgeons engage vigorously in this issue. We need equal administrative status with cardiac and general surgery in our institutions,” Dr. Veith advised.
In discussing the technological future, Dr. Veith said that by 2026, 75%-95% of all vascular cases requiring more than medical therapy will be treated endovascularly, with perhaps 5% in a hybrid fashion (open plus endovascular), and between 5% and 15% being treated fully by open surgery. This shift away from open surgery is and will continue to cause challenges in training and patient access to open treatment.
He asked the question: How should vascular surgery deal with the decreasing numbers of complex open procedures and who should do them?
“One solution is to have centers to which these patients are sent and in which vascular surgeons seeking this skill can get adequate open training,” he answered.
But the technological future he painted was bright. Not only was the future likely to be filled with new advances in medical therapy, but he also highlighted computer-assisted 3-D–device navigational tools to aid endovascular treatment; advances in robotic guidance to decrease radiation exposure and facilitate device placement; computer-enhanced simulation to improve training and, when patient specific, to allow procedure planning and rehearsal; and even 3-D printed modeling of lesions and blood vessels.
He predicted that the endovascular problems of intimal hyperplasia will be overcome by antiproliferative drugs in all vascular beds – once the best way of getting the best drug to the proper location is found – and that computer-enabled remote monitoring of flows within grafts and stents, perhaps using miniaturized piezoelectric sensors, will allow corrective treatment before occlusion occurs.
Dr. Veith stated that, in his view, to take its proper place, vascular surgery should rise above its subspecialty status in the shadow of general surgery and in its competition with cardiology.
This “will help vascular surgery to flourish and be recognized as the main specialty devoted to patients with noncardiac vascular diseases. Vascular surgery can then fulfill its potential for a brighter future. More importantly, patients and society will be the ultimate beneficiaries,” he concluded.
Dr. Veith reported that he had no conflicts to disclose with regard to his remarks.
On Twitter @VascularTweets
NATIONAL HARBOR, MD – “Specialties are like species,” said Frank J. Veith, MD, “they must evolve or go extinct.”
Dr. Veith of the New York University Langone Medical Center made this comparison in his 2016 Homans Lecture on the topic of “The future of vascular surgery,” at this year’s annual meeting hosted by the Society for Vascular Surgery.
Dr. Veith reviewed the history of vascular surgery, touched on its present status, and speculated on its potentially bright future. The vascular specialty has evolved dramatically over the past decades, especially in the area of embracing the endovascular revolution, said Dr. Veith, with that revolution putting vascular surgery at the forefront of research to develop new techniques.
His witnessing such innovations as those developed by Dr. Juan Parodi, and being a part of the early history of endovascular surgery, convinced Dr. Veith of its long-term importance to the development and survival of the specialty.
In his 1996 SVS Presidential Address, he predicted that 40%-70% of the open operations being done then would be replaced by endovascular procedures. “Accordingly, to survive, I recommended that vascular surgeons become endocompetent, learn how to do these procedures, and embrace them.” Dr. Veith added that, although his recommendation was not greeted with open arms by everyone, endovascular techniques moved forward.
In fact, “vascular surgeons often lead in developing many evolving endovascular procedures that are currently the standard of care,” he said.
Dr. Veith pointed out that a wide variety of conditions are now amenable to endovascular treatment, although some, including carotid disease, remain controversial. He listed examples of those conditions that he felt were still best treated with open surgery: thoracic outlet and entrapment syndromes, some ascending aorta and arch lesions, a few rare aneurysms not suited for endovascular treatment, some Takayasu’s lesions, some congenital and genetic aortic and renal artery lesions, some infected arteries and arterial grafts, a rare recurrent or complex lower-extremity lesion, some carotid lesions, and some failed endovascular treatments.
“Our specialty has embraced the endovascular revolution and become endocompetent,” he said. “It is why vascular surgery is doing as well as it is today.” He added. “Vascular surgery is presently an exciting, vibrant specialty in the United States.”
Dr. Veith noted, “Well-trained vascular surgeons are the only ones who can provide the most appropriate, full spectrum of care for patients with vascular disease, outside the head and the heart – whether that treatment be medical, endovascular, or open. There are abundant numbers of patients who require our skills. In addition, we use fascinating technology and have good industry relationships. And finally, many patients regard their vascular surgeon as a key doctor who they see regularly. As a result of these advantages, many bright medical students and general surgery residents are choosing to train as vascular surgeons. Vascular surgery should be flourishing.”
However, despite the fact vascular surgery is an exciting and vibrant specialty, and the best for treating vascular disease outside of the heart and the brain, the vascular specialty has significant problems competing with other specialties, he said.
He blamed in part the size and structure of the specialty, in particular with regard to its competition.
“Vascular surgery competes, as it always has, with general and cardiac surgeons. However, general surgeons have become less competitive, but cardiac surgeons have become more in need of work, and thus more active beyond the heart and thoracic aorta – as their open operations are replaced by coronary stents and transcatheter valves. More importantly, as vascular treatments become increasingly endovascular, vascular surgery will be competing with interventional radiology and, importantly, interventional cardiology.”
He outlined a number of major challenges these other disciplines create, in part, because of the DRG/RVU/dollar orientation of institutions, and the fact that most institutions still consider vascular surgery a subspecialty of general or cardiac surgery, or a subordinate part of a Heart & Vascular Center, with administrative control of these centers rarely in the hands of vascular surgeons. Moreover, when institutional resources – like angiography suites or hybrid operating rooms are distributed, the interests of vascular surgery are often represented by a general or cardiac surgeon – or worse a cardiologist,” he added.
He stated that these conditions limit vascular surgery’s ability to get its fair share of institutional resources.
“The competitive playing field is not level, and vascular surgeons are disadvantaged in the Darwinian struggle to survive,” he stated.
“To survive, vascular surgery needs to unify, recognize this inequity, and fix it. This can only be done if all vascular surgeons engage vigorously in this issue. We need equal administrative status with cardiac and general surgery in our institutions,” Dr. Veith advised.
In discussing the technological future, Dr. Veith said that by 2026, 75%-95% of all vascular cases requiring more than medical therapy will be treated endovascularly, with perhaps 5% in a hybrid fashion (open plus endovascular), and between 5% and 15% being treated fully by open surgery. This shift away from open surgery is and will continue to cause challenges in training and patient access to open treatment.
