User login
Hysteroscopy: Managing and minimizing operative complications
- Perform endometrial sampling for abnormal uterine bleeding before scheduling operative hysteroscopy.
- Most uterine perforations do not require treatment— even those involving large dilators—although further assessment may be necessary to rule out bowel injury.
- Most complications of electrosurgery involve activating an electrode at the time of perforation, or diverting current to the outer sheath.
- Scrupulously monitor fluid intake and output to prevent hyponatremic complications.
WHAT WENT WRONG?
A 44-year-old woman undergoing resection of a submucous myoma from the left cornual region has persistent bleeding at the resection site. The surgeon continues coagulation at the bleeding site, using a rollerball electrode in an attempt to achieve hemostasis, but perforates the uterus. Immediate laparoscopy to identify collateral injury reveals some thermal damage on the posterior leaf of the broad ligament, but no bowel injury. After 24 hours of observation, she is afebrile without leukocytosis. She is discharged with explicit instructions to return if she has symptoms suggesting bowel injury. She returns in 72 hours, with abdominal pain and low-grade fever. CT reveals extravasation of contrast from the left ureter in the pelvis. Immediate laparotomy finds perforation of the left ureter secondary to a thermal injury. She undergoes ureteroneocystotomy and recovers.
This case illustrates one of the most common complications of operative hysteroscopy: uterine perforation with collateral injury. Both could have been avoided if the Ob/Gyn had stopped the procedure when bleeding first occurred, removed the instruments, and allowed the uterus to contract spontaneously.
This is just one of the strategies that can reduce the risks of hysteroscopic surgery. Numerous reports confirm that operative hysteroscopy is safe and effective, but as more gynecologists perform an increasing number of procedures, we must be aware of potential complications and do our best to minimize risk to our patients.
Complications cannot be completely avoided, and may occur when a procedure is done correctly by experienced doctors. They are far more likely if techniques or equipment are used improperly. This article describes ways to minimize risk.
When the American Association of Gynecologic Laparoscopists (AAGL) surveyed its members in 1993, it found a complication rate of 2% for operative hysteroscopy.1 The rate of major complications—perforation, hemorrhage, fluid overload, and bowel or urinary tract injury—was less than 1%. A prospective multicenter trial2 of 13,600 procedures in the Netherlands found a higher complication rate for operative (0.95%) than for diagnostic hysteroscopy (0.13%).
Preoperative precautions
We can reduce the risk of complications if contraindications are not ignored, equipment is thoroughly inspected and understood, and the surgeon goes through a mental checklist and plans each procedure. A “time out”before the operation begins, when every member of the team is briefed, is also valuable in preventing errors.
A hands-on course necessary before undertaking advanced resectoscopic surgery, to become familiar with equipment and techniques, followed by proctoring by a surgeon credentialed for the procedure.
Contraindications
Ignoring contraindications to hysteroscopic surgery increases the risk of complications and is the single greatest factor leading to patient injury and physician liability.
Contraindications include:
- Unfamiliarity with equipment, instruments, or technique
- Lack of appropriate equipment or staff familiar with the equipment
- Acute pelvic inflammatory disease
- Pregnancy
- Genital tract malignancies
- Lack of informed consent
- Inability to dilate the cervix
- Inability to distend the uterus to obtain visualization
- Poor surgical candidates who may not tolerate fluid overload because of renal disease, or radiofrequency current when a cardiac pacemaker is present
- The patient desires and expects complete amenorrhea3
Mechanical or traumatic complications
These types of complications are among the most common. Other categories include preoperative complications (ie, improper patient selection and lack of informed consent), electrosurgical and gaseous, complications related to distention media, and postoperative complications (ie, infection and late sequelae).
Inability to insert the hysteroscope
This may be caused by a stenotic, nulliparous cervix; menopause; GnRH agonists; previous cone biopsy, laceration, or cryosurgery; or an acutely retroflexed or anteflexed uterus.
Acute flexion problems can be corrected using a long-bladed, open-sided Graves speculum deep in the anterior or posterior fornix. The speculum pushes the fundus to the midposition and facilitates dilation. Once the hysteroscope is inserted, remove the speculum.
Placing a tenaculum on the posterior lip of the cervix of an acutely retroflexed uterus will straighten the cervical canal when traction is applied.
Inserting a laminaria tent the evening before surgery helps dilate the cervix easily and atraumatically.4 However, the laminaria can sometimes create a false passage, leading to perforation.
Cervical ripening agents such as intravaginal or oral misoprostol (200 μg inserted vaginally or 400 μg orally 8 to 12 hours preoperatively) also can facilitate dilation.
Intracervical injection of vasopressin solution (4 IU in 100 cc sodium chloride, injected at the 4 and 8 o’clock positions) can reduce the force needed to dilate the cervix.5 Half-size dilators may help; they also reduce the risk of cervical laceration.
Laceration of the cervix
Although this is a minor complication, substantial bleeding sometimes occurs when the cervix is lacerated by the tenaculum. In these cases, suture the cervix.
Occasionally, a touch of cautery from the rollerball electrode at low power (20 to 30 W) can control the bleeding.
Silver nitrate sticks or ferric subsulfate (Monsel’s) paste are also effective on superficial lacerations.
Bleeding from lower uterus or cervical canal can obscure view
In some cases, bleeding is delayed, necessitating additional surgery. Intravasation of distention fluid also can occur at these lacerations. Coagulation with the electrode may be necessary when bleeding is heavy.
Check for collateral injury when uterine perforation occurs
Perforation is a well-documented risk of operative hysteroscopy and should be discussed with the patient when obtaining informed consent. In the AAGL survey,1 the incidence of perforation was 14 per 1,000. It was even higher during transection of lateral and fundal adhesions: 2 to 3 per 100.6
Although perforation is more common with thermal energy sources, it may occur mechanically when scissors are used to transect a uterine septum, synechiae, or polyps.
When the cervix is stenotic or the uterus is acutely ante- or retroflexed, sounds and dilators can perforate the uterus.
Most perforations—even those involving large dilators—usually do not require treatment, although further assessment may be necessary to rule out bowel injury. Most perforations occur in the fundal region or posterior lower segment.
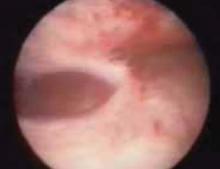
A false passage can be created when entering the uterus. Occasionally the surgeon may be fooled into thinking the hysteroscope is in the uterine cavity, since the false passage distends (FIGURE 1). If muscle fibers are visible and the tubal ostea are not, assume the passage is false. Slowly remove the hysteroscope and identify the true cavity for confirmation. Discontinue the procedure—even if no perforation is detected—to prevent distention fluid from being absorbed into the circulation through the injury. Adequate distention is not possible at this time.
Delay repeat hysteroscopy for 2 to 3 months.
To avoid creating a false passage, dilate the cervix with slow, steady pressure and stop as soon as the internal os opens; do not attempt to push the dilator to the uterine fundus.
Often the external os opens, but the internal os cannot be dilated the extra 1 to 2 mm necessary to accommodate the 27-French resectoscope. Rather than exert more force and risk perforation or laceration, simply turn on the resectoscope’s inflow with the outflow shut off, and let the fluid pressure dilate the cervix.
Always insert the hysteroscope or resectoscope under direct vision rather than use an obturator. Keep the “dark circle” in the center of the field and slowly advance the hysteroscope toward it until the cavity is reached.
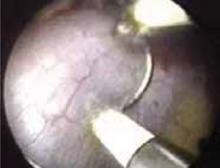
Avulsion of the myometrium sometimes occurs during removal of incompletely resected myomas (FIGURE 2). Keep the myoma grasper away from the fundus when removing myoma segments, and avoid excessive traction on what may be a thin segment of myometrium. Injuries can occur when the grasper perforates the uterus and bowel is inadvertently grasped. Large injuries require laparoscopic repair.
Perforation is more likely in repeat procedures. In a report of 80 repeat endometrial ablations, Townsend and colleagues7 noted 8 perforations that prevented completion of the procedure. In a series of 75 repeat ablations compared with 800 primary ablations by the same surgeon, the rate of serious perioperative complications was significantly higher in the repeat ablation group (9.3% versus 2.0%).8
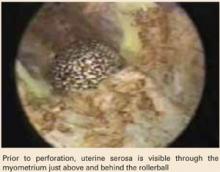
When perforation occurs during the use of thermal energy, laparoscopy is necessary to assess the organs overlying the site.9 During setup for laparoscopy, bring the hysteroscope near the area of perforation to inspect the bowel beyond the uterus. Since the pelvis fills quickly with distention fluid, the hysteroscope can even be placed through the perforation to yield an excellent view of the undersurfaces of the bowel immediately adjacent to the injured area (FIGURE 3). (Disconnect the electrosurgical cord before doing this!)
Thorough laparoscopic inspection of the bowel in the pelvis often reveals thermal injury, which appears as a whitish patch on the bowel serosa. To repair bowel injuries, bring the injured segment out through a minilaparotomy and excise the damage with a 2- to 3-cm border. A general surgeon should be called in to consult.
A 42-year-old woman who underwent endometrial ablation 2 years earlier presents with persistent menorrhagia and a 12-week–size fibroid uterus and expresses a desire for repeat ablation. At the second surgery, the uterine cavity appears scarred, with multiple synechiae.
As the procedure progresses, the uterine serosa becomes visible from within the cavity, appearing as a smooth, bluish structure that can be moved with only slight pressure. As the ablation continues, the uterus perforates, necessitating laparoscopic inspection of the organs overlying the site.
Although the patient recovers, her menorrhagia eventually returns, and she opts for laparoscopically assisted vaginal hysterectomy 1 year later.
Uterine perforation is more likely during repeat procedures
This case illustrates one of the most common risks of operative hysteroscopy: uterine perforation, which occurs more frequenly in repeat procedures.
The case also highlights an important indicator of perforation: the serosal sign, which I first described in 1996.24 When the smooth, bluish structure appears, cease ablation in the region immediately.
If no injury is apparent, discharge the patient but follow her closely, including daily white blood cell counts for 4 to 5 days. Instruct her to take her temperature twice daily and return to the hospital immediately if any signs of bowel perforation develop. Delayed perforation from thermal injury can occur as late as 2 weeks following surgery, and the patient should be apprised of this possibility.
FIGURE 1 Signs of a false passage
Myometrial fibers signal that a false passage has been created. Stop the procedure even if no perforation is detected, to prevent distention fluid from being absorbed into the circulation through the injury.
FIGURE 2 Risk of myomectomy: Myometrial avulsion
Small bowel visible within the uterine cavity after avulsion of uterine wall at the time of myomectomy
FIGURE 3 Use the hysteroscope to assess perforation site
Hysteroscopic view of perforation at the fundus. The small bowel is visible beyond the perforation at left.
Intraoperative bleeding is rare
Bleeding is unlikely unless vessels are lacerated or injured in the cervical canal or lower uterine segment during dilation or deep ablation or vaporization. Bleeding is more common when endomyometrial resection is performed with the wire loop electrode or during ablation or vaporization of fibroids. Bleeding sufficient to require intervention occurs at a rate of 0.5% to 1.9% in several reported series.
To achieve hemostasis via balloon tamponade, insert a Foley catheter with a 30-cc balloon into the uterine cavity, inject 15 to 20 mL (or more for a larger cavity) of fluid into the balloon, and observe the patient.10 If there is no bleeding in 1 hour, remove half the fluid. Remove the remainder of the fluid and the catheter over the next hour if no further bleeding occurs.
Alternative method: Pack the uterus. I prefer 1/2-inch–gauge packing that has been soaked in a dilute vasopressin solution (20 U [1 mL] in 60 mL normal saline).11
The benefits of vasopressin. Before balloon tamponade or packing the uterus, I inject very dilute vasopressin (4 U [0.2 mL] in 60 mL normal saline) directly into the cervix 2 cm deep, at the 4 and 8 o’clock positions. Phillips12 demonstrated a marked decrease in blood loss during resectoscopic surgery using this approach. I also do this routinely prior to operative hysteroscopy, since the vasopressin-induced vasoconstriction reduces intravasation of distention media.
