User login
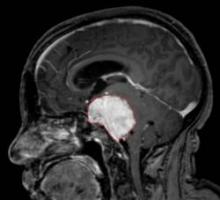
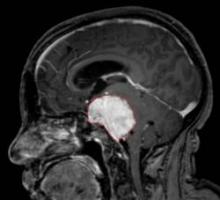
Bevacizumab Effective in Glioblastoma Trials
MONTREAL – Bevacizumab-containing regimens continued to show efficacy against glioblastoma in recent reports of phase II trial results, but the trials' designs have come under fire by some neuro-oncologists.
Investigators from Duke University, Durham, N.C., reported at the meeting that adding bevacizumab (Avastin) and irinotecan (Camptosar) to a standard temozolomide (Temodar)–based chemoradiation regimen for newly diagnosed glioblastoma increased progression-free and overall survival by about 6 months, compared with historical controls.
In a separate RTOG (Radiation Therapy Oncology Group) study, investigators defined efficacy as a progression-free survival rate of 35% at 6 months in patients with recurrent glioblastoma. Both regimens in the noncomparative trial – bevacizumab with dose-dense temozolomide and bevacizumab with irinotecan – cleared the mark at 40% and 39%, respectively. Median overall survival was longer with the temozolomide partnership (9.4 months vs. 7.7 months with irinotecan), but the difference was not significant.
In 2009, the Food and Drug Administration approved bevacizumab as a single agent for the second-line treatment of glioblastoma, based on objective response rates in two single-arm trials.
Newly Diagnosed in Duke Study
The Genentech-sponsored study from Duke began with 125 patients (mean age, 56 years; 59% male) with newly diagnosed grade IV malignant glioblastoma multiforme (GBM), reported Dr. Annick Desjardins of the university's brain tumor center. Most (70%) had Karnofsky performance scores greater than 90%.
Between 2 and 4 weeks after resection, patients started 6 weeks of radiotherapy and daily temozolomide at 75 mg/m
In the second phase of the trial, 113 patients went on to receive 6–12 more weeks of bevacizumab at the same dosage, combined with temozolomide at 200 mg/m
The first phase of treatment was associated with minimal toxicity, the investigators recently reported (Int. J. Radiat. Oncol. Biol. Phys. 2010 Oct 30 [doi: 10.1016/j.ijrobp.2010.08.058]). Grade 4 thrombocytopenia occurred in 2.4%, neutropenia in 0.8%, central nervous system hemorrhage in 0.8%, and deep vein thrombosis and pulmonary embolism in 1.6%, said Dr. Desjardins.
Five patients did not complete the first phase (one patient with grade 2 CNS hemorrhage, two with pulmonary emboli, one with grade 4 pancytopenia, and one with wound dehiscence). Seven other patients did not go on to the second phase (three with tumor progression, two withdrawing because of fatigue, and one each with a bowel perforation and a rectal abscess). Patients in the second phase have been followed for a median of 28 months, said Dr. Desjardins.
A final analysis for the original cohort of 125 shows that median progression-free survival reached 14.2 months and median overall survival was 21.3 months. This compares with medians of 6.9 months and 15.9 months, respectively, that had been reported in the literature, she said.
Additionally, progression-free survival rates were 88% at 6 months, 64% at 1 year, and 16% at 2 years in the Duke cohort; overall survival rates were 94%, 82%, and 44%, respectively.
For all 125 patients enrolled, the overall serious toxicities included 1 CNS hemorrhage, 9 VTEs, 2 wound dehiscences, 1 bowel perforation, 17 grade 4 hematologic toxicities, 1 secondary malignancy, and 2 cases of pneumocystis pneumonia. There were four toxicity-related deaths.
RTOG Trial in Recurrent GBM
The noncomparative RTOG study enrolled patients with recurrent glioblastoma who had failed previous chemoradiation with temozolomide. In all, 57 patients were assigned to bevacizumab 10 mg/kg IV plus irinotecan 200 mg/kg every 2 weeks, and 58 were assigned to the same bevacizumab dose plus dose-dense temozolomide 75–100 mg/m
The two arms had different end points: safety with temozolomide and efficacy in the irinotecan arm, noted the presenter, Dr. Mark Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston.
Hematologic toxicities were seen more in the temozolomide arm, whereas gastrointestinal toxicities predominated in the irinotecan arm, said Dr. Gilbert. One case of gastrointestinal perforation resulted in death.
“The primary objectives were met,” he reported. “We found that administering bevacizumab regimens in a cooperative group setting was feasible. We had acceptable toxicity with the combination of bevacizumab and dose-dense temozolomide, and it supported our use of this type of regimen in the up-front setting. And, in fact, the efficacy of both arms reached our target.”