He asked the question: How should vascular surgery deal with the decreasing numbers of complex open procedures and who should do them?
“One solution is to have centers to which these patients are sent and in which vascular surgeons seeking this skill can get adequate open training,” he answered.
But the technological future he painted was bright. Not only was the future likely to be filled with new advances in medical therapy, but he also highlighted computer-assisted 3-D–device navigational tools to aid endovascular treatment; advances in robotic guidance to decrease radiation exposure and facilitate device placement; computer-enhanced simulation to improve training and, when patient specific, to allow procedure planning and rehearsal; and even 3-D printed modeling of lesions and blood vessels.
He predicted that the endovascular problems of intimal hyperplasia will be overcome by antiproliferative drugs in all vascular beds – once the best way of getting the best drug to the proper location is found – and that computer-enabled remote monitoring of flows within grafts and stents, perhaps using miniaturized piezoelectric sensors, will allow corrective treatment before occlusion occurs.
Dr. Veith stated that, in his view, to take its proper place, vascular surgery should rise above its subspecialty status in the shadow of general surgery and in its competition with cardiology.
This “will help vascular surgery to flourish and be recognized as the main specialty devoted to patients with noncardiac vascular diseases. Vascular surgery can then fulfill its potential for a brighter future. More importantly, patients and society will be the ultimate beneficiaries,” he concluded.
Dr. Veith reported that he had no conflicts to disclose with regard to his remarks.
On Twitter @VascularTweets
AT THE 2016 VASCULAR ANNUAL MEETING
NSQIP Study: Symptomatic AAAs have a twofold increased periop mortality risk over asymptomatic
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Outcomes for repair of abdominal aortic aneurysm repair were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
Major finding: Patients with symptomatic AAA had a twofold increased risk of perioperative mortality, compared with patients with asymptomatic AAA undergoing repair.
Data source: The study assessed all patients undergoing AAA repair in the 2011-2013 American College of Surgeons NSQIP data set.
Disclosures: The authors reported that they had no relevant disclosures.
Abdominal compartment syndrome – a common adverse event after rAAA repair
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Abdominal compartment syndrome is a dangerous and frequent complication after treatment of ruptured abdominal aortic aneurysms.
Major finding: After ruptured AAA repair, ACS developed in 6.8% of patients with open repair and 6.9% of patients with EVAR.
Data source: Researchers performed a population-based study using the Swedish vascular registry and the Swedish national population registry.
Disclosures: The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
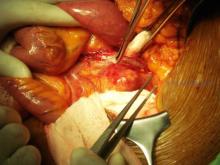
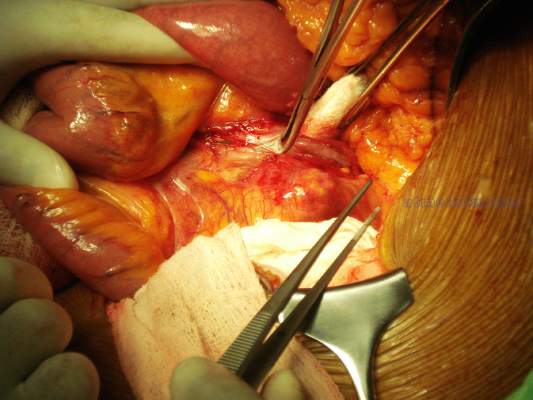
Subtotal fenestrating cholecystectomy: Optimal ‘bailout’ for difficult cases
Subtotal fenestrating cholecystectomy with drain placement appears optimal, compared with the reconstituting procedure, for experienced surgeons seeking a “bailout” operation in both open and minimally invasive cholecystectomy where the critical view of safety (CVS) is not easily attainable, according to a report written on behalf of the SAGES Safe Cholecystectomy Task Force 2015.
The rise in laparoscopic cholecystectomy has been associated with an increase in the rate of bile duct injury, most commonly when secure ductal identification using CVS is not possible because of an inflamed hepatocystic triangle occluding the cystic duct, cystic artery, and cystic plate. In such cases, a safe and effective bailout technique (one not requiring a second operation) must be decided upon in preference to simply closing and proceeding to a later open procedure, according to Steven M. Strasberg, MD, of Washington University in Saint Louis and his colleagues (J Am Coll Surg. 2016;222:89-96).
In order to clarify the two most common and effective “partial cholecystectomy” procedures being performed, Dr. Strasburg and his colleagues have suggested the use of the term “subtotal” in place of “partial” and the terms “fenestrating” vs. “reconstituting,” to define whether there is an open or closed gallbladder remnant, respectively, after the procedure.
In subtotal fenestrating cholecystectomy, the free peritonealized portion of the gallbladder is excised, except for a tip at the lowest portion that acts as a shield to protect against inadvertently entering the hepatocystic triangle, according to the authors. There is no sealed lumen remaining, thus the cystic duct requires closure. The cystic duct may be closed from the inside with a purse-string suture. Attempts to ligate the cystic duct outside the gallbladder may injure the common bile duct and can potentially result in fistulas.
In subtotal reconstituting cholecystectomy, the free peritonealized portion of the gallbladder is excised, but the lowest portion of the gallbladder is closed with sutures or staples and reconstitutes an intact lumen in which stones may be re-formed, which can in turn require reoperation.
“Whether the subtotal cholecystectomy is ‘fenestrating’ or ‘reconstituting’ depends on whether the lowest part of the gallbladder is left open (fenestrating) or closed (reconstituting) and not on the amount of gallbladder that is left attached to the liver,” according to the authors.
Subtotal fenestrating cholecystectomy is most likely done when an open approach is used, whereas subtotal reconstituting cholecystectomies are probably easier to do laparoscopically and are preferred by surgeons doing minimally invasive procedures, they said.
Despite the fact that there have been no head-to-head comparisons of fenestrating vs. reconstituting techniques, the authors said they prefer the fenestrating method, although the technique chosen may be based on the experience of the surgeon, they noted.