A vaporizing electrode may prevent significant blood loss during myoma resection by sealing blood vessels as the tissue is vaporized.13 All major manufacturers of hysteroscopic equipment produce these electrodes.
In my series of 44 endometrial ablations and hysteroscopic myomectomies performed with the vaporizing electrode, blood loss was “minimal” or less than 50 mL in 29 cases. The maximum blood loss was 300 mL in a patient with a 4-cm submucous myoma who was managed emergently for hemorrhage.14 In another case, during resection of a 5-cm submucous myoma, I encountered significant bleeding from large vessels at the base of the myoma, which required intrauterine tamponade with a vasopressin-soaked pack.
Preoperative measures may decrease vascularity. In their analysis of 16 randomized and nonrandomized controlled trials published in the English literature between 1990 and 1996, Parazzini and colleagues15 found that preoperative danazol or GnRH agonists decreased the thickness and vascularity of the endometrium and shrank myomata, resulting in shorter operating times, less blood loss, and less intravasation of distending fluid.
Electrosurgical and gaseous complications
Most electrosurgical complications involve activation of an electrode at the time of perforation, or current diversion to the outer sheath.
Thermal injuries also can be caused by overheating of the return pad or use of a weighted speculum that has not fully cooled after removal from the autoclave. The latter can be avoided by immersing the entire speculum in cool saline for at least 1 to 2 minutes prior to inserting it into the vagina. The blade cools much faster than the weighted ball, so be sure to check both to prevent a perineal or buttock burn.
Perforation with an active electrode
This usually occurs when current is applied as the electrode is extended or the resectoscope is moved toward the fundus. It can be avoided if the electrode is activated only when moving it toward the operator.
Perforations with intraabdominal burns also have occurred during attempts to coagulate bleeders—especially in the cornual regions.
Diversion of current can be destructive
Genital tract injuries have occurred as a result of current diversion. Vilos and colleagues16 reported 13 electrical burns during endometrial ablation, and mention many more anecdotal reports. The usual cause: electrode insulation failure, which allows current to jump to the outer sheath of the resectoscope.
To avoid this, inspect all electrodes thoroughly before surgery and use them only once.
Capacitative coupling also diverts current
Since the sheath-within-a-sheath design of the resectoscope resembles a capacitor, high-voltage current can jump to the outer sheath without direct contact from the electrode. When Munro17 bench-tested electrosurgical generators and electrodes with and without insulation defects, he found that capacitative coupling with intact electrodes occurred more frequently with high-voltage coagulation current than with lower-voltage cutting current.
One way to avoid these injuries is to activate the electrode intermittently, with short bursts, rather than rolling back and forth over an area with continuous current. Another strategy is placing a damp sponge in the posterior vagina extending out the introitus; this protects the mucosa and perineal skin—especially in obese patients.
How to avoid return-pad injuries
Keep the patient’s thigh completely dry; ensure that the pad is flat against the skin at application, with no bubbles or creases; and use only return electrode monitor (REM) dispersive pads.
Especially when using vaporization electrodes, avoid prolonged activation of the electrode at high power. To minimize risk of vaporization, use a second dispersive pad connected to the first via a “y” connector to further disperse current and heat at the return pad.
Also, limit the use of coagulation current and use a maximum generator setting of 60 to 80 W in the coagulation mode.
Take steps to avert gas embolism, but watch closely for signs
Initial reports of this potentially fatal complication came mostly from laser ablation procedures, but gas embolism can occur during all diagnostic and operative hysteroscopic procedures, especially the latter.
Sources of gas embolism: room air, carbon dioxide, carbon monoxide, and other gaseous products of vaporization or tissue combustion. The anesthesiologist is usually the first to identify the signs.
Signs of gas embolism. The surgeon should ask to be immediately alerted to any sudden fall in oxygen saturation, as well as to hypotension, hypercarbia, arrhythmias, tachypnea, or a “mill wheel” murmur. If any of these signs are detected and a gas embolism is suspected, stop the procedure and ventilate the patient with 100% oxygen.
Carbon dioxide is a soluble gas, so these emboli generally resolve rapidly. In contrast, room air emboli are more likely to be fatal.
Reduce the risk of air embolism by avoiding the Trendelenburg position and leaving the last dilator in the cervix until just before inserting the resectoscope.
Also limit repetitive removal and reinsertion of the resectoscope, as often occurs during myoma resection. By vaporizing rather than resecting myomas, it is possible to eliminate the need to continually remove fibroid chips. Preoperative GnRH agonists narrow venous sinuses and help prevent this complication.
Intracervical injection of dilute vasopressin prior to dilatation of the cervix creates vascular spasm and may help prevent gas from entering the circulation.
Complications from distention media
Excess absorption of distention media is one of the most frequent complications. Most surgeons use low-viscosity, sodium-free fluids for operative hysteroscopy, since fluids that contain electrolytes are incompatible with monopolar electrosurgical instruments. The use of 3% sorbitol, 1.5% glycine, or sorbitol-mannitol solutions can lead to dilutional hyponatremia and hypoosmolality.18 Although the vast majority of women quickly recover from these conditions, some cases of permanent morbidity and even death have been reported.19 The overall incidence of dilutional hyponatremia was 0.2% in 1993, according to the AAGL member survey.1
Hyponatremia and hypoosmolality more likely in premenopausal women
These conditions may have catastrophic consequences if they are not recognized and corrected promptly. The brain swells as it attempts to become isoosmotic with the vascular system. If swelling exceeds 5%, the risk of severe neurological damage dramatically increases.
This is an important problem in premenopausal women, since estrogen and progesterone inhibit sodium-potassium adenosine triphosphatase (ATPase) activity in the brain. This sodium pump protects the brain against cerebral edema, which can cause herniation of the brain stem and death. Although men and postmenopausal women develop dilutional hyponatremia, they are less likely to suffer brain damage because the sodium pump is intact.
Taskin et al20 conducted a randomized trial showing an increase in the sodium-potassium ATPase pump activity and decreased volume deficit during hysteroscopic surgery in patients pretreated with a GnRH agonist, compared with a control group. This increased pump activity in the brain and endometrium may decrease women’s susceptibility to hyponatremic complications and brain damage.
Vigilant monitoring of fluid intake/output during hysteroscopic surgery is necessary to prevent hyponatremic complications. Avoid the pitfall of erroneously attributing deficits to fluid “in the drapes” by using drapes with a fluid-collection pouch.
The standard of care is use of electronic inflow-outflow measuring systems. Manufacturers of hysteroscopic equipment offer highly accurate electronic fluid monitoring systems that measure the weight of the distending fluid infused and collected rather than relying on manual estimation of deficit. The latter method may be inaccurate since the volume of the supply bag can vary by as much as 10%.
Adjust intrauterine pressure to reduce the likelihood of intravasation. High intrauterine pressure may be desirable for visualization, but it greatly increases the risk of intravasation.
I adjust pressure and flow rates by opening or closing the inflow and outflow valves of the resectoscope until slight amounts of bleeding from resected tissue can be visualized. Since intrauterine pressure is extremely difficult to monitor accurately during operative hysteroscopy, this practice ensures that it remains below the patient’s mean arterial pressure, thus minimizing the risk of intravasation.
Use a dilute vasopressin injection to constrict blood vessels and decrease the chance of intravasation.
Vaporizing electrodes for myoma resection and ablation seal blood vessels and reduce fluid absorption.
Guidelines for distention media
To reduce the likelihood of these complications, I recommend that surgeons:
- Draw preoperative serum electrolytes for a baseline in all patients.
- Give all patients with myomas 2 monthly injections of depot leuprolide acetate (3.75 mg intramuscularly). Give patients without fibroids a single injection 4 to 6 weeks prior to the procedure.
- Place a fluid-collection drape or a larger, plastic Mayo stand cover with the bottom cut off under the patient’s buttocks so that fluid drains into a “kick” bucket. Also adjust the resectoscope’s outflow tubing to drain into the collection bag, which should be kept on constant suction to the flow-stat electronic fluid monitor.
- Continuously record inflow and outflow using the electronic monitor with the deficit alarm set to 500 mL.
- Keep distention fluid at room temperature and monitor the patient’s core temperature continuously. Significant fluid intravasation will lower the patient’s temperature, and this may be the first sign of fluid overload.
- Perform operative hysteroscopy under spinal or epidural anesthesia so the anesthesiologist can continually assess the patient’s sensorium. Confusion and irritability are early signs of dilutional hyponatremia.
- If the fluid deficit reaches 750 mL, immediately give 20 to 40 mg of intravenous furosemide and draw a serum sodium. Do not wait for the result of the sodium level before treatment, since a 5- to 20-minute delay can be catastrophic.
- Interrupt the procedure for 5 to 10 minutes to allow the uterus to contract and to seal off small blood vessels.
- Discontinue the procedure if the fluid deficit reaches 1,500 mL or if the serum sodium level is below 125 mEq/L.
I do not limit the duration of resectoscopic procedures as long as fluid deficits are below 750 mL, as measured by electronic fluid monitor. I also ensure that the operating room staff is well educated in the use of the monitor and able to troubleshoot intraoperatively.
If the machine fails during the procedure, reset it with the alarm limit lowered to reflect the deficit recorded before failure.
Monitor the color of the outflow fluid. Excessive blood loss counted as part of the outflow can occasionally mask a distention fluid deficit.
Choosing a distention medium
There is no ideal distention medium for monopolar operative hysteroscopy. Several authors have suggested that 5% mannitol is advantageous since it is isosmolar and acts as an osmotic diuretic. However, it does not prevent hyponatremia. The main disadvantage of 5% mannitol is its high cost and limited availability in 3-L bags or 4-L bottles.
The use of bipolar devices in normal saline prevents dilutional hyponatremia, but fluid deficits must still be monitored electronically so they do not exceed 2,000 mL. The false sense of security that may occur when normal saline is used for distention may lead to inaction when a large deficit occurs. This can lead to pulmonary edema and death.
Postoperative and late complications
These include infection, endometrial cancer, iatrogenic adenomyosis, hematometria, post-ablation tubal ligation syndrome, and pregnancy.
Infection rate is 0.3% to 2%
Infection is relatively rare following endometrial ablation, with a rate of 0.3% to 0.5% reported in most series. Endometritis, parametritis, and pyometra are more common following resection of submucous myomas, with rates as high as 2% reported.
Infection is more likely after prolonged procedures, especially when the hysteroscope is repeatedly inserted and removed. It also is more likely if the patient has a history of pelvic inflammatory disease. I generally administer prophylactic antibiotics (1 dose of intravenous ceftizoxime, 1 g, approximately 30 to 60 minutes prior to surgery).
I also insert a laminaria tent the evening prior to surgery. Patients with a history of pelvic inflammatory disease are discharged on doxycycline (100 mg twice daily for 7 days).
Be alert for endometrial cancer
This malignancy has been diagnosed at the time of endometrial ablation and reported in patients who have undergone prior endometrial ablations or fibroid resections. Thus, endometrial sampling should be part of the workup of abnormal uterine bleeding before the patient is scheduled for operative hysteroscopy. In women at high risk for endometrial cancer, perform office diagnostic hysteroscopy, with directed biopsy of any suspicious areas.
When viable endometrial glands are “buried” during ablation, or synechiae develop, preventing the egress of blood, there is a chance that diagnosis of endometrial cancer will be delayed. However, this theoretical fear has not been proven clinically.
Patients whose abnormal bleeding recurs after ablation should undergo sampling and office hysteroscopy, just as if they had not undergone a previous ablation. Theoretically, women who undergo endomyometrial resection or vaporization should have a lower incidence of endometrial cancer, since the tissue most susceptible to malignancy is removed. This has not yet been proven scientifically.