Trials' Protocols Criticized
Both study designs were challenged at the meeting.
Session moderator Dr. Martin van den Bent contended that exposing all patients to the bevacizumab/irinotecan regimen in the Duke study – rather than randomizing patients to one agent or the other – makes it impossible to know which drug is preferable.
“It's flatly outrageous. It should not have been done,” Dr. van den Bent elaborated in an interview after the session. “What I find very disturbing is they did a very big study of 120 patients … but by doing it in an uncontrolled fashion, they ended up with an impossible interpretation of whether the irinotecan added to the bevacizumab made any difference.”
Dr. van den Bent, professor of neuro-oncology at the cancer center of Erasmus University in Rotterdam, the Netherlands, charged that Duke has a history of doing uncontrolled trials, but he also criticized the field's eagerness to embrace bevacizumab based on such trials.
“The use of bevacizumab at present is based on uncontrolled studies; it's been FDA approved on a scientifically not valid end point,” said Dr. van den Bent, a past chair of the EORTC (European Organisation for Research and Treatment of Cancer) Brain Tumor Group.
Study design also triggered a complaint with the RTOG trial. Dr. Gilbert cautioned that randomization was not consistent because of safety concerns with the temozolomide regimen. Until these were resolved, the initial 90 patients were randomized 2:1 favoring irinotecan. Consequently, the final 30 temozolomide patients were assigned to that arm without randomization.
For these reasons, “we cannot on the basis of this study tell which of the two treatments” is better, “or in fact whether a combination of chemotherapy with bevacizumab is better than bevacizumab alone,” he said, stressing that the study was not powered for comparison.
A member of the audience asked whether this wasn't “kind of a charade,” since comparisons were being made anyway.
“It's not a charade,” Dr. Gilbert replied, reiterating that the investigators had two separate goals: safety with temozolomide and efficacy with irinotecan. “It is what it is,” he said. “We certainly weren't going to power it, because we weren't interested particularly in the question of which was the better regimen.”
Genentech sponsored the study from Duke University. Dr. Desjardins reported no conflicts of interest. Dr. Gilbert disclosed research support from Merck and Genentech, and honoraria/advisory board participation with Merck and Genentech. Dr. van den Bent said he is a consultant for eight companies, including F. Hoffmann-La Roche, parent company of Genentech.
MONTREAL – Bevacizumab-containing regimens continued to show efficacy against glioblastoma in recent reports of phase II trial results, but the trials' designs have come under fire by some neuro-oncologists.
Investigators from Duke University, Durham, N.C., reported at the meeting that adding bevacizumab (Avastin) and irinotecan (Camptosar) to a standard temozolomide (Temodar)–based chemoradiation regimen for newly diagnosed glioblastoma increased progression-free and overall survival by about 6 months, compared with historical controls.
In a separate RTOG (Radiation Therapy Oncology Group) study, investigators defined efficacy as a progression-free survival rate of 35% at 6 months in patients with recurrent glioblastoma. Both regimens in the noncomparative trial – bevacizumab with dose-dense temozolomide and bevacizumab with irinotecan – cleared the mark at 40% and 39%, respectively. Median overall survival was longer with the temozolomide partnership (9.4 months vs. 7.7 months with irinotecan), but the difference was not significant.
In 2009, the Food and Drug Administration approved bevacizumab as a single agent for the second-line treatment of glioblastoma, based on objective response rates in two single-arm trials.
Newly Diagnosed in Duke Study
The Genentech-sponsored study from Duke began with 125 patients (mean age, 56 years; 59% male) with newly diagnosed grade IV malignant glioblastoma multiforme (GBM), reported Dr. Annick Desjardins of the university's brain tumor center. Most (70%) had Karnofsky performance scores greater than 90%.
Between 2 and 4 weeks after resection, patients started 6 weeks of radiotherapy and daily temozolomide at 75 mg/m
In the second phase of the trial, 113 patients went on to receive 6–12 more weeks of bevacizumab at the same dosage, combined with temozolomide at 200 mg/m
The first phase of treatment was associated with minimal toxicity, the investigators recently reported (Int. J. Radiat. Oncol. Biol. Phys. 2010 Oct 30 [doi: 10.1016/j.ijrobp.2010.08.058]). Grade 4 thrombocytopenia occurred in 2.4%, neutropenia in 0.8%, central nervous system hemorrhage in 0.8%, and deep vein thrombosis and pulmonary embolism in 1.6%, said Dr. Desjardins.