“The principle is that a subtotal fenestrating cholecystectomy is a standard operation that should be used liberally when surgeons encounter difficulty getting to the CVS,” the authors wrote. “We believe that clarification of the procedures and what they are called will help to choose which type of procedure to select, and it will also facilitate the performance of clinical studies in this area,“ they concluded.
The authors reported having no relevant financial disclosures.
A transcript of an interactive discussion of this paper and topic is available online (www.journalacs.org/RAS-ACS-discussion-2016).
Subtotal fenestrating cholecystectomy with drain placement appears optimal, compared with the reconstituting procedure, for experienced surgeons seeking a “bailout” operation in both open and minimally invasive cholecystectomy where the critical view of safety (CVS) is not easily attainable, according to a report written on behalf of the SAGES Safe Cholecystectomy Task Force 2015.
The rise in laparoscopic cholecystectomy has been associated with an increase in the rate of bile duct injury, most commonly when secure ductal identification using CVS is not possible because of an inflamed hepatocystic triangle occluding the cystic duct, cystic artery, and cystic plate. In such cases, a safe and effective bailout technique (one not requiring a second operation) must be decided upon in preference to simply closing and proceeding to a later open procedure, according to Steven M. Strasberg, MD, of Washington University in Saint Louis and his colleagues (J Am Coll Surg. 2016;222:89-96).
In order to clarify the two most common and effective “partial cholecystectomy” procedures being performed, Dr. Strasburg and his colleagues have suggested the use of the term “subtotal” in place of “partial” and the terms “fenestrating” vs. “reconstituting,” to define whether there is an open or closed gallbladder remnant, respectively, after the procedure.
In subtotal fenestrating cholecystectomy, the free peritonealized portion of the gallbladder is excised, except for a tip at the lowest portion that acts as a shield to protect against inadvertently entering the hepatocystic triangle, according to the authors. There is no sealed lumen remaining, thus the cystic duct requires closure. The cystic duct may be closed from the inside with a purse-string suture. Attempts to ligate the cystic duct outside the gallbladder may injure the common bile duct and can potentially result in fistulas.
In subtotal reconstituting cholecystectomy, the free peritonealized portion of the gallbladder is excised, but the lowest portion of the gallbladder is closed with sutures or staples and reconstitutes an intact lumen in which stones may be re-formed, which can in turn require reoperation.
“Whether the subtotal cholecystectomy is ‘fenestrating’ or ‘reconstituting’ depends on whether the lowest part of the gallbladder is left open (fenestrating) or closed (reconstituting) and not on the amount of gallbladder that is left attached to the liver,” according to the authors.
Subtotal fenestrating cholecystectomy is most likely done when an open approach is used, whereas subtotal reconstituting cholecystectomies are probably easier to do laparoscopically and are preferred by surgeons doing minimally invasive procedures, they said.
Despite the fact that there have been no head-to-head comparisons of fenestrating vs. reconstituting techniques, the authors said they prefer the fenestrating method, although the technique chosen may be based on the experience of the surgeon, they noted.
“The principle is that a subtotal fenestrating cholecystectomy is a standard operation that should be used liberally when surgeons encounter difficulty getting to the CVS,” the authors wrote. “We believe that clarification of the procedures and what they are called will help to choose which type of procedure to select, and it will also facilitate the performance of clinical studies in this area,“ they concluded.
The authors reported having no relevant financial disclosures.
A transcript of an interactive discussion of this paper and topic is available online (www.journalacs.org/RAS-ACS-discussion-2016).
Subtotal fenestrating cholecystectomy with drain placement appears optimal, compared with the reconstituting procedure, for experienced surgeons seeking a “bailout” operation in both open and minimally invasive cholecystectomy where the critical view of safety (CVS) is not easily attainable, according to a report written on behalf of the SAGES Safe Cholecystectomy Task Force 2015.
The rise in laparoscopic cholecystectomy has been associated with an increase in the rate of bile duct injury, most commonly when secure ductal identification using CVS is not possible because of an inflamed hepatocystic triangle occluding the cystic duct, cystic artery, and cystic plate. In such cases, a safe and effective bailout technique (one not requiring a second operation) must be decided upon in preference to simply closing and proceeding to a later open procedure, according to Steven M. Strasberg, MD, of Washington University in Saint Louis and his colleagues (J Am Coll Surg. 2016;222:89-96).
In order to clarify the two most common and effective “partial cholecystectomy” procedures being performed, Dr. Strasburg and his colleagues have suggested the use of the term “subtotal” in place of “partial” and the terms “fenestrating” vs. “reconstituting,” to define whether there is an open or closed gallbladder remnant, respectively, after the procedure.
In subtotal fenestrating cholecystectomy, the free peritonealized portion of the gallbladder is excised, except for a tip at the lowest portion that acts as a shield to protect against inadvertently entering the hepatocystic triangle, according to the authors. There is no sealed lumen remaining, thus the cystic duct requires closure. The cystic duct may be closed from the inside with a purse-string suture. Attempts to ligate the cystic duct outside the gallbladder may injure the common bile duct and can potentially result in fistulas.
In subtotal reconstituting cholecystectomy, the free peritonealized portion of the gallbladder is excised, but the lowest portion of the gallbladder is closed with sutures or staples and reconstitutes an intact lumen in which stones may be re-formed, which can in turn require reoperation.
“Whether the subtotal cholecystectomy is ‘fenestrating’ or ‘reconstituting’ depends on whether the lowest part of the gallbladder is left open (fenestrating) or closed (reconstituting) and not on the amount of gallbladder that is left attached to the liver,” according to the authors.
Subtotal fenestrating cholecystectomy is most likely done when an open approach is used, whereas subtotal reconstituting cholecystectomies are probably easier to do laparoscopically and are preferred by surgeons doing minimally invasive procedures, they said.
Despite the fact that there have been no head-to-head comparisons of fenestrating vs. reconstituting techniques, the authors said they prefer the fenestrating method, although the technique chosen may be based on the experience of the surgeon, they noted.
“The principle is that a subtotal fenestrating cholecystectomy is a standard operation that should be used liberally when surgeons encounter difficulty getting to the CVS,” the authors wrote. “We believe that clarification of the procedures and what they are called will help to choose which type of procedure to select, and it will also facilitate the performance of clinical studies in this area,“ they concluded.