In their comprehensive review of late complications of operative hysteroscopy, Cooper and Brady21 suggest that patients at high risk for endometrial cancer who present with abnormal uterine bleeding not controlled by hormones might be better served by hysterectomy. Unfortunately, these patients tend to be high-risk surgical candidates.
If atypia is present, do not perform endometrial ablation or resection. I do perform ablation and resection in patients with complex hyperplasia without atypia if it has been reversed with progestin and does not recur for at least 6 months without progestin therapy. These patients undergo office hysteroscopy and sampling of the endometrium before operative hysteroscopy is scheduled.
Iatrogenic adenomyosis
Two theories suggest this is a late complication of operative hysteroscopy. According to the first, when the endometrium is incompletely resected, scarring over this tissue causes the viable glands to grow into the myometrium. The other theory suggests that viable endometrial debris is transported into the myometrium by vessels opened at resection.
I have found that using the vaporization electrode followed by application of a rollerball over the surface of the cavity most effectively reaches maximal tissue depth and, theoretically, prevents adenomyosis. Since most adenomyosis occurs on the posterior wall, I take a strip from this area for pathologic analysis to determine whether adenomyosis preceded the ablation or developed subsequent to it.
Hematometria
This can occur following operative hysteroscopy if viable glands are left in the fundal or cornual region and synechiae develop in the lower segment, preventing egress of blood. It also can occur if the upper endocervix is ablated and subsequently scars, causing stenosis. To avoid this, ablate only to the level of the internal os.
Diagnosis and treatment. Hematometria can be diagnosed easily by ultrasound and treated with office hysteroscopy using a narrow-diameter, rigid, continuous-flow hysteroscope with an operating channel to pass small instruments.
Post-ablation tubal ligation syndrome
This is cornual hematometria that develops when viable endometrial cells are left in the cornua when the cavity also contains synechiae, causing cyclic bleeding. Since there is no egress from the cervix or tubes, blood gradually builds up, leading to hematosalpinx and pain. One way to avoid this is to ensure complete ablation of the cornual endometrium.
Prevention. Some experts recommend that the small rollerball electrode be placed in the cornua, with slightly reduced intrauterine pressure, to allow the cornual endometrium to collapse around the rollerball. A short burst of current is then applied to ablate the tissue.
This complication is less likely after hydrothermablation, since the free-flowing saline ablates the cornua completely. After more than 50 hydrothermablations performed in patients with prior tubal sterilizations, I have not seen any cases of post-ablation tubal ligation syndrome.
Treatment consists of bilateral salpingectomy or tubal fulguration at the cornual region and repeat ablation or resection of viable endometrial tissue. Another option is hysterectomy.
Post-ablation pregnancy can be very complicated
Pregnancy after endometrial ablation occurs at a rate of 0.2% to 1.6%. Counsel patients that this procedure does not prevent pregnancy and that contraception is vital. Uterine rupture after fibroid resection has been reported.
In a review of 37 post-ablation pregnancies, only 11 of 17 women who chose not to terminate carried the gestation beyond 28 weeks. In addition, there was a high incidence of intrauterine growth restriction, prematurity, and placenta accreta.22
Hysteroscopic tubal sterilization with the Essure system (Conceptus, San Carlos, Calif), or laparoscopic tubal fulguration performed at the time of ablation averts these complications.
Complications of global ablation
Global ablation technologies were developed to enable gynecologists with limited operative hysteroscopy skills to perform endometrial ablation and to make ablation safer for the patient. These technologies completely eliminate the risk of distention-media complications, but widespread use has resulted in other complications that have been reported in the literature to only a limited extent.
Most published articles on global endometrial ablation are from the original US Food and Drug Administration (FDA) trials, in which the complication rates were extraordinarily low. Widespread commercial use of these technologies since FDA approval, especially by practitioners with limited skills, has increased complications.
Do not override safety systems
Complications are more frequent when devices are misused or safety systems overridden. And, fear of litigation makes physicians unwilling to report complications.
In the FDA Manufacturer and User Facility Device Experience (MAUDE) database (www.fda.gov.cdrh/maude.html), complications include bowel burns after unrecognized perforation, and bowel burns associated with electrosurgical, microwave energy, or heat transferred through intact myometrium.23 Vaginal burns, uterine necrosis myometritis requiring hysterectomy, and death from unrecognized bowel burn also have been reported.
Most global procedures are performed blindly, and some doctors fail to perform diagnostic hysteroscopy before and after surgery, which I feel is mandatory with any endometrial ablation. Hydrothermablation is the only global technique that has the advantage of direct observation. In more than 150 procedures done in my office under local anesthesia, the only complications were 2 false passages. Both were promptly identified during diagnostic hysteroscopy, and the surgery was rescheduled 2 to 3 months later.
The author has served on the speakers’ bureau for Boston Scientific.
1. Hulka JF, Peterson HA, Philips JM, Surrey MW. Operative hysteroscopy: American Association of Gynecologic Laparoscopists’ 1993 Member Survey. J Am Assoc Gynecol Laparos. 1995;2:131-132.
2. Jansen FW, Vredevoogd CB, Van Ulzen K, et al. Complications of hysteroscopy: a prospective multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Vilos GA. Hysteroscopic surgery: indications, contraindications, and complications. In: Pasic and Levine’s A Practical Manual of Hysteroscopy and Endometrial Ablation Techniques. London and New York: Taylor and Francis; 2004;237-258.
4. Ostrzenski A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. Int J Fertil. 1994;39:111-113
5. Phillips DA, Nathanson HG, Millim SJ, et al. The effect of dilute vasopressin solution on the force needed for cervical dilatation: a randomized controlled trial. Obstet Gynecol. 1997;89:507-511
6. Valle RF, Sciarra JJ. Intrauterine adhesions; hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459-1470
7. Townsend DE, Quinlan DJ, Johnson GH. Repeat endometrial ablation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.
8. MacLean-Fraser E, Penava D, Vilos GA. Perioperative complication rates of primary and repeat hysteroscopic endometrial ablations. J Am Assoc Gynecol Laparosc. 2002;9:175-177.
9. Loffer FD. Complications of hysteroscopy—their cause, prevention, and correction. J Am Assoc Gynecol Laparosc; 1995;3:11-26
10. Goldrath MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol. 1983;147:869-872
11. Townsend DE. Vasopressin pack for treatment of bleeding after myoma resection. Am J Obstet Gynecol. 1991;165:1405-1407
12. Phillips DR, Nathanson HG, Milim SJ, et al. The effect of dilute vasopressin solution on intraoperative blood loss during operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 1996;88:761-766
13. Brooks PG. Resectoscopic myoma vaporizer. J Reprod Med. 1995;40:791-795
14. Glasser MH. Endometrial ablation and hysteroscopic myomectomy by electrosurgical vaporization. J Am Assoc Gynecol Laparosc. 1997;4:369-374
15. Parazzini F, Vercellini P, Di Giorgio O, et al. Efficacy of preoperative medical treatment in facilitating endometrial resection, myomectomy and metroplasty: literature review. Hum Reprod. 1998;13:2592-2597
16. Vilos GA, Brown S, Graham G, et al. Genital tract electrical burns during hysteroscopic endometrial ablation: report of 13 cases in the United States and Canada. J Am Assoc Gynecol Laparosc. 2000;7:141-147
17. Munro MG. Factors affecting capacitative current diversion with a uterine resectoscope: an in vitro study. J Am Assoc Gynecol Laparosc. 2003;10:450-460
18. Istre O, Shajaa K, Schjoensky AP, et al. Changes in serum electrolytes after transcervical resection of endometrium and submucous fibroids with the use of 1.5% glycine for irrigation. Obstet Gynecol. 1992;80:218-222
19. Arieff AI, Azus JC. Hyponatremic encephalopathy after endometrial ablation. JAMA. 1994;271:345.-
20. Taskin O, Buhur A, Birincioglu M, et al. Endometrial Na+, K+–ATPase pump function and vasopressin levels during hysteroscopic surgery in patients pretreated with GnRH agonist. J Am Assoc Gynecol Laparosc. 1998;5:119-124
21. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374
22. Rogerson L, Gannon, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160
23. Gurtcheff SE, Sharp MT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282
24. Glasser MH. The serosal sign: the hysteroscopic appearance of the uterine cavity just prior to perforation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.
- Perform endometrial sampling for abnormal uterine bleeding before scheduling operative hysteroscopy.
- Most uterine perforations do not require treatment— even those involving large dilators—although further assessment may be necessary to rule out bowel injury.
- Most complications of electrosurgery involve activating an electrode at the time of perforation, or diverting current to the outer sheath.
- Scrupulously monitor fluid intake and output to prevent hyponatremic complications.
WHAT WENT WRONG?
A 44-year-old woman undergoing resection of a submucous myoma from the left cornual region has persistent bleeding at the resection site. The surgeon continues coagulation at the bleeding site, using a rollerball electrode in an attempt to achieve hemostasis, but perforates the uterus. Immediate laparoscopy to identify collateral injury reveals some thermal damage on the posterior leaf of the broad ligament, but no bowel injury. After 24 hours of observation, she is afebrile without leukocytosis. She is discharged with explicit instructions to return if she has symptoms suggesting bowel injury. She returns in 72 hours, with abdominal pain and low-grade fever. CT reveals extravasation of contrast from the left ureter in the pelvis. Immediate laparotomy finds perforation of the left ureter secondary to a thermal injury. She undergoes ureteroneocystotomy and recovers.
This case illustrates one of the most common complications of operative hysteroscopy: uterine perforation with collateral injury. Both could have been avoided if the Ob/Gyn had stopped the procedure when bleeding first occurred, removed the instruments, and allowed the uterus to contract spontaneously.
This is just one of the strategies that can reduce the risks of hysteroscopic surgery. Numerous reports confirm that operative hysteroscopy is safe and effective, but as more gynecologists perform an increasing number of procedures, we must be aware of potential complications and do our best to minimize risk to our patients.
Complications cannot be completely avoided, and may occur when a procedure is done correctly by experienced doctors. They are far more likely if techniques or equipment are used improperly. This article describes ways to minimize risk.
When the American Association of Gynecologic Laparoscopists (AAGL) surveyed its members in 1993, it found a complication rate of 2% for operative hysteroscopy.1 The rate of major complications—perforation, hemorrhage, fluid overload, and bowel or urinary tract injury—was less than 1%. A prospective multicenter trial2 of 13,600 procedures in the Netherlands found a higher complication rate for operative (0.95%) than for diagnostic hysteroscopy (0.13%).
Preoperative precautions
We can reduce the risk of complications if contraindications are not ignored, equipment is thoroughly inspected and understood, and the surgeon goes through a mental checklist and plans each procedure. A “time out”before the operation begins, when every member of the team is briefed, is also valuable in preventing errors.
A hands-on course necessary before undertaking advanced resectoscopic surgery, to become familiar with equipment and techniques, followed by proctoring by a surgeon credentialed for the procedure.
Contraindications
Ignoring contraindications to hysteroscopic surgery increases the risk of complications and is the single greatest factor leading to patient injury and physician liability.
Contraindications include:
- Unfamiliarity with equipment, instruments, or technique
- Lack of appropriate equipment or staff familiar with the equipment
- Acute pelvic inflammatory disease
- Pregnancy
- Genital tract malignancies
- Lack of informed consent
- Inability to dilate the cervix
- Inability to distend the uterus to obtain visualization
- Poor surgical candidates who may not tolerate fluid overload because of renal disease, or radiofrequency current when a cardiac pacemaker is present
- The patient desires and expects complete amenorrhea3
Mechanical or traumatic complications
These types of complications are among the most common. Other categories include preoperative complications (ie, improper patient selection and lack of informed consent), electrosurgical and gaseous, complications related to distention media, and postoperative complications (ie, infection and late sequelae).
Inability to insert the hysteroscope
This may be caused by a stenotic, nulliparous cervix; menopause; GnRH agonists; previous cone biopsy, laceration, or cryosurgery; or an acutely retroflexed or anteflexed uterus.