Five patients did not complete the first phase (one patient with grade 2 CNS hemorrhage, two with pulmonary emboli, one with grade 4 pancytopenia, and one with wound dehiscence). Seven other patients did not go on to the second phase (three with tumor progression, two withdrawing because of fatigue, and one each with a bowel perforation and a rectal abscess). Patients in the second phase have been followed for a median of 28 months, said Dr. Desjardins.
A final analysis for the original cohort of 125 shows that median progression-free survival reached 14.2 months and median overall survival was 21.3 months. This compares with medians of 6.9 months and 15.9 months, respectively, that had been reported in the literature, she said.
Additionally, progression-free survival rates were 88% at 6 months, 64% at 1 year, and 16% at 2 years in the Duke cohort; overall survival rates were 94%, 82%, and 44%, respectively.
For all 125 patients enrolled, the overall serious toxicities included 1 CNS hemorrhage, 9 VTEs, 2 wound dehiscences, 1 bowel perforation, 17 grade 4 hematologic toxicities, 1 secondary malignancy, and 2 cases of pneumocystis pneumonia. There were four toxicity-related deaths.
RTOG Trial in Recurrent GBM
The noncomparative RTOG study enrolled patients with recurrent glioblastoma who had failed previous chemoradiation with temozolomide. In all, 57 patients were assigned to bevacizumab 10 mg/kg IV plus irinotecan 200 mg/kg every 2 weeks, and 58 were assigned to the same bevacizumab dose plus dose-dense temozolomide 75–100 mg/m
The two arms had different end points: safety with temozolomide and efficacy in the irinotecan arm, noted the presenter, Dr. Mark Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston.
Hematologic toxicities were seen more in the temozolomide arm, whereas gastrointestinal toxicities predominated in the irinotecan arm, said Dr. Gilbert. One case of gastrointestinal perforation resulted in death.
“The primary objectives were met,” he reported. “We found that administering bevacizumab regimens in a cooperative group setting was feasible. We had acceptable toxicity with the combination of bevacizumab and dose-dense temozolomide, and it supported our use of this type of regimen in the up-front setting. And, in fact, the efficacy of both arms reached our target.”
Trials' Protocols Criticized
Both study designs were challenged at the meeting.
Session moderator Dr. Martin van den Bent contended that exposing all patients to the bevacizumab/irinotecan regimen in the Duke study – rather than randomizing patients to one agent or the other – makes it impossible to know which drug is preferable.
“It's flatly outrageous. It should not have been done,” Dr. van den Bent elaborated in an interview after the session. “What I find very disturbing is they did a very big study of 120 patients … but by doing it in an uncontrolled fashion, they ended up with an impossible interpretation of whether the irinotecan added to the bevacizumab made any difference.”
Dr. van den Bent, professor of neuro-oncology at the cancer center of Erasmus University in Rotterdam, the Netherlands, charged that Duke has a history of doing uncontrolled trials, but he also criticized the field's eagerness to embrace bevacizumab based on such trials.
“The use of bevacizumab at present is based on uncontrolled studies; it's been FDA approved on a scientifically not valid end point,” said Dr. van den Bent, a past chair of the EORTC (European Organisation for Research and Treatment of Cancer) Brain Tumor Group.
Study design also triggered a complaint with the RTOG trial. Dr. Gilbert cautioned that randomization was not consistent because of safety concerns with the temozolomide regimen. Until these were resolved, the initial 90 patients were randomized 2:1 favoring irinotecan. Consequently, the final 30 temozolomide patients were assigned to that arm without randomization.
For these reasons, “we cannot on the basis of this study tell which of the two treatments” is better, “or in fact whether a combination of chemotherapy with bevacizumab is better than bevacizumab alone,” he said, stressing that the study was not powered for comparison.
A member of the audience asked whether this wasn't “kind of a charade,” since comparisons were being made anyway.
“It's not a charade,” Dr. Gilbert replied, reiterating that the investigators had two separate goals: safety with temozolomide and efficacy with irinotecan. “It is what it is,” he said. “We certainly weren't going to power it, because we weren't interested particularly in the question of which was the better regimen.”
Genentech sponsored the study from Duke University. Dr. Desjardins reported no conflicts of interest. Dr. Gilbert disclosed research support from Merck and Genentech, and honoraria/advisory board participation with Merck and Genentech. Dr. van den Bent said he is a consultant for eight companies, including F. Hoffmann-La Roche, parent company of Genentech.
MONTREAL – Bevacizumab-containing regimens continued to show efficacy against glioblastoma in recent reports of phase II trial results, but the trials' designs have come under fire by some neuro-oncologists.