The authors reported having no relevant financial disclosures.
A transcript of an interactive discussion of this paper and topic is available online (www.journalacs.org/RAS-ACS-discussion-2016).
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Subtotal fenestrating cholecystectomy should be used liberally when surgeons have difficulty getting to the critical view of safety (CVS).
Major finding: Subtotal fenestrating cholecystectomy with drain placement, despite its difficulty in laparoscopic cases, should be the procedure of choice for experienced surgeons in both open and minimally invasive procedures where the CVS is not safely attainable.
Data source: An expert analysis of historical data and the literature to determine optimal surgical technique, on behalf of the SAGES Safe Cholecystectomy Task Force 2015.
Disclosures: The authors reported having no relevant financial disclosures.
Anatomic repair of ccTGA did not yield superior survival
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Performing an anatomic repair for ccTGA in patients without associated lesions could be counterproductive.
Major finding: There was no significant difference in transplant-free survival at 20 years among anatomic repair (58%), physiologic repair (71%), and single-ventricle repair (78%).
Data source: A single-institution study assessing 200 patients with ccGTA/Fontan who were managed from 1982 to 2015.
Disclosures: Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
Wedge resection showed improved survival over SBRT for early-stage NSCLC
BALTIMORE – Wedge resection was associated with significantly improved overall 5-year survival, compared with stereotactic body radiation therapy (SBRT) in patients with operable clinical Stage IA non–small cell lung cancer, according to a study of more than 8,000 patients.
Despite the fact that surgical resection has been the standard of care for early-stage non–small cell lung cancer (NSCLC), an increasing number of patients with potentially operable early-stage NSCLC are now being treated with SBRT, study investigator Dr. Babatunde A. Yerokun said at the annual meeting of the American Association for Thoracic Surgery.
“Our data show that thoracic surgeons should be included in the evaluation of these patients, and operative candidates with ct1A NO MO NSCLC should continue to receive a wedge resection vs. SBRT when technically feasible,” said Dr. Yerokun of Duke University, Durham, N.C. “Prospective studies are needed to determine the appropriate role of SBRT in management of these patients,” he concluded.
Dr. Yerokun and his colleagues examined overall survival of patients with cT1N0 lung cancer who underwent SBRT or wedge resection as reported in the National Cancer Data Base from 2003 to 2011. Survival was assessed using Kaplan-Meier and propensity-score matched analysis. The researchers matched groups according to common prognostic covariates, including age, sex, race, education, insurance status, facility type, and Charlson/Deyo comorbidity score, as well as tumor histology, size, and location.
Patients identified as having cT1N0 NSCLC with a tumor less than 2 cm underwent SBRT (1,514 patients) or wedge resection (6,923). Compared with the wedge resection cohort, the SBRT patients were significantly older (74 vs. 69 years) and significantly more likely to be treated at an academic comprehensive cancer program (47% vs. 37%). The median Charlson/Deyo score was lower in the SBRT patients (0 vs. 1).
In unmatched analysis, SBRT was associated with significantly lower survival than wedge resection (5-year overall survival: 32% vs. 55%). In the propensity matching, all baseline covariates, including co-morbidity scores, facility type, and tumor size, were well balanced between the SBRT and wedge groups, with 1,398 patients in each group.
However, even in the matched groups, SBRT was associated with significantly lower 5-year overall survival than wedge (33% vs. 52%). When the investigators performed a propensity matched subgroup analysis in younger patients (age less than 60 years) who had a Charlson/Deyo score of 0, SBRT was associated with worse survival with a 5-year overall survival of 59% vs. 82% for SBRT and wedge resection, respectively.
Additionally, Dr. Yerokun and his colleagues conducted a sensitivity analysis comparing centers that performed predominately wedge resection with centers that performed predominately SBRT. After the exclusion of centers with low-volume and centers that conducted either 100% wedge resection or 100% SBRT only, centers that performed predominately wedge resection were more likely to have significantly better 3-year survival.
A video of the original presentation from the AATS Annual Meeting is available online.
Dr. Yerokun reported that he had no disclosures related to this presentation.
On Twitter @ThoracicTweets
BALTIMORE – Wedge resection was associated with significantly improved overall 5-year survival, compared with stereotactic body radiation therapy (SBRT) in patients with operable clinical Stage IA non–small cell lung cancer, according to a study of more than 8,000 patients.
Despite the fact that surgical resection has been the standard of care for early-stage non–small cell lung cancer (NSCLC), an increasing number of patients with potentially operable early-stage NSCLC are now being treated with SBRT, study investigator Dr. Babatunde A. Yerokun said at the annual meeting of the American Association for Thoracic Surgery.
“Our data show that thoracic surgeons should be included in the evaluation of these patients, and operative candidates with ct1A NO MO NSCLC should continue to receive a wedge resection vs. SBRT when technically feasible,” said Dr. Yerokun of Duke University, Durham, N.C. “Prospective studies are needed to determine the appropriate role of SBRT in management of these patients,” he concluded.
Dr. Yerokun and his colleagues examined overall survival of patients with cT1N0 lung cancer who underwent SBRT or wedge resection as reported in the National Cancer Data Base from 2003 to 2011. Survival was assessed using Kaplan-Meier and propensity-score matched analysis. The researchers matched groups according to common prognostic covariates, including age, sex, race, education, insurance status, facility type, and Charlson/Deyo comorbidity score, as well as tumor histology, size, and location.
Patients identified as having cT1N0 NSCLC with a tumor less than 2 cm underwent SBRT (1,514 patients) or wedge resection (6,923). Compared with the wedge resection cohort, the SBRT patients were significantly older (74 vs. 69 years) and significantly more likely to be treated at an academic comprehensive cancer program (47% vs. 37%). The median Charlson/Deyo score was lower in the SBRT patients (0 vs. 1).
In unmatched analysis, SBRT was associated with significantly lower survival than wedge resection (5-year overall survival: 32% vs. 55%). In the propensity matching, all baseline covariates, including co-morbidity scores, facility type, and tumor size, were well balanced between the SBRT and wedge groups, with 1,398 patients in each group.