Acute flexion problems can be corrected using a long-bladed, open-sided Graves speculum deep in the anterior or posterior fornix. The speculum pushes the fundus to the midposition and facilitates dilation. Once the hysteroscope is inserted, remove the speculum.
Placing a tenaculum on the posterior lip of the cervix of an acutely retroflexed uterus will straighten the cervical canal when traction is applied.
Inserting a laminaria tent the evening before surgery helps dilate the cervix easily and atraumatically.4 However, the laminaria can sometimes create a false passage, leading to perforation.
Cervical ripening agents such as intravaginal or oral misoprostol (200 μg inserted vaginally or 400 μg orally 8 to 12 hours preoperatively) also can facilitate dilation.
Intracervical injection of vasopressin solution (4 IU in 100 cc sodium chloride, injected at the 4 and 8 o’clock positions) can reduce the force needed to dilate the cervix.5 Half-size dilators may help; they also reduce the risk of cervical laceration.
Laceration of the cervix
Although this is a minor complication, substantial bleeding sometimes occurs when the cervix is lacerated by the tenaculum. In these cases, suture the cervix.
Occasionally, a touch of cautery from the rollerball electrode at low power (20 to 30 W) can control the bleeding.
Silver nitrate sticks or ferric subsulfate (Monsel’s) paste are also effective on superficial lacerations.
Bleeding from lower uterus or cervical canal can obscure view
In some cases, bleeding is delayed, necessitating additional surgery. Intravasation of distention fluid also can occur at these lacerations. Coagulation with the electrode may be necessary when bleeding is heavy.
Check for collateral injury when uterine perforation occurs
Perforation is a well-documented risk of operative hysteroscopy and should be discussed with the patient when obtaining informed consent. In the AAGL survey,1 the incidence of perforation was 14 per 1,000. It was even higher during transection of lateral and fundal adhesions: 2 to 3 per 100.6
Although perforation is more common with thermal energy sources, it may occur mechanically when scissors are used to transect a uterine septum, synechiae, or polyps.
When the cervix is stenotic or the uterus is acutely ante- or retroflexed, sounds and dilators can perforate the uterus.
Most perforations—even those involving large dilators—usually do not require treatment, although further assessment may be necessary to rule out bowel injury. Most perforations occur in the fundal region or posterior lower segment.
A false passage can be created when entering the uterus. Occasionally the surgeon may be fooled into thinking the hysteroscope is in the uterine cavity, since the false passage distends (FIGURE 1). If muscle fibers are visible and the tubal ostea are not, assume the passage is false. Slowly remove the hysteroscope and identify the true cavity for confirmation. Discontinue the procedure—even if no perforation is detected—to prevent distention fluid from being absorbed into the circulation through the injury. Adequate distention is not possible at this time.
Delay repeat hysteroscopy for 2 to 3 months.
To avoid creating a false passage, dilate the cervix with slow, steady pressure and stop as soon as the internal os opens; do not attempt to push the dilator to the uterine fundus.
Often the external os opens, but the internal os cannot be dilated the extra 1 to 2 mm necessary to accommodate the 27-French resectoscope. Rather than exert more force and risk perforation or laceration, simply turn on the resectoscope’s inflow with the outflow shut off, and let the fluid pressure dilate the cervix.
Always insert the hysteroscope or resectoscope under direct vision rather than use an obturator. Keep the “dark circle” in the center of the field and slowly advance the hysteroscope toward it until the cavity is reached.
Avulsion of the myometrium sometimes occurs during removal of incompletely resected myomas (FIGURE 2). Keep the myoma grasper away from the fundus when removing myoma segments, and avoid excessive traction on what may be a thin segment of myometrium. Injuries can occur when the grasper perforates the uterus and bowel is inadvertently grasped. Large injuries require laparoscopic repair.
Perforation is more likely in repeat procedures. In a report of 80 repeat endometrial ablations, Townsend and colleagues7 noted 8 perforations that prevented completion of the procedure. In a series of 75 repeat ablations compared with 800 primary ablations by the same surgeon, the rate of serious perioperative complications was significantly higher in the repeat ablation group (9.3% versus 2.0%).8
When perforation occurs during the use of thermal energy, laparoscopy is necessary to assess the organs overlying the site.9 During setup for laparoscopy, bring the hysteroscope near the area of perforation to inspect the bowel beyond the uterus. Since the pelvis fills quickly with distention fluid, the hysteroscope can even be placed through the perforation to yield an excellent view of the undersurfaces of the bowel immediately adjacent to the injured area (FIGURE 3). (Disconnect the electrosurgical cord before doing this!)
Thorough laparoscopic inspection of the bowel in the pelvis often reveals thermal injury, which appears as a whitish patch on the bowel serosa. To repair bowel injuries, bring the injured segment out through a minilaparotomy and excise the damage with a 2- to 3-cm border. A general surgeon should be called in to consult.
A 42-year-old woman who underwent endometrial ablation 2 years earlier presents with persistent menorrhagia and a 12-week–size fibroid uterus and expresses a desire for repeat ablation. At the second surgery, the uterine cavity appears scarred, with multiple synechiae.
As the procedure progresses, the uterine serosa becomes visible from within the cavity, appearing as a smooth, bluish structure that can be moved with only slight pressure. As the ablation continues, the uterus perforates, necessitating laparoscopic inspection of the organs overlying the site.
Although the patient recovers, her menorrhagia eventually returns, and she opts for laparoscopically assisted vaginal hysterectomy 1 year later.
Uterine perforation is more likely during repeat procedures
This case illustrates one of the most common risks of operative hysteroscopy: uterine perforation, which occurs more frequenly in repeat procedures.
The case also highlights an important indicator of perforation: the serosal sign, which I first described in 1996.24 When the smooth, bluish structure appears, cease ablation in the region immediately.
If no injury is apparent, discharge the patient but follow her closely, including daily white blood cell counts for 4 to 5 days. Instruct her to take her temperature twice daily and return to the hospital immediately if any signs of bowel perforation develop. Delayed perforation from thermal injury can occur as late as 2 weeks following surgery, and the patient should be apprised of this possibility.
FIGURE 1 Signs of a false passage
Myometrial fibers signal that a false passage has been created. Stop the procedure even if no perforation is detected, to prevent distention fluid from being absorbed into the circulation through the injury.
FIGURE 2 Risk of myomectomy: Myometrial avulsion
Small bowel visible within the uterine cavity after avulsion of uterine wall at the time of myomectomy
FIGURE 3 Use the hysteroscope to assess perforation site
Hysteroscopic view of perforation at the fundus. The small bowel is visible beyond the perforation at left.
Intraoperative bleeding is rare
Bleeding is unlikely unless vessels are lacerated or injured in the cervical canal or lower uterine segment during dilation or deep ablation or vaporization. Bleeding is more common when endomyometrial resection is performed with the wire loop electrode or during ablation or vaporization of fibroids. Bleeding sufficient to require intervention occurs at a rate of 0.5% to 1.9% in several reported series.
To achieve hemostasis via balloon tamponade, insert a Foley catheter with a 30-cc balloon into the uterine cavity, inject 15 to 20 mL (or more for a larger cavity) of fluid into the balloon, and observe the patient.10 If there is no bleeding in 1 hour, remove half the fluid. Remove the remainder of the fluid and the catheter over the next hour if no further bleeding occurs.
Alternative method: Pack the uterus. I prefer 1/2-inch–gauge packing that has been soaked in a dilute vasopressin solution (20 U [1 mL] in 60 mL normal saline).11
The benefits of vasopressin. Before balloon tamponade or packing the uterus, I inject very dilute vasopressin (4 U [0.2 mL] in 60 mL normal saline) directly into the cervix 2 cm deep, at the 4 and 8 o’clock positions. Phillips12 demonstrated a marked decrease in blood loss during resectoscopic surgery using this approach. I also do this routinely prior to operative hysteroscopy, since the vasopressin-induced vasoconstriction reduces intravasation of distention media.
A vaporizing electrode may prevent significant blood loss during myoma resection by sealing blood vessels as the tissue is vaporized.13 All major manufacturers of hysteroscopic equipment produce these electrodes.
In my series of 44 endometrial ablations and hysteroscopic myomectomies performed with the vaporizing electrode, blood loss was “minimal” or less than 50 mL in 29 cases. The maximum blood loss was 300 mL in a patient with a 4-cm submucous myoma who was managed emergently for hemorrhage.14 In another case, during resection of a 5-cm submucous myoma, I encountered significant bleeding from large vessels at the base of the myoma, which required intrauterine tamponade with a vasopressin-soaked pack.
Preoperative measures may decrease vascularity. In their analysis of 16 randomized and nonrandomized controlled trials published in the English literature between 1990 and 1996, Parazzini and colleagues15 found that preoperative danazol or GnRH agonists decreased the thickness and vascularity of the endometrium and shrank myomata, resulting in shorter operating times, less blood loss, and less intravasation of distending fluid.
Electrosurgical and gaseous complications
Most electrosurgical complications involve activation of an electrode at the time of perforation, or current diversion to the outer sheath.
Thermal injuries also can be caused by overheating of the return pad or use of a weighted speculum that has not fully cooled after removal from the autoclave. The latter can be avoided by immersing the entire speculum in cool saline for at least 1 to 2 minutes prior to inserting it into the vagina. The blade cools much faster than the weighted ball, so be sure to check both to prevent a perineal or buttock burn.
Perforation with an active electrode
This usually occurs when current is applied as the electrode is extended or the resectoscope is moved toward the fundus. It can be avoided if the electrode is activated only when moving it toward the operator.
Perforations with intraabdominal burns also have occurred during attempts to coagulate bleeders—especially in the cornual regions.
Diversion of current can be destructive
Genital tract injuries have occurred as a result of current diversion. Vilos and colleagues16 reported 13 electrical burns during endometrial ablation, and mention many more anecdotal reports. The usual cause: electrode insulation failure, which allows current to jump to the outer sheath of the resectoscope.
To avoid this, inspect all electrodes thoroughly before surgery and use them only once.
Capacitative coupling also diverts current
Since the sheath-within-a-sheath design of the resectoscope resembles a capacitor, high-voltage current can jump to the outer sheath without direct contact from the electrode. When Munro17 bench-tested electrosurgical generators and electrodes with and without insulation defects, he found that capacitative coupling with intact electrodes occurred more frequently with high-voltage coagulation current than with lower-voltage cutting current.
One way to avoid these injuries is to activate the electrode intermittently, with short bursts, rather than rolling back and forth over an area with continuous current. Another strategy is placing a damp sponge in the posterior vagina extending out the introitus; this protects the mucosa and perineal skin—especially in obese patients.
How to avoid return-pad injuries
Keep the patient’s thigh completely dry; ensure that the pad is flat against the skin at application, with no bubbles or creases; and use only return electrode monitor (REM) dispersive pads.
Especially when using vaporization electrodes, avoid prolonged activation of the electrode at high power. To minimize risk of vaporization, use a second dispersive pad connected to the first via a “y” connector to further disperse current and heat at the return pad.
Also, limit the use of coagulation current and use a maximum generator setting of 60 to 80 W in the coagulation mode.
Take steps to avert gas embolism, but watch closely for signs
Initial reports of this potentially fatal complication came mostly from laser ablation procedures, but gas embolism can occur during all diagnostic and operative hysteroscopic procedures, especially the latter.
Sources of gas embolism: room air, carbon dioxide, carbon monoxide, and other gaseous products of vaporization or tissue combustion. The anesthesiologist is usually the first to identify the signs.
Signs of gas embolism. The surgeon should ask to be immediately alerted to any sudden fall in oxygen saturation, as well as to hypotension, hypercarbia, arrhythmias, tachypnea, or a “mill wheel” murmur. If any of these signs are detected and a gas embolism is suspected, stop the procedure and ventilate the patient with 100% oxygen.