However, even in the matched groups, SBRT was associated with significantly lower 5-year overall survival than wedge (33% vs. 52%). When the investigators performed a propensity matched subgroup analysis in younger patients (age less than 60 years) who had a Charlson/Deyo score of 0, SBRT was associated with worse survival with a 5-year overall survival of 59% vs. 82% for SBRT and wedge resection, respectively.
Additionally, Dr. Yerokun and his colleagues conducted a sensitivity analysis comparing centers that performed predominately wedge resection with centers that performed predominately SBRT. After the exclusion of centers with low-volume and centers that conducted either 100% wedge resection or 100% SBRT only, centers that performed predominately wedge resection were more likely to have significantly better 3-year survival.
A video of the original presentation from the AATS Annual Meeting is available online.
Dr. Yerokun reported that he had no disclosures related to this presentation.
On Twitter @ThoracicTweets
BALTIMORE – Wedge resection was associated with significantly improved overall 5-year survival, compared with stereotactic body radiation therapy (SBRT) in patients with operable clinical Stage IA non–small cell lung cancer, according to a study of more than 8,000 patients.
Despite the fact that surgical resection has been the standard of care for early-stage non–small cell lung cancer (NSCLC), an increasing number of patients with potentially operable early-stage NSCLC are now being treated with SBRT, study investigator Dr. Babatunde A. Yerokun said at the annual meeting of the American Association for Thoracic Surgery.
“Our data show that thoracic surgeons should be included in the evaluation of these patients, and operative candidates with ct1A NO MO NSCLC should continue to receive a wedge resection vs. SBRT when technically feasible,” said Dr. Yerokun of Duke University, Durham, N.C. “Prospective studies are needed to determine the appropriate role of SBRT in management of these patients,” he concluded.
Dr. Yerokun and his colleagues examined overall survival of patients with cT1N0 lung cancer who underwent SBRT or wedge resection as reported in the National Cancer Data Base from 2003 to 2011. Survival was assessed using Kaplan-Meier and propensity-score matched analysis. The researchers matched groups according to common prognostic covariates, including age, sex, race, education, insurance status, facility type, and Charlson/Deyo comorbidity score, as well as tumor histology, size, and location.
Patients identified as having cT1N0 NSCLC with a tumor less than 2 cm underwent SBRT (1,514 patients) or wedge resection (6,923). Compared with the wedge resection cohort, the SBRT patients were significantly older (74 vs. 69 years) and significantly more likely to be treated at an academic comprehensive cancer program (47% vs. 37%). The median Charlson/Deyo score was lower in the SBRT patients (0 vs. 1).
In unmatched analysis, SBRT was associated with significantly lower survival than wedge resection (5-year overall survival: 32% vs. 55%). In the propensity matching, all baseline covariates, including co-morbidity scores, facility type, and tumor size, were well balanced between the SBRT and wedge groups, with 1,398 patients in each group.
However, even in the matched groups, SBRT was associated with significantly lower 5-year overall survival than wedge (33% vs. 52%). When the investigators performed a propensity matched subgroup analysis in younger patients (age less than 60 years) who had a Charlson/Deyo score of 0, SBRT was associated with worse survival with a 5-year overall survival of 59% vs. 82% for SBRT and wedge resection, respectively.
Additionally, Dr. Yerokun and his colleagues conducted a sensitivity analysis comparing centers that performed predominately wedge resection with centers that performed predominately SBRT. After the exclusion of centers with low-volume and centers that conducted either 100% wedge resection or 100% SBRT only, centers that performed predominately wedge resection were more likely to have significantly better 3-year survival.
A video of the original presentation from the AATS Annual Meeting is available online.
Dr. Yerokun reported that he had no disclosures related to this presentation.
On Twitter @ThoracicTweets
AT THE AATS ANNUAL MEETING
Key clinical point: Wedge resection outperformed SBRT in terms of mortality for early-stage NSCLC.
Major finding: In matched groups, SBRT was associated with significantly lower 5-year overall survival than was wedge resection (32% vs. 50%).
Data source: The study assessed more than 8.000 patients with early stage NSCLC who had either wedge resection or SBRT from the National Cancer Database from 2003 to 2011.
Disclosures: Dr. Yerokun had no relevant disclosures.
Esophagectomy 30-day readmission rate pegged at 19%
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
AT THE AATS ANNUAL MEETING
Key clinical point: Operative length, perioperative blood transfusions, and postoperative ICU admission were significant risk factors for readmission after esophagectomy.
Major finding: The rate of unplanned 30-day readmission was 19%.
Data source: The study assessed 86 patients who underwent esophagectomy at the Mayo Clinic between August 2012 and July 2014.
Disclosures: Dr. Dickinson and her colleagues reported having no relevant disclosures.
IASLC lung cancer staging project proposes changes for new TNM classification
The International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee has developed proposals for revision of the T, N, and M categories of the 8th edition of the TNM Classification for lung cancer due to be published in late 2016. The new classification will be enacted in January 2017.
The changes proposed were based on the results of an analysis of a new database of 94,708 cases donated from 35 sources in 16 countries around the world.
The methods used and the proposals made were published in the Journal of Thoracic Oncology (2016;11:39-51).
Candidate proposals for the TNM stage groups were developed in conjunction with proposed changes to the T and M categories, which were previously published (J Thorac Oncol 2015;10:990-1003, and 2015;10:1515-22). There were no proposed changes to the N.
Changes to some T and M descriptors will result in these cases being assigned to a different stage than that to which they would have been assigned in the 7th edition. In addition, some TNM subsets have been moved to a new stage grouping, according to Dr. Peter Goldstraw of Imperial College, London, and his colleagues on behalf of the IASLC Staging and Prognostic Factors Committee.
Major new proposals
T1 changes: Size cut points have further proliferated in the proposals for the 8th edition, and outgrowth of the emphasis on tumor size in the 7th edition, such that size will now be a descriptor in all T categories, according to the authors. New stage groupings proposed divide stage T1 into T1a, T1b, and T1c, based on the new size cut points of 1 cm and 2 cm. This results in these cases (when associated with the categories N0 and M0) being assigned to stages 1A1, 1A2, and 1A3, respectively, which reflects the statistically different prognosis of these cases.
T3, T4 changes: A new group has been created for the most advanced local disease categories, T3 and T4 associated with N3 disease, but category M0. Such cases will now be classified as stage IIIC, reflecting their worse outcomes than seen in cases involving tumors that remain in stage IIIB. The prognosis for stage IIIC cases is similar to that of stage IVA cases, however the researchers justified the separation, based upon the different treatment approaches used for such cases.
M changes: Although cases with intrathoracic metastatic disease to the contralateral lung or with pleural/pericardial dissemination remain classified as M1a disease, the category M1b will now be assigned to cases with a single metastatic deposit (in one organ) and M1a and M1b cases will be moored to a new stage grouping called IVA. The more common situation of multiple metastatic deposits, usually in more than one organ, will be classified as M1c and staged as IVB. Separation of the M1a and M1b categories was maintained both for further data analysis and because some patients with oligometastatic disease are now receiving more aggressive local therapy in addition to systemic treatment, according to the authors.
Other proposals
A variety of more minor changes to stage groupings has also been proposed, some of which will result in a T descriptor being allocated to a higher stage. In some cases, tumors may be allocated to a different T category entirely, leading to a reclassification of stage. Among the examples given were tumors associated with diaphragmatic invasion to TV, which, when associated with N0 disease, will move from stage IIB to IIA.
Impact on treatment
The relationship of the proposed classification changes to treatment decisions is not direct, the authors stated in their discussion. “Although such changes might raise the issue of whether consequent changes to treatment algorithms are needed, it is important to remind ourselves that stage does not dictate treatment. Stage is one, and perhaps the most important, of several prognostic factors that guide the appropriate treatment option[s] to offer the patient. Any change to established treatment algorithms should be based on clinical judgment informed by prospective trials,” they emphasized.
New stage groupings should be used in any trials of novel therapies, they added.
“We hope that the thoracic oncology community finds the proposals of value and that, when accepted, will have a positive impact on the effectiveness of treatment for lung cancer, which will benefit patients around the globe,” the researchers concluded.
The research to develop the new proposals was funded by the IASLC, including funds obtained through unrestricted grants obtained from the pharmaceutical industry. The authors reported no other disclosures.
The 8th edition of the TNM staging is upon us. It is the summary of analysis of 90,000 cases and data collected over 11 years. It behooves every thoracic surgeon taking care of patients with lung cancer to familiarize themselves with the new version. The staging proposal is available as an open access article on the Journal of Thoracic Oncology website.
From a statistical viewpoint, this edition fits the data better than previous editions did. However, from a practical application, it is more cumbersome to use routinely in a busy clinic. One hopes that we can soon say, “There’s an app for that!” Such interfacing will enhance the application of this edition significantly.

|
Dr. Sai Yendamuri |
The new edition of the staging system is particularly important for surgeons for two reasons. The first is the formal recognition that patients with oligometastatic disease have a better prognosis than other stage IV disease and may be amenable to multimodality therapies with curative intent, as is currently performed by select clinical teams. The second is the further refinement of stage I disease with respect to tumor size. Combined with the new histologic classification of adenocarcinoma and its proposed integration with the TNM classification, the debate of sublobar vs. lobar resection for stage I NSCLC will become more nuanced. These implications for the practicing thoracic surgeon make the manuscript mandatory reading.
Dr. Sai Yendamuri is chair, department of thoracic surgery, and director, thoracic surgery research laboratory, and a professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also the general thoracic editor for Thoracic Surgery News.
The 8th edition of the TNM staging is upon us. It is the summary of analysis of 90,000 cases and data collected over 11 years. It behooves every thoracic surgeon taking care of patients with lung cancer to familiarize themselves with the new version. The staging proposal is available as an open access article on the Journal of Thoracic Oncology website.
From a statistical viewpoint, this edition fits the data better than previous editions did. However, from a practical application, it is more cumbersome to use routinely in a busy clinic. One hopes that we can soon say, “There’s an app for that!” Such interfacing will enhance the application of this edition significantly.

|
Dr. Sai Yendamuri |
The new edition of the staging system is particularly important for surgeons for two reasons. The first is the formal recognition that patients with oligometastatic disease have a better prognosis than other stage IV disease and may be amenable to multimodality therapies with curative intent, as is currently performed by select clinical teams. The second is the further refinement of stage I disease with respect to tumor size. Combined with the new histologic classification of adenocarcinoma and its proposed integration with the TNM classification, the debate of sublobar vs. lobar resection for stage I NSCLC will become more nuanced. These implications for the practicing thoracic surgeon make the manuscript mandatory reading.
Dr. Sai Yendamuri is chair, department of thoracic surgery, and director, thoracic surgery research laboratory, and a professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also the general thoracic editor for Thoracic Surgery News.
The 8th edition of the TNM staging is upon us. It is the summary of analysis of 90,000 cases and data collected over 11 years. It behooves every thoracic surgeon taking care of patients with lung cancer to familiarize themselves with the new version. The staging proposal is available as an open access article on the Journal of Thoracic Oncology website.
From a statistical viewpoint, this edition fits the data better than previous editions did. However, from a practical application, it is more cumbersome to use routinely in a busy clinic. One hopes that we can soon say, “There’s an app for that!” Such interfacing will enhance the application of this edition significantly.

|
Dr. Sai Yendamuri |
The new edition of the staging system is particularly important for surgeons for two reasons. The first is the formal recognition that patients with oligometastatic disease have a better prognosis than other stage IV disease and may be amenable to multimodality therapies with curative intent, as is currently performed by select clinical teams. The second is the further refinement of stage I disease with respect to tumor size. Combined with the new histologic classification of adenocarcinoma and its proposed integration with the TNM classification, the debate of sublobar vs. lobar resection for stage I NSCLC will become more nuanced. These implications for the practicing thoracic surgeon make the manuscript mandatory reading.
Dr. Sai Yendamuri is chair, department of thoracic surgery, and director, thoracic surgery research laboratory, and a professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also the general thoracic editor for Thoracic Surgery News.
The International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee has developed proposals for revision of the T, N, and M categories of the 8th edition of the TNM Classification for lung cancer due to be published in late 2016. The new classification will be enacted in January 2017.