Carbon dioxide is a soluble gas, so these emboli generally resolve rapidly. In contrast, room air emboli are more likely to be fatal.
Reduce the risk of air embolism by avoiding the Trendelenburg position and leaving the last dilator in the cervix until just before inserting the resectoscope.
Also limit repetitive removal and reinsertion of the resectoscope, as often occurs during myoma resection. By vaporizing rather than resecting myomas, it is possible to eliminate the need to continually remove fibroid chips. Preoperative GnRH agonists narrow venous sinuses and help prevent this complication.
Intracervical injection of dilute vasopressin prior to dilatation of the cervix creates vascular spasm and may help prevent gas from entering the circulation.
Complications from distention media
Excess absorption of distention media is one of the most frequent complications. Most surgeons use low-viscosity, sodium-free fluids for operative hysteroscopy, since fluids that contain electrolytes are incompatible with monopolar electrosurgical instruments. The use of 3% sorbitol, 1.5% glycine, or sorbitol-mannitol solutions can lead to dilutional hyponatremia and hypoosmolality.18 Although the vast majority of women quickly recover from these conditions, some cases of permanent morbidity and even death have been reported.19 The overall incidence of dilutional hyponatremia was 0.2% in 1993, according to the AAGL member survey.1
Hyponatremia and hypoosmolality more likely in premenopausal women
These conditions may have catastrophic consequences if they are not recognized and corrected promptly. The brain swells as it attempts to become isoosmotic with the vascular system. If swelling exceeds 5%, the risk of severe neurological damage dramatically increases.
This is an important problem in premenopausal women, since estrogen and progesterone inhibit sodium-potassium adenosine triphosphatase (ATPase) activity in the brain. This sodium pump protects the brain against cerebral edema, which can cause herniation of the brain stem and death. Although men and postmenopausal women develop dilutional hyponatremia, they are less likely to suffer brain damage because the sodium pump is intact.
Taskin et al20 conducted a randomized trial showing an increase in the sodium-potassium ATPase pump activity and decreased volume deficit during hysteroscopic surgery in patients pretreated with a GnRH agonist, compared with a control group. This increased pump activity in the brain and endometrium may decrease women’s susceptibility to hyponatremic complications and brain damage.
Vigilant monitoring of fluid intake/output during hysteroscopic surgery is necessary to prevent hyponatremic complications. Avoid the pitfall of erroneously attributing deficits to fluid “in the drapes” by using drapes with a fluid-collection pouch.
The standard of care is use of electronic inflow-outflow measuring systems. Manufacturers of hysteroscopic equipment offer highly accurate electronic fluid monitoring systems that measure the weight of the distending fluid infused and collected rather than relying on manual estimation of deficit. The latter method may be inaccurate since the volume of the supply bag can vary by as much as 10%.
Adjust intrauterine pressure to reduce the likelihood of intravasation. High intrauterine pressure may be desirable for visualization, but it greatly increases the risk of intravasation.
I adjust pressure and flow rates by opening or closing the inflow and outflow valves of the resectoscope until slight amounts of bleeding from resected tissue can be visualized. Since intrauterine pressure is extremely difficult to monitor accurately during operative hysteroscopy, this practice ensures that it remains below the patient’s mean arterial pressure, thus minimizing the risk of intravasation.
Use a dilute vasopressin injection to constrict blood vessels and decrease the chance of intravasation.
Vaporizing electrodes for myoma resection and ablation seal blood vessels and reduce fluid absorption.
Guidelines for distention media
To reduce the likelihood of these complications, I recommend that surgeons:
- Draw preoperative serum electrolytes for a baseline in all patients.
- Give all patients with myomas 2 monthly injections of depot leuprolide acetate (3.75 mg intramuscularly). Give patients without fibroids a single injection 4 to 6 weeks prior to the procedure.
- Place a fluid-collection drape or a larger, plastic Mayo stand cover with the bottom cut off under the patient’s buttocks so that fluid drains into a “kick” bucket. Also adjust the resectoscope’s outflow tubing to drain into the collection bag, which should be kept on constant suction to the flow-stat electronic fluid monitor.
- Continuously record inflow and outflow using the electronic monitor with the deficit alarm set to 500 mL.
- Keep distention fluid at room temperature and monitor the patient’s core temperature continuously. Significant fluid intravasation will lower the patient’s temperature, and this may be the first sign of fluid overload.
- Perform operative hysteroscopy under spinal or epidural anesthesia so the anesthesiologist can continually assess the patient’s sensorium. Confusion and irritability are early signs of dilutional hyponatremia.
- If the fluid deficit reaches 750 mL, immediately give 20 to 40 mg of intravenous furosemide and draw a serum sodium. Do not wait for the result of the sodium level before treatment, since a 5- to 20-minute delay can be catastrophic.
- Interrupt the procedure for 5 to 10 minutes to allow the uterus to contract and to seal off small blood vessels.
- Discontinue the procedure if the fluid deficit reaches 1,500 mL or if the serum sodium level is below 125 mEq/L.
I do not limit the duration of resectoscopic procedures as long as fluid deficits are below 750 mL, as measured by electronic fluid monitor. I also ensure that the operating room staff is well educated in the use of the monitor and able to troubleshoot intraoperatively.
If the machine fails during the procedure, reset it with the alarm limit lowered to reflect the deficit recorded before failure.
Monitor the color of the outflow fluid. Excessive blood loss counted as part of the outflow can occasionally mask a distention fluid deficit.
Choosing a distention medium
There is no ideal distention medium for monopolar operative hysteroscopy. Several authors have suggested that 5% mannitol is advantageous since it is isosmolar and acts as an osmotic diuretic. However, it does not prevent hyponatremia. The main disadvantage of 5% mannitol is its high cost and limited availability in 3-L bags or 4-L bottles.
The use of bipolar devices in normal saline prevents dilutional hyponatremia, but fluid deficits must still be monitored electronically so they do not exceed 2,000 mL. The false sense of security that may occur when normal saline is used for distention may lead to inaction when a large deficit occurs. This can lead to pulmonary edema and death.
Postoperative and late complications
These include infection, endometrial cancer, iatrogenic adenomyosis, hematometria, post-ablation tubal ligation syndrome, and pregnancy.
Infection rate is 0.3% to 2%
Infection is relatively rare following endometrial ablation, with a rate of 0.3% to 0.5% reported in most series. Endometritis, parametritis, and pyometra are more common following resection of submucous myomas, with rates as high as 2% reported.
Infection is more likely after prolonged procedures, especially when the hysteroscope is repeatedly inserted and removed. It also is more likely if the patient has a history of pelvic inflammatory disease. I generally administer prophylactic antibiotics (1 dose of intravenous ceftizoxime, 1 g, approximately 30 to 60 minutes prior to surgery).
I also insert a laminaria tent the evening prior to surgery. Patients with a history of pelvic inflammatory disease are discharged on doxycycline (100 mg twice daily for 7 days).
Be alert for endometrial cancer
This malignancy has been diagnosed at the time of endometrial ablation and reported in patients who have undergone prior endometrial ablations or fibroid resections. Thus, endometrial sampling should be part of the workup of abnormal uterine bleeding before the patient is scheduled for operative hysteroscopy. In women at high risk for endometrial cancer, perform office diagnostic hysteroscopy, with directed biopsy of any suspicious areas.
When viable endometrial glands are “buried” during ablation, or synechiae develop, preventing the egress of blood, there is a chance that diagnosis of endometrial cancer will be delayed. However, this theoretical fear has not been proven clinically.
Patients whose abnormal bleeding recurs after ablation should undergo sampling and office hysteroscopy, just as if they had not undergone a previous ablation. Theoretically, women who undergo endomyometrial resection or vaporization should have a lower incidence of endometrial cancer, since the tissue most susceptible to malignancy is removed. This has not yet been proven scientifically.
In their comprehensive review of late complications of operative hysteroscopy, Cooper and Brady21 suggest that patients at high risk for endometrial cancer who present with abnormal uterine bleeding not controlled by hormones might be better served by hysterectomy. Unfortunately, these patients tend to be high-risk surgical candidates.
If atypia is present, do not perform endometrial ablation or resection. I do perform ablation and resection in patients with complex hyperplasia without atypia if it has been reversed with progestin and does not recur for at least 6 months without progestin therapy. These patients undergo office hysteroscopy and sampling of the endometrium before operative hysteroscopy is scheduled.
Iatrogenic adenomyosis
Two theories suggest this is a late complication of operative hysteroscopy. According to the first, when the endometrium is incompletely resected, scarring over this tissue causes the viable glands to grow into the myometrium. The other theory suggests that viable endometrial debris is transported into the myometrium by vessels opened at resection.
I have found that using the vaporization electrode followed by application of a rollerball over the surface of the cavity most effectively reaches maximal tissue depth and, theoretically, prevents adenomyosis. Since most adenomyosis occurs on the posterior wall, I take a strip from this area for pathologic analysis to determine whether adenomyosis preceded the ablation or developed subsequent to it.
Hematometria
This can occur following operative hysteroscopy if viable glands are left in the fundal or cornual region and synechiae develop in the lower segment, preventing egress of blood. It also can occur if the upper endocervix is ablated and subsequently scars, causing stenosis. To avoid this, ablate only to the level of the internal os.
Diagnosis and treatment. Hematometria can be diagnosed easily by ultrasound and treated with office hysteroscopy using a narrow-diameter, rigid, continuous-flow hysteroscope with an operating channel to pass small instruments.
Post-ablation tubal ligation syndrome
This is cornual hematometria that develops when viable endometrial cells are left in the cornua when the cavity also contains synechiae, causing cyclic bleeding. Since there is no egress from the cervix or tubes, blood gradually builds up, leading to hematosalpinx and pain. One way to avoid this is to ensure complete ablation of the cornual endometrium.
Prevention. Some experts recommend that the small rollerball electrode be placed in the cornua, with slightly reduced intrauterine pressure, to allow the cornual endometrium to collapse around the rollerball. A short burst of current is then applied to ablate the tissue.
This complication is less likely after hydrothermablation, since the free-flowing saline ablates the cornua completely. After more than 50 hydrothermablations performed in patients with prior tubal sterilizations, I have not seen any cases of post-ablation tubal ligation syndrome.
Treatment consists of bilateral salpingectomy or tubal fulguration at the cornual region and repeat ablation or resection of viable endometrial tissue. Another option is hysterectomy.
Post-ablation pregnancy can be very complicated
Pregnancy after endometrial ablation occurs at a rate of 0.2% to 1.6%. Counsel patients that this procedure does not prevent pregnancy and that contraception is vital. Uterine rupture after fibroid resection has been reported.
In a review of 37 post-ablation pregnancies, only 11 of 17 women who chose not to terminate carried the gestation beyond 28 weeks. In addition, there was a high incidence of intrauterine growth restriction, prematurity, and placenta accreta.22
Hysteroscopic tubal sterilization with the Essure system (Conceptus, San Carlos, Calif), or laparoscopic tubal fulguration performed at the time of ablation averts these complications.
Complications of global ablation
Global ablation technologies were developed to enable gynecologists with limited operative hysteroscopy skills to perform endometrial ablation and to make ablation safer for the patient. These technologies completely eliminate the risk of distention-media complications, but widespread use has resulted in other complications that have been reported in the literature to only a limited extent.
Most published articles on global endometrial ablation are from the original US Food and Drug Administration (FDA) trials, in which the complication rates were extraordinarily low. Widespread commercial use of these technologies since FDA approval, especially by practitioners with limited skills, has increased complications.
Do not override safety systems
Complications are more frequent when devices are misused or safety systems overridden. And, fear of litigation makes physicians unwilling to report complications.
In the FDA Manufacturer and User Facility Device Experience (MAUDE) database (www.fda.gov.cdrh/maude.html), complications include bowel burns after unrecognized perforation, and bowel burns associated with electrosurgical, microwave energy, or heat transferred through intact myometrium.23 Vaginal burns, uterine necrosis myometritis requiring hysterectomy, and death from unrecognized bowel burn also have been reported.