The changes proposed were based on the results of an analysis of a new database of 94,708 cases donated from 35 sources in 16 countries around the world.
The methods used and the proposals made were published in the Journal of Thoracic Oncology (2016;11:39-51).
Candidate proposals for the TNM stage groups were developed in conjunction with proposed changes to the T and M categories, which were previously published (J Thorac Oncol 2015;10:990-1003, and 2015;10:1515-22). There were no proposed changes to the N.
Changes to some T and M descriptors will result in these cases being assigned to a different stage than that to which they would have been assigned in the 7th edition. In addition, some TNM subsets have been moved to a new stage grouping, according to Dr. Peter Goldstraw of Imperial College, London, and his colleagues on behalf of the IASLC Staging and Prognostic Factors Committee.
Major new proposals
T1 changes: Size cut points have further proliferated in the proposals for the 8th edition, and outgrowth of the emphasis on tumor size in the 7th edition, such that size will now be a descriptor in all T categories, according to the authors. New stage groupings proposed divide stage T1 into T1a, T1b, and T1c, based on the new size cut points of 1 cm and 2 cm. This results in these cases (when associated with the categories N0 and M0) being assigned to stages 1A1, 1A2, and 1A3, respectively, which reflects the statistically different prognosis of these cases.
T3, T4 changes: A new group has been created for the most advanced local disease categories, T3 and T4 associated with N3 disease, but category M0. Such cases will now be classified as stage IIIC, reflecting their worse outcomes than seen in cases involving tumors that remain in stage IIIB. The prognosis for stage IIIC cases is similar to that of stage IVA cases, however the researchers justified the separation, based upon the different treatment approaches used for such cases.
M changes: Although cases with intrathoracic metastatic disease to the contralateral lung or with pleural/pericardial dissemination remain classified as M1a disease, the category M1b will now be assigned to cases with a single metastatic deposit (in one organ) and M1a and M1b cases will be moored to a new stage grouping called IVA. The more common situation of multiple metastatic deposits, usually in more than one organ, will be classified as M1c and staged as IVB. Separation of the M1a and M1b categories was maintained both for further data analysis and because some patients with oligometastatic disease are now receiving more aggressive local therapy in addition to systemic treatment, according to the authors.
Other proposals
A variety of more minor changes to stage groupings has also been proposed, some of which will result in a T descriptor being allocated to a higher stage. In some cases, tumors may be allocated to a different T category entirely, leading to a reclassification of stage. Among the examples given were tumors associated with diaphragmatic invasion to TV, which, when associated with N0 disease, will move from stage IIB to IIA.
Impact on treatment
The relationship of the proposed classification changes to treatment decisions is not direct, the authors stated in their discussion. “Although such changes might raise the issue of whether consequent changes to treatment algorithms are needed, it is important to remind ourselves that stage does not dictate treatment. Stage is one, and perhaps the most important, of several prognostic factors that guide the appropriate treatment option[s] to offer the patient. Any change to established treatment algorithms should be based on clinical judgment informed by prospective trials,” they emphasized.
New stage groupings should be used in any trials of novel therapies, they added.
“We hope that the thoracic oncology community finds the proposals of value and that, when accepted, will have a positive impact on the effectiveness of treatment for lung cancer, which will benefit patients around the globe,” the researchers concluded.
The research to develop the new proposals was funded by the IASLC, including funds obtained through unrestricted grants obtained from the pharmaceutical industry. The authors reported no other disclosures.
The International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee has developed proposals for revision of the T, N, and M categories of the 8th edition of the TNM Classification for lung cancer due to be published in late 2016. The new classification will be enacted in January 2017.
The changes proposed were based on the results of an analysis of a new database of 94,708 cases donated from 35 sources in 16 countries around the world.
The methods used and the proposals made were published in the Journal of Thoracic Oncology (2016;11:39-51).
Candidate proposals for the TNM stage groups were developed in conjunction with proposed changes to the T and M categories, which were previously published (J Thorac Oncol 2015;10:990-1003, and 2015;10:1515-22). There were no proposed changes to the N.
Changes to some T and M descriptors will result in these cases being assigned to a different stage than that to which they would have been assigned in the 7th edition. In addition, some TNM subsets have been moved to a new stage grouping, according to Dr. Peter Goldstraw of Imperial College, London, and his colleagues on behalf of the IASLC Staging and Prognostic Factors Committee.
Major new proposals
T1 changes: Size cut points have further proliferated in the proposals for the 8th edition, and outgrowth of the emphasis on tumor size in the 7th edition, such that size will now be a descriptor in all T categories, according to the authors. New stage groupings proposed divide stage T1 into T1a, T1b, and T1c, based on the new size cut points of 1 cm and 2 cm. This results in these cases (when associated with the categories N0 and M0) being assigned to stages 1A1, 1A2, and 1A3, respectively, which reflects the statistically different prognosis of these cases.
T3, T4 changes: A new group has been created for the most advanced local disease categories, T3 and T4 associated with N3 disease, but category M0. Such cases will now be classified as stage IIIC, reflecting their worse outcomes than seen in cases involving tumors that remain in stage IIIB. The prognosis for stage IIIC cases is similar to that of stage IVA cases, however the researchers justified the separation, based upon the different treatment approaches used for such cases.
M changes: Although cases with intrathoracic metastatic disease to the contralateral lung or with pleural/pericardial dissemination remain classified as M1a disease, the category M1b will now be assigned to cases with a single metastatic deposit (in one organ) and M1a and M1b cases will be moored to a new stage grouping called IVA. The more common situation of multiple metastatic deposits, usually in more than one organ, will be classified as M1c and staged as IVB. Separation of the M1a and M1b categories was maintained both for further data analysis and because some patients with oligometastatic disease are now receiving more aggressive local therapy in addition to systemic treatment, according to the authors.
Other proposals
A variety of more minor changes to stage groupings has also been proposed, some of which will result in a T descriptor being allocated to a higher stage. In some cases, tumors may be allocated to a different T category entirely, leading to a reclassification of stage. Among the examples given were tumors associated with diaphragmatic invasion to TV, which, when associated with N0 disease, will move from stage IIB to IIA.
Impact on treatment
The relationship of the proposed classification changes to treatment decisions is not direct, the authors stated in their discussion. “Although such changes might raise the issue of whether consequent changes to treatment algorithms are needed, it is important to remind ourselves that stage does not dictate treatment. Stage is one, and perhaps the most important, of several prognostic factors that guide the appropriate treatment option[s] to offer the patient. Any change to established treatment algorithms should be based on clinical judgment informed by prospective trials,” they emphasized.
New stage groupings should be used in any trials of novel therapies, they added.
“We hope that the thoracic oncology community finds the proposals of value and that, when accepted, will have a positive impact on the effectiveness of treatment for lung cancer, which will benefit patients around the globe,” the researchers concluded.
The research to develop the new proposals was funded by the IASLC, including funds obtained through unrestricted grants obtained from the pharmaceutical industry. The authors reported no other disclosures.
FROM THE JOURNAL OF THORACIC ONCOLOGY
Key clinical point: New lung cancer classification to become effective January 2017.
Major finding: Size will now be a descriptor in all T categories, according to the authors. New stage groupings proposed dividing stage T1 into T1a, T1b, and T1c, based on the new size cut points of 1 cm and 2 cm.
Data source: The International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee has developed proposals for revision of the T, N, and M categories of the 8th edition of the TNM Classification for lung cancer.
Disclosures: The research to develop the new proposals was funded by the IASLC, including funds obtained through unrestricted grants obtained from the pharmaceutical industry. The authors reported no other disclosures.
Esophageal perforation severity scoring system reliably stratifies patients
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).

|
Dr. Mara B. Antonoff |
Schweigert and his colleagues suggest that the Pittsburgh scoring system may identify patients suitable for nonoperative management. The authors retrospectively found less morbidity/mortality and less-frequent operative management among patients in Group 1, and thus, a recommendation was formulated favoring less-invasive management for these individuals.
The additional step of evaluating the success of nonoperative management in each group, either through further analyses of the current study or with future prospective studies is needed in order to make such recommendations.
Further demonstrating the utility of the Pittsburgh esophageal PSS, this study supports the notion that prospective, large-scale studies are in need, and that such scoring systems will be instrumental in standardizing data across centers.
Dr. Mara B. Antonoff is from the department of thoracic and cardiothoracic surgery at the University of Texas MD Anderson Cancer Center, Houston. Her remarks were made as part of an invited commentary on the article (J Thorac Cardiovasc Surg. 2016 Apr;151:1012-3).
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.
The Pittsburgh perforation severity score (PSS) can be used to improve decision making in the management of esophageal perforation, findings from a retrospective, multicenter study have shown.
Dr. Michael Schweigert and his colleagues performed a study of 288 patients with esophageal perforation treated at 11 centers between 1990 and 2014, using them as a completely independent population to validate whether the PSS could be used to stratify such patients into discrete subgroups with differential outcomes.
The PSS was analyzed using logistic regression as a continuous variable and stratified into low, intermediate and high score groups, according to their report published in the Journal of Thoracic and Cardiovascular Surgery (2016 Apr;151:1002-11).
Operative management was more frequent than nonoperative management (200 patients, or 69.4% vs. 30.6%), according to Dr. Schweigert of the Städtisches Klinikum Dresden Friedrichstadt, Germany, and his colleagues. Patients with esophageal cancer (34/43; 79%) and stricture (18/23; 78.3%) mainly were treated operatively. The most common type of surgery was primary repair (83 patients), followed by surgical drainage (38 patients).
Perforation-related morbidity was seen in 180 patients (65%), with sepsis (21%) and pneumonia (19%) being most common. Overall in-hospital mortality was 20%, and the median length of stay was 27 days.
Patients with fatal outcomes had a significantly higher median PSS score (11 vs. 1) and the median PSS was significantly higher in operatively managed cases, compared with nonoperative cases (5 vs. 4, P = .0001). The researchers found that the PSS score predicted morbidity well, with an area under the curve (AUC) of 0.77, as well as mortality (AUC = 0.83). However, prediction of the need for operative management was not as good (AUC = 0.65).
Based upon their analysis, the researchers proposed a treatment decision tree in which group I (low PSS patients) should have a focus of nonoperative management. Group 2 patients (medium PSS) with non–contained leak preferably should be managed by surgery.
They found that the high-risk group (PSS greater than 5) had the worst prognosis and highest mortality, with the odds for mortality being 8 times higher than that the intermediate group and 18 times higher than the low-risk group. “Because these patients are most endangered by esophageal perforation, early and aggressive treatment is mandatory to avoid fatal outcomes,” the authors stated.
They found that nonoperative management was not associated with higher mortality or more unfavorable outcome regarding perforation-related morbidity or length of stay, but they pointed out that nonoperative treatment was only successful in 60% of cases, with 36 out of the 88 nonoperative patients eventually undergoing surgery and 8 undergoing esophagectomy. But patients with a high perforation severity score were 3.37 times more likely to have operative management compared to low-scoring patients. “Better selective criteria for nonoperative management are urgently required,” they stated.
“The Pittsburgh PSS is helpful to assess the severity and potential consequences of esophageal injury and stratifies patients into low-, intermediate-, and high-risk groups with differential morbidity and mortality outcomes. Prospective studies are required to analyze the influence of the Pittsburgh scoring system on the treatment of esophageal perforation,” the researchers concluded.
The authors reported having no disclosures.
A webcast of the AATS Annual Meeting presentation of this paper is available.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Scoring system reliably stratifies patients into low-, intermediate-, and high-risk groups.
Major finding: Patients with a high perforation severity score were 3.37 times more likely to have operative management, compared with low-scoring patients.
Data source: A retrospective study was performed on 288 patients with esophageal perforation at 11 centers since 1990.
Disclosures: The authors presented no relevant disclosures.
Pulmonary function testing adds little to STS risk scores
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Additional pulmonary function testing adds little predictive value to STS risk scoring when available.
Major finding: There was no significant added benefit to the predictive ability of STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied, as determined by AUC analysis.
Data source: A retrospective, database analysis of 1,685 patients undergoing index cardiac surgery at a single center between April 2004 and January 2014.
Disclosures: The authors reported that they had no disclosures.