Most global procedures are performed blindly, and some doctors fail to perform diagnostic hysteroscopy before and after surgery, which I feel is mandatory with any endometrial ablation. Hydrothermablation is the only global technique that has the advantage of direct observation. In more than 150 procedures done in my office under local anesthesia, the only complications were 2 false passages. Both were promptly identified during diagnostic hysteroscopy, and the surgery was rescheduled 2 to 3 months later.
The author has served on the speakers’ bureau for Boston Scientific.
- Perform endometrial sampling for abnormal uterine bleeding before scheduling operative hysteroscopy.
- Most uterine perforations do not require treatment— even those involving large dilators—although further assessment may be necessary to rule out bowel injury.
- Most complications of electrosurgery involve activating an electrode at the time of perforation, or diverting current to the outer sheath.
- Scrupulously monitor fluid intake and output to prevent hyponatremic complications.
WHAT WENT WRONG?
A 44-year-old woman undergoing resection of a submucous myoma from the left cornual region has persistent bleeding at the resection site. The surgeon continues coagulation at the bleeding site, using a rollerball electrode in an attempt to achieve hemostasis, but perforates the uterus. Immediate laparoscopy to identify collateral injury reveals some thermal damage on the posterior leaf of the broad ligament, but no bowel injury. After 24 hours of observation, she is afebrile without leukocytosis. She is discharged with explicit instructions to return if she has symptoms suggesting bowel injury. She returns in 72 hours, with abdominal pain and low-grade fever. CT reveals extravasation of contrast from the left ureter in the pelvis. Immediate laparotomy finds perforation of the left ureter secondary to a thermal injury. She undergoes ureteroneocystotomy and recovers.
This case illustrates one of the most common complications of operative hysteroscopy: uterine perforation with collateral injury. Both could have been avoided if the Ob/Gyn had stopped the procedure when bleeding first occurred, removed the instruments, and allowed the uterus to contract spontaneously.
This is just one of the strategies that can reduce the risks of hysteroscopic surgery. Numerous reports confirm that operative hysteroscopy is safe and effective, but as more gynecologists perform an increasing number of procedures, we must be aware of potential complications and do our best to minimize risk to our patients.
Complications cannot be completely avoided, and may occur when a procedure is done correctly by experienced doctors. They are far more likely if techniques or equipment are used improperly. This article describes ways to minimize risk.
When the American Association of Gynecologic Laparoscopists (AAGL) surveyed its members in 1993, it found a complication rate of 2% for operative hysteroscopy.1 The rate of major complications—perforation, hemorrhage, fluid overload, and bowel or urinary tract injury—was less than 1%. A prospective multicenter trial2 of 13,600 procedures in the Netherlands found a higher complication rate for operative (0.95%) than for diagnostic hysteroscopy (0.13%).
Preoperative precautions
We can reduce the risk of complications if contraindications are not ignored, equipment is thoroughly inspected and understood, and the surgeon goes through a mental checklist and plans each procedure. A “time out”before the operation begins, when every member of the team is briefed, is also valuable in preventing errors.
A hands-on course necessary before undertaking advanced resectoscopic surgery, to become familiar with equipment and techniques, followed by proctoring by a surgeon credentialed for the procedure.
Contraindications
Ignoring contraindications to hysteroscopic surgery increases the risk of complications and is the single greatest factor leading to patient injury and physician liability.
Contraindications include:
- Unfamiliarity with equipment, instruments, or technique
- Lack of appropriate equipment or staff familiar with the equipment
- Acute pelvic inflammatory disease
- Pregnancy
- Genital tract malignancies
- Lack of informed consent
- Inability to dilate the cervix
- Inability to distend the uterus to obtain visualization
- Poor surgical candidates who may not tolerate fluid overload because of renal disease, or radiofrequency current when a cardiac pacemaker is present
- The patient desires and expects complete amenorrhea3
Mechanical or traumatic complications
These types of complications are among the most common. Other categories include preoperative complications (ie, improper patient selection and lack of informed consent), electrosurgical and gaseous, complications related to distention media, and postoperative complications (ie, infection and late sequelae).
Inability to insert the hysteroscope
This may be caused by a stenotic, nulliparous cervix; menopause; GnRH agonists; previous cone biopsy, laceration, or cryosurgery; or an acutely retroflexed or anteflexed uterus.
Acute flexion problems can be corrected using a long-bladed, open-sided Graves speculum deep in the anterior or posterior fornix. The speculum pushes the fundus to the midposition and facilitates dilation. Once the hysteroscope is inserted, remove the speculum.
Placing a tenaculum on the posterior lip of the cervix of an acutely retroflexed uterus will straighten the cervical canal when traction is applied.
Inserting a laminaria tent the evening before surgery helps dilate the cervix easily and atraumatically.4 However, the laminaria can sometimes create a false passage, leading to perforation.
Cervical ripening agents such as intravaginal or oral misoprostol (200 μg inserted vaginally or 400 μg orally 8 to 12 hours preoperatively) also can facilitate dilation.
Intracervical injection of vasopressin solution (4 IU in 100 cc sodium chloride, injected at the 4 and 8 o’clock positions) can reduce the force needed to dilate the cervix.5 Half-size dilators may help; they also reduce the risk of cervical laceration.
Laceration of the cervix
Although this is a minor complication, substantial bleeding sometimes occurs when the cervix is lacerated by the tenaculum. In these cases, suture the cervix.
Occasionally, a touch of cautery from the rollerball electrode at low power (20 to 30 W) can control the bleeding.
Silver nitrate sticks or ferric subsulfate (Monsel’s) paste are also effective on superficial lacerations.
Bleeding from lower uterus or cervical canal can obscure view
In some cases, bleeding is delayed, necessitating additional surgery. Intravasation of distention fluid also can occur at these lacerations. Coagulation with the electrode may be necessary when bleeding is heavy.
Check for collateral injury when uterine perforation occurs
Perforation is a well-documented risk of operative hysteroscopy and should be discussed with the patient when obtaining informed consent. In the AAGL survey,1 the incidence of perforation was 14 per 1,000. It was even higher during transection of lateral and fundal adhesions: 2 to 3 per 100.6
Although perforation is more common with thermal energy sources, it may occur mechanically when scissors are used to transect a uterine septum, synechiae, or polyps.
When the cervix is stenotic or the uterus is acutely ante- or retroflexed, sounds and dilators can perforate the uterus.
Most perforations—even those involving large dilators—usually do not require treatment, although further assessment may be necessary to rule out bowel injury. Most perforations occur in the fundal region or posterior lower segment.
A false passage can be created when entering the uterus. Occasionally the surgeon may be fooled into thinking the hysteroscope is in the uterine cavity, since the false passage distends (FIGURE 1). If muscle fibers are visible and the tubal ostea are not, assume the passage is false. Slowly remove the hysteroscope and identify the true cavity for confirmation. Discontinue the procedure—even if no perforation is detected—to prevent distention fluid from being absorbed into the circulation through the injury. Adequate distention is not possible at this time.
Delay repeat hysteroscopy for 2 to 3 months.
To avoid creating a false passage, dilate the cervix with slow, steady pressure and stop as soon as the internal os opens; do not attempt to push the dilator to the uterine fundus.
Often the external os opens, but the internal os cannot be dilated the extra 1 to 2 mm necessary to accommodate the 27-French resectoscope. Rather than exert more force and risk perforation or laceration, simply turn on the resectoscope’s inflow with the outflow shut off, and let the fluid pressure dilate the cervix.
Always insert the hysteroscope or resectoscope under direct vision rather than use an obturator. Keep the “dark circle” in the center of the field and slowly advance the hysteroscope toward it until the cavity is reached.
Avulsion of the myometrium sometimes occurs during removal of incompletely resected myomas (FIGURE 2). Keep the myoma grasper away from the fundus when removing myoma segments, and avoid excessive traction on what may be a thin segment of myometrium. Injuries can occur when the grasper perforates the uterus and bowel is inadvertently grasped. Large injuries require laparoscopic repair.
Perforation is more likely in repeat procedures. In a report of 80 repeat endometrial ablations, Townsend and colleagues7 noted 8 perforations that prevented completion of the procedure. In a series of 75 repeat ablations compared with 800 primary ablations by the same surgeon, the rate of serious perioperative complications was significantly higher in the repeat ablation group (9.3% versus 2.0%).8
When perforation occurs during the use of thermal energy, laparoscopy is necessary to assess the organs overlying the site.9 During setup for laparoscopy, bring the hysteroscope near the area of perforation to inspect the bowel beyond the uterus. Since the pelvis fills quickly with distention fluid, the hysteroscope can even be placed through the perforation to yield an excellent view of the undersurfaces of the bowel immediately adjacent to the injured area (FIGURE 3). (Disconnect the electrosurgical cord before doing this!)
Thorough laparoscopic inspection of the bowel in the pelvis often reveals thermal injury, which appears as a whitish patch on the bowel serosa. To repair bowel injuries, bring the injured segment out through a minilaparotomy and excise the damage with a 2- to 3-cm border. A general surgeon should be called in to consult.
A 42-year-old woman who underwent endometrial ablation 2 years earlier presents with persistent menorrhagia and a 12-week–size fibroid uterus and expresses a desire for repeat ablation. At the second surgery, the uterine cavity appears scarred, with multiple synechiae.
As the procedure progresses, the uterine serosa becomes visible from within the cavity, appearing as a smooth, bluish structure that can be moved with only slight pressure. As the ablation continues, the uterus perforates, necessitating laparoscopic inspection of the organs overlying the site.
Although the patient recovers, her menorrhagia eventually returns, and she opts for laparoscopically assisted vaginal hysterectomy 1 year later.
Uterine perforation is more likely during repeat procedures
This case illustrates one of the most common risks of operative hysteroscopy: uterine perforation, which occurs more frequenly in repeat procedures.
The case also highlights an important indicator of perforation: the serosal sign, which I first described in 1996.24 When the smooth, bluish structure appears, cease ablation in the region immediately.
If no injury is apparent, discharge the patient but follow her closely, including daily white blood cell counts for 4 to 5 days. Instruct her to take her temperature twice daily and return to the hospital immediately if any signs of bowel perforation develop. Delayed perforation from thermal injury can occur as late as 2 weeks following surgery, and the patient should be apprised of this possibility.
FIGURE 1 Signs of a false passage
Myometrial fibers signal that a false passage has been created. Stop the procedure even if no perforation is detected, to prevent distention fluid from being absorbed into the circulation through the injury.
FIGURE 2 Risk of myomectomy: Myometrial avulsion
Small bowel visible within the uterine cavity after avulsion of uterine wall at the time of myomectomy
FIGURE 3 Use the hysteroscope to assess perforation site
Hysteroscopic view of perforation at the fundus. The small bowel is visible beyond the perforation at left.
Intraoperative bleeding is rare
Bleeding is unlikely unless vessels are lacerated or injured in the cervical canal or lower uterine segment during dilation or deep ablation or vaporization. Bleeding is more common when endomyometrial resection is performed with the wire loop electrode or during ablation or vaporization of fibroids. Bleeding sufficient to require intervention occurs at a rate of 0.5% to 1.9% in several reported series.
To achieve hemostasis via balloon tamponade, insert a Foley catheter with a 30-cc balloon into the uterine cavity, inject 15 to 20 mL (or more for a larger cavity) of fluid into the balloon, and observe the patient.10 If there is no bleeding in 1 hour, remove half the fluid. Remove the remainder of the fluid and the catheter over the next hour if no further bleeding occurs.
Alternative method: Pack the uterus. I prefer 1/2-inch–gauge packing that has been soaked in a dilute vasopressin solution (20 U [1 mL] in 60 mL normal saline).11
The benefits of vasopressin. Before balloon tamponade or packing the uterus, I inject very dilute vasopressin (4 U [0.2 mL] in 60 mL normal saline) directly into the cervix 2 cm deep, at the 4 and 8 o’clock positions. Phillips12 demonstrated a marked decrease in blood loss during resectoscopic surgery using this approach. I also do this routinely prior to operative hysteroscopy, since the vasopressin-induced vasoconstriction reduces intravasation of distention media.
A vaporizing electrode may prevent significant blood loss during myoma resection by sealing blood vessels as the tissue is vaporized.13 All major manufacturers of hysteroscopic equipment produce these electrodes.
In my series of 44 endometrial ablations and hysteroscopic myomectomies performed with the vaporizing electrode, blood loss was “minimal” or less than 50 mL in 29 cases. The maximum blood loss was 300 mL in a patient with a 4-cm submucous myoma who was managed emergently for hemorrhage.14 In another case, during resection of a 5-cm submucous myoma, I encountered significant bleeding from large vessels at the base of the myoma, which required intrauterine tamponade with a vasopressin-soaked pack.
Preoperative measures may decrease vascularity. In their analysis of 16 randomized and nonrandomized controlled trials published in the English literature between 1990 and 1996, Parazzini and colleagues15 found that preoperative danazol or GnRH agonists decreased the thickness and vascularity of the endometrium and shrank myomata, resulting in shorter operating times, less blood loss, and less intravasation of distending fluid.
Electrosurgical and gaseous complications
Most electrosurgical complications involve activation of an electrode at the time of perforation, or current diversion to the outer sheath.
Thermal injuries also can be caused by overheating of the return pad or use of a weighted speculum that has not fully cooled after removal from the autoclave. The latter can be avoided by immersing the entire speculum in cool saline for at least 1 to 2 minutes prior to inserting it into the vagina. The blade cools much faster than the weighted ball, so be sure to check both to prevent a perineal or buttock burn.
Perforation with an active electrode
This usually occurs when current is applied as the electrode is extended or the resectoscope is moved toward the fundus. It can be avoided if the electrode is activated only when moving it toward the operator.
Perforations with intraabdominal burns also have occurred during attempts to coagulate bleeders—especially in the cornual regions.
Diversion of current can be destructive
Genital tract injuries have occurred as a result of current diversion. Vilos and colleagues16 reported 13 electrical burns during endometrial ablation, and mention many more anecdotal reports. The usual cause: electrode insulation failure, which allows current to jump to the outer sheath of the resectoscope.
To avoid this, inspect all electrodes thoroughly before surgery and use them only once.
Capacitative coupling also diverts current
Since the sheath-within-a-sheath design of the resectoscope resembles a capacitor, high-voltage current can jump to the outer sheath without direct contact from the electrode. When Munro17 bench-tested electrosurgical generators and electrodes with and without insulation defects, he found that capacitative coupling with intact electrodes occurred more frequently with high-voltage coagulation current than with lower-voltage cutting current.
One way to avoid these injuries is to activate the electrode intermittently, with short bursts, rather than rolling back and forth over an area with continuous current. Another strategy is placing a damp sponge in the posterior vagina extending out the introitus; this protects the mucosa and perineal skin—especially in obese patients.
How to avoid return-pad injuries
Keep the patient’s thigh completely dry; ensure that the pad is flat against the skin at application, with no bubbles or creases; and use only return electrode monitor (REM) dispersive pads.
Especially when using vaporization electrodes, avoid prolonged activation of the electrode at high power. To minimize risk of vaporization, use a second dispersive pad connected to the first via a “y” connector to further disperse current and heat at the return pad.
Also, limit the use of coagulation current and use a maximum generator setting of 60 to 80 W in the coagulation mode.
Take steps to avert gas embolism, but watch closely for signs
Initial reports of this potentially fatal complication came mostly from laser ablation procedures, but gas embolism can occur during all diagnostic and operative hysteroscopic procedures, especially the latter.
Sources of gas embolism: room air, carbon dioxide, carbon monoxide, and other gaseous products of vaporization or tissue combustion. The anesthesiologist is usually the first to identify the signs.
Signs of gas embolism. The surgeon should ask to be immediately alerted to any sudden fall in oxygen saturation, as well as to hypotension, hypercarbia, arrhythmias, tachypnea, or a “mill wheel” murmur. If any of these signs are detected and a gas embolism is suspected, stop the procedure and ventilate the patient with 100% oxygen.
Carbon dioxide is a soluble gas, so these emboli generally resolve rapidly. In contrast, room air emboli are more likely to be fatal.
Reduce the risk of air embolism by avoiding the Trendelenburg position and leaving the last dilator in the cervix until just before inserting the resectoscope.
Also limit repetitive removal and reinsertion of the resectoscope, as often occurs during myoma resection. By vaporizing rather than resecting myomas, it is possible to eliminate the need to continually remove fibroid chips. Preoperative GnRH agonists narrow venous sinuses and help prevent this complication.
Intracervical injection of dilute vasopressin prior to dilatation of the cervix creates vascular spasm and may help prevent gas from entering the circulation.
Complications from distention media
Excess absorption of distention media is one of the most frequent complications. Most surgeons use low-viscosity, sodium-free fluids for operative hysteroscopy, since fluids that contain electrolytes are incompatible with monopolar electrosurgical instruments. The use of 3% sorbitol, 1.5% glycine, or sorbitol-mannitol solutions can lead to dilutional hyponatremia and hypoosmolality.18 Although the vast majority of women quickly recover from these conditions, some cases of permanent morbidity and even death have been reported.19 The overall incidence of dilutional hyponatremia was 0.2% in 1993, according to the AAGL member survey.1
Hyponatremia and hypoosmolality more likely in premenopausal women
These conditions may have catastrophic consequences if they are not recognized and corrected promptly. The brain swells as it attempts to become isoosmotic with the vascular system. If swelling exceeds 5%, the risk of severe neurological damage dramatically increases.
This is an important problem in premenopausal women, since estrogen and progesterone inhibit sodium-potassium adenosine triphosphatase (ATPase) activity in the brain. This sodium pump protects the brain against cerebral edema, which can cause herniation of the brain stem and death. Although men and postmenopausal women develop dilutional hyponatremia, they are less likely to suffer brain damage because the sodium pump is intact.
Taskin et al20 conducted a randomized trial showing an increase in the sodium-potassium ATPase pump activity and decreased volume deficit during hysteroscopic surgery in patients pretreated with a GnRH agonist, compared with a control group. This increased pump activity in the brain and endometrium may decrease women’s susceptibility to hyponatremic complications and brain damage.
Vigilant monitoring of fluid intake/output during hysteroscopic surgery is necessary to prevent hyponatremic complications. Avoid the pitfall of erroneously attributing deficits to fluid “in the drapes” by using drapes with a fluid-collection pouch.
The standard of care is use of electronic inflow-outflow measuring systems. Manufacturers of hysteroscopic equipment offer highly accurate electronic fluid monitoring systems that measure the weight of the distending fluid infused and collected rather than relying on manual estimation of deficit. The latter method may be inaccurate since the volume of the supply bag can vary by as much as 10%.
Adjust intrauterine pressure to reduce the likelihood of intravasation. High intrauterine pressure may be desirable for visualization, but it greatly increases the risk of intravasation.
I adjust pressure and flow rates by opening or closing the inflow and outflow valves of the resectoscope until slight amounts of bleeding from resected tissue can be visualized. Since intrauterine pressure is extremely difficult to monitor accurately during operative hysteroscopy, this practice ensures that it remains below the patient’s mean arterial pressure, thus minimizing the risk of intravasation.
Use a dilute vasopressin injection to constrict blood vessels and decrease the chance of intravasation.
Vaporizing electrodes for myoma resection and ablation seal blood vessels and reduce fluid absorption.
Guidelines for distention media
To reduce the likelihood of these complications, I recommend that surgeons:
- Draw preoperative serum electrolytes for a baseline in all patients.
- Give all patients with myomas 2 monthly injections of depot leuprolide acetate (3.75 mg intramuscularly). Give patients without fibroids a single injection 4 to 6 weeks prior to the procedure.
- Place a fluid-collection drape or a larger, plastic Mayo stand cover with the bottom cut off under the patient’s buttocks so that fluid drains into a “kick” bucket. Also adjust the resectoscope’s outflow tubing to drain into the collection bag, which should be kept on constant suction to the flow-stat electronic fluid monitor.
- Continuously record inflow and outflow using the electronic monitor with the deficit alarm set to 500 mL.
- Keep distention fluid at room temperature and monitor the patient’s core temperature continuously. Significant fluid intravasation will lower the patient’s temperature, and this may be the first sign of fluid overload.
- Perform operative hysteroscopy under spinal or epidural anesthesia so the anesthesiologist can continually assess the patient’s sensorium. Confusion and irritability are early signs of dilutional hyponatremia.
- If the fluid deficit reaches 750 mL, immediately give 20 to 40 mg of intravenous furosemide and draw a serum sodium. Do not wait for the result of the sodium level before treatment, since a 5- to 20-minute delay can be catastrophic.
- Interrupt the procedure for 5 to 10 minutes to allow the uterus to contract and to seal off small blood vessels.
- Discontinue the procedure if the fluid deficit reaches 1,500 mL or if the serum sodium level is below 125 mEq/L.
I do not limit the duration of resectoscopic procedures as long as fluid deficits are below 750 mL, as measured by electronic fluid monitor. I also ensure that the operating room staff is well educated in the use of the monitor and able to troubleshoot intraoperatively.
If the machine fails during the procedure, reset it with the alarm limit lowered to reflect the deficit recorded before failure.
Monitor the color of the outflow fluid. Excessive blood loss counted as part of the outflow can occasionally mask a distention fluid deficit.
Choosing a distention medium
There is no ideal distention medium for monopolar operative hysteroscopy. Several authors have suggested that 5% mannitol is advantageous since it is isosmolar and acts as an osmotic diuretic. However, it does not prevent hyponatremia. The main disadvantage of 5% mannitol is its high cost and limited availability in 3-L bags or 4-L bottles.
The use of bipolar devices in normal saline prevents dilutional hyponatremia, but fluid deficits must still be monitored electronically so they do not exceed 2,000 mL. The false sense of security that may occur when normal saline is used for distention may lead to inaction when a large deficit occurs. This can lead to pulmonary edema and death.
Postoperative and late complications
These include infection, endometrial cancer, iatrogenic adenomyosis, hematometria, post-ablation tubal ligation syndrome, and pregnancy.
Infection rate is 0.3% to 2%
Infection is relatively rare following endometrial ablation, with a rate of 0.3% to 0.5% reported in most series. Endometritis, parametritis, and pyometra are more common following resection of submucous myomas, with rates as high as 2% reported.
Infection is more likely after prolonged procedures, especially when the hysteroscope is repeatedly inserted and removed. It also is more likely if the patient has a history of pelvic inflammatory disease. I generally administer prophylactic antibiotics (1 dose of intravenous ceftizoxime, 1 g, approximately 30 to 60 minutes prior to surgery).
I also insert a laminaria tent the evening prior to surgery. Patients with a history of pelvic inflammatory disease are discharged on doxycycline (100 mg twice daily for 7 days).
Be alert for endometrial cancer
This malignancy has been diagnosed at the time of endometrial ablation and reported in patients who have undergone prior endometrial ablations or fibroid resections. Thus, endometrial sampling should be part of the workup of abnormal uterine bleeding before the patient is scheduled for operative hysteroscopy. In women at high risk for endometrial cancer, perform office diagnostic hysteroscopy, with directed biopsy of any suspicious areas.
When viable endometrial glands are “buried” during ablation, or synechiae develop, preventing the egress of blood, there is a chance that diagnosis of endometrial cancer will be delayed. However, this theoretical fear has not been proven clinically.
Patients whose abnormal bleeding recurs after ablation should undergo sampling and office hysteroscopy, just as if they had not undergone a previous ablation. Theoretically, women who undergo endomyometrial resection or vaporization should have a lower incidence of endometrial cancer, since the tissue most susceptible to malignancy is removed. This has not yet been proven scientifically.
In their comprehensive review of late complications of operative hysteroscopy, Cooper and Brady21 suggest that patients at high risk for endometrial cancer who present with abnormal uterine bleeding not controlled by hormones might be better served by hysterectomy. Unfortunately, these patients tend to be high-risk surgical candidates.
If atypia is present, do not perform endometrial ablation or resection. I do perform ablation and resection in patients with complex hyperplasia without atypia if it has been reversed with progestin and does not recur for at least 6 months without progestin therapy. These patients undergo office hysteroscopy and sampling of the endometrium before operative hysteroscopy is scheduled.
Iatrogenic adenomyosis
Two theories suggest this is a late complication of operative hysteroscopy. According to the first, when the endometrium is incompletely resected, scarring over this tissue causes the viable glands to grow into the myometrium. The other theory suggests that viable endometrial debris is transported into the myometrium by vessels opened at resection.
I have found that using the vaporization electrode followed by application of a rollerball over the surface of the cavity most effectively reaches maximal tissue depth and, theoretically, prevents adenomyosis. Since most adenomyosis occurs on the posterior wall, I take a strip from this area for pathologic analysis to determine whether adenomyosis preceded the ablation or developed subsequent to it.
Hematometria
This can occur following operative hysteroscopy if viable glands are left in the fundal or cornual region and synechiae develop in the lower segment, preventing egress of blood. It also can occur if the upper endocervix is ablated and subsequently scars, causing stenosis. To avoid this, ablate only to the level of the internal os.
Diagnosis and treatment. Hematometria can be diagnosed easily by ultrasound and treated with office hysteroscopy using a narrow-diameter, rigid, continuous-flow hysteroscope with an operating channel to pass small instruments.
Post-ablation tubal ligation syndrome
This is cornual hematometria that develops when viable endometrial cells are left in the cornua when the cavity also contains synechiae, causing cyclic bleeding. Since there is no egress from the cervix or tubes, blood gradually builds up, leading to hematosalpinx and pain. One way to avoid this is to ensure complete ablation of the cornual endometrium.
Prevention. Some experts recommend that the small rollerball electrode be placed in the cornua, with slightly reduced intrauterine pressure, to allow the cornual endometrium to collapse around the rollerball. A short burst of current is then applied to ablate the tissue.
This complication is less likely after hydrothermablation, since the free-flowing saline ablates the cornua completely. After more than 50 hydrothermablations performed in patients with prior tubal sterilizations, I have not seen any cases of post-ablation tubal ligation syndrome.
Treatment consists of bilateral salpingectomy or tubal fulguration at the cornual region and repeat ablation or resection of viable endometrial tissue. Another option is hysterectomy.
Post-ablation pregnancy can be very complicated
Pregnancy after endometrial ablation occurs at a rate of 0.2% to 1.6%. Counsel patients that this procedure does not prevent pregnancy and that contraception is vital. Uterine rupture after fibroid resection has been reported.
In a review of 37 post-ablation pregnancies, only 11 of 17 women who chose not to terminate carried the gestation beyond 28 weeks. In addition, there was a high incidence of intrauterine growth restriction, prematurity, and placenta accreta.22
Hysteroscopic tubal sterilization with the Essure system (Conceptus, San Carlos, Calif), or laparoscopic tubal fulguration performed at the time of ablation averts these complications.
Complications of global ablation
Global ablation technologies were developed to enable gynecologists with limited operative hysteroscopy skills to perform endometrial ablation and to make ablation safer for the patient. These technologies completely eliminate the risk of distention-media complications, but widespread use has resulted in other complications that have been reported in the literature to only a limited extent.
Most published articles on global endometrial ablation are from the original US Food and Drug Administration (FDA) trials, in which the complication rates were extraordinarily low. Widespread commercial use of these technologies since FDA approval, especially by practitioners with limited skills, has increased complications.
Do not override safety systems
Complications are more frequent when devices are misused or safety systems overridden. And, fear of litigation makes physicians unwilling to report complications.
In the FDA Manufacturer and User Facility Device Experience (MAUDE) database (www.fda.gov.cdrh/maude.html), complications include bowel burns after unrecognized perforation, and bowel burns associated with electrosurgical, microwave energy, or heat transferred through intact myometrium.23 Vaginal burns, uterine necrosis myometritis requiring hysterectomy, and death from unrecognized bowel burn also have been reported.
Most global procedures are performed blindly, and some doctors fail to perform diagnostic hysteroscopy before and after surgery, which I feel is mandatory with any endometrial ablation. Hydrothermablation is the only global technique that has the advantage of direct observation. In more than 150 procedures done in my office under local anesthesia, the only complications were 2 false passages. Both were promptly identified during diagnostic hysteroscopy, and the surgery was rescheduled 2 to 3 months later.
The author has served on the speakers’ bureau for Boston Scientific.
1. Hulka JF, Peterson HA, Philips JM, Surrey MW. Operative hysteroscopy: American Association of Gynecologic Laparoscopists’ 1993 Member Survey. J Am Assoc Gynecol Laparos. 1995;2:131-132.
2. Jansen FW, Vredevoogd CB, Van Ulzen K, et al. Complications of hysteroscopy: a prospective multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Vilos GA. Hysteroscopic surgery: indications, contraindications, and complications. In: Pasic and Levine’s A Practical Manual of Hysteroscopy and Endometrial Ablation Techniques. London and New York: Taylor and Francis; 2004;237-258.
4. Ostrzenski A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. Int J Fertil. 1994;39:111-113
5. Phillips DA, Nathanson HG, Millim SJ, et al. The effect of dilute vasopressin solution on the force needed for cervical dilatation: a randomized controlled trial. Obstet Gynecol. 1997;89:507-511
6. Valle RF, Sciarra JJ. Intrauterine adhesions; hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459-1470
7. Townsend DE, Quinlan DJ, Johnson GH. Repeat endometrial ablation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.
8. MacLean-Fraser E, Penava D, Vilos GA. Perioperative complication rates of primary and repeat hysteroscopic endometrial ablations. J Am Assoc Gynecol Laparosc. 2002;9:175-177.
9. Loffer FD. Complications of hysteroscopy—their cause, prevention, and correction. J Am Assoc Gynecol Laparosc; 1995;3:11-26
10. Goldrath MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol. 1983;147:869-872
11. Townsend DE. Vasopressin pack for treatment of bleeding after myoma resection. Am J Obstet Gynecol. 1991;165:1405-1407
12. Phillips DR, Nathanson HG, Milim SJ, et al. The effect of dilute vasopressin solution on intraoperative blood loss during operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 1996;88:761-766
13. Brooks PG. Resectoscopic myoma vaporizer. J Reprod Med. 1995;40:791-795
14. Glasser MH. Endometrial ablation and hysteroscopic myomectomy by electrosurgical vaporization. J Am Assoc Gynecol Laparosc. 1997;4:369-374
15. Parazzini F, Vercellini P, Di Giorgio O, et al. Efficacy of preoperative medical treatment in facilitating endometrial resection, myomectomy and metroplasty: literature review. Hum Reprod. 1998;13:2592-2597
16. Vilos GA, Brown S, Graham G, et al. Genital tract electrical burns during hysteroscopic endometrial ablation: report of 13 cases in the United States and Canada. J Am Assoc Gynecol Laparosc. 2000;7:141-147
17. Munro MG. Factors affecting capacitative current diversion with a uterine resectoscope: an in vitro study. J Am Assoc Gynecol Laparosc. 2003;10:450-460
18. Istre O, Shajaa K, Schjoensky AP, et al. Changes in serum electrolytes after transcervical resection of endometrium and submucous fibroids with the use of 1.5% glycine for irrigation. Obstet Gynecol. 1992;80:218-222
19. Arieff AI, Azus JC. Hyponatremic encephalopathy after endometrial ablation. JAMA. 1994;271:345.-
20. Taskin O, Buhur A, Birincioglu M, et al. Endometrial Na+, K+–ATPase pump function and vasopressin levels during hysteroscopic surgery in patients pretreated with GnRH agonist. J Am Assoc Gynecol Laparosc. 1998;5:119-124
21. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374
22. Rogerson L, Gannon, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160
23. Gurtcheff SE, Sharp MT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282
24. Glasser MH. The serosal sign: the hysteroscopic appearance of the uterine cavity just prior to perforation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.
1. Hulka JF, Peterson HA, Philips JM, Surrey MW. Operative hysteroscopy: American Association of Gynecologic Laparoscopists’ 1993 Member Survey. J Am Assoc Gynecol Laparos. 1995;2:131-132.
2. Jansen FW, Vredevoogd CB, Van Ulzen K, et al. Complications of hysteroscopy: a prospective multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Vilos GA. Hysteroscopic surgery: indications, contraindications, and complications. In: Pasic and Levine’s A Practical Manual of Hysteroscopy and Endometrial Ablation Techniques. London and New York: Taylor and Francis; 2004;237-258.
4. Ostrzenski A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. Int J Fertil. 1994;39:111-113
5. Phillips DA, Nathanson HG, Millim SJ, et al. The effect of dilute vasopressin solution on the force needed for cervical dilatation: a randomized controlled trial. Obstet Gynecol. 1997;89:507-511
6. Valle RF, Sciarra JJ. Intrauterine adhesions; hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459-1470
7. Townsend DE, Quinlan DJ, Johnson GH. Repeat endometrial ablation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.
8. MacLean-Fraser E, Penava D, Vilos GA. Perioperative complication rates of primary and repeat hysteroscopic endometrial ablations. J Am Assoc Gynecol Laparosc. 2002;9:175-177.
9. Loffer FD. Complications of hysteroscopy—their cause, prevention, and correction. J Am Assoc Gynecol Laparosc; 1995;3:11-26
10. Goldrath MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol. 1983;147:869-872
11. Townsend DE. Vasopressin pack for treatment of bleeding after myoma resection. Am J Obstet Gynecol. 1991;165:1405-1407
12. Phillips DR, Nathanson HG, Milim SJ, et al. The effect of dilute vasopressin solution on intraoperative blood loss during operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 1996;88:761-766
13. Brooks PG. Resectoscopic myoma vaporizer. J Reprod Med. 1995;40:791-795
14. Glasser MH. Endometrial ablation and hysteroscopic myomectomy by electrosurgical vaporization. J Am Assoc Gynecol Laparosc. 1997;4:369-374
15. Parazzini F, Vercellini P, Di Giorgio O, et al. Efficacy of preoperative medical treatment in facilitating endometrial resection, myomectomy and metroplasty: literature review. Hum Reprod. 1998;13:2592-2597
16. Vilos GA, Brown S, Graham G, et al. Genital tract electrical burns during hysteroscopic endometrial ablation: report of 13 cases in the United States and Canada. J Am Assoc Gynecol Laparosc. 2000;7:141-147
17. Munro MG. Factors affecting capacitative current diversion with a uterine resectoscope: an in vitro study. J Am Assoc Gynecol Laparosc. 2003;10:450-460
18. Istre O, Shajaa K, Schjoensky AP, et al. Changes in serum electrolytes after transcervical resection of endometrium and submucous fibroids with the use of 1.5% glycine for irrigation. Obstet Gynecol. 1992;80:218-222
19. Arieff AI, Azus JC. Hyponatremic encephalopathy after endometrial ablation. JAMA. 1994;271:345.-
20. Taskin O, Buhur A, Birincioglu M, et al. Endometrial Na+, K+–ATPase pump function and vasopressin levels during hysteroscopic surgery in patients pretreated with GnRH agonist. J Am Assoc Gynecol Laparosc. 1998;5:119-124
21. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374
22. Rogerson L, Gannon, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160
23. Gurtcheff SE, Sharp MT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282
24. Glasser MH. The serosal sign: the hysteroscopic appearance of the uterine cavity just prior to perforation. Presented at the World Congress of Hysteroscopy, Miami, Florida, 1996.