Investigators from Duke University, Durham, N.C., reported at the meeting that adding bevacizumab (Avastin) and irinotecan (Camptosar) to a standard temozolomide (Temodar)–based chemoradiation regimen for newly diagnosed glioblastoma increased progression-free and overall survival by about 6 months, compared with historical controls.
In a separate RTOG (Radiation Therapy Oncology Group) study, investigators defined efficacy as a progression-free survival rate of 35% at 6 months in patients with recurrent glioblastoma. Both regimens in the noncomparative trial – bevacizumab with dose-dense temozolomide and bevacizumab with irinotecan – cleared the mark at 40% and 39%, respectively. Median overall survival was longer with the temozolomide partnership (9.4 months vs. 7.7 months with irinotecan), but the difference was not significant.
In 2009, the Food and Drug Administration approved bevacizumab as a single agent for the second-line treatment of glioblastoma, based on objective response rates in two single-arm trials.
Newly Diagnosed in Duke Study
The Genentech-sponsored study from Duke began with 125 patients (mean age, 56 years; 59% male) with newly diagnosed grade IV malignant glioblastoma multiforme (GBM), reported Dr. Annick Desjardins of the university's brain tumor center. Most (70%) had Karnofsky performance scores greater than 90%.
Between 2 and 4 weeks after resection, patients started 6 weeks of radiotherapy and daily temozolomide at 75 mg/m
In the second phase of the trial, 113 patients went on to receive 6–12 more weeks of bevacizumab at the same dosage, combined with temozolomide at 200 mg/m
The first phase of treatment was associated with minimal toxicity, the investigators recently reported (Int. J. Radiat. Oncol. Biol. Phys. 2010 Oct 30 [doi: 10.1016/j.ijrobp.2010.08.058]). Grade 4 thrombocytopenia occurred in 2.4%, neutropenia in 0.8%, central nervous system hemorrhage in 0.8%, and deep vein thrombosis and pulmonary embolism in 1.6%, said Dr. Desjardins.
Five patients did not complete the first phase (one patient with grade 2 CNS hemorrhage, two with pulmonary emboli, one with grade 4 pancytopenia, and one with wound dehiscence). Seven other patients did not go on to the second phase (three with tumor progression, two withdrawing because of fatigue, and one each with a bowel perforation and a rectal abscess). Patients in the second phase have been followed for a median of 28 months, said Dr. Desjardins.
A final analysis for the original cohort of 125 shows that median progression-free survival reached 14.2 months and median overall survival was 21.3 months. This compares with medians of 6.9 months and 15.9 months, respectively, that had been reported in the literature, she said.
Additionally, progression-free survival rates were 88% at 6 months, 64% at 1 year, and 16% at 2 years in the Duke cohort; overall survival rates were 94%, 82%, and 44%, respectively.
For all 125 patients enrolled, the overall serious toxicities included 1 CNS hemorrhage, 9 VTEs, 2 wound dehiscences, 1 bowel perforation, 17 grade 4 hematologic toxicities, 1 secondary malignancy, and 2 cases of pneumocystis pneumonia. There were four toxicity-related deaths.
RTOG Trial in Recurrent GBM
The noncomparative RTOG study enrolled patients with recurrent glioblastoma who had failed previous chemoradiation with temozolomide. In all, 57 patients were assigned to bevacizumab 10 mg/kg IV plus irinotecan 200 mg/kg every 2 weeks, and 58 were assigned to the same bevacizumab dose plus dose-dense temozolomide 75–100 mg/m
The two arms had different end points: safety with temozolomide and efficacy in the irinotecan arm, noted the presenter, Dr. Mark Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston.
Hematologic toxicities were seen more in the temozolomide arm, whereas gastrointestinal toxicities predominated in the irinotecan arm, said Dr. Gilbert. One case of gastrointestinal perforation resulted in death.
“The primary objectives were met,” he reported. “We found that administering bevacizumab regimens in a cooperative group setting was feasible. We had acceptable toxicity with the combination of bevacizumab and dose-dense temozolomide, and it supported our use of this type of regimen in the up-front setting. And, in fact, the efficacy of both arms reached our target.”
Trials' Protocols Criticized
Both study designs were challenged at the meeting.
Session moderator Dr. Martin van den Bent contended that exposing all patients to the bevacizumab/irinotecan regimen in the Duke study – rather than randomizing patients to one agent or the other – makes it impossible to know which drug is preferable.
“It's flatly outrageous. It should not have been done,” Dr. van den Bent elaborated in an interview after the session. “What I find very disturbing is they did a very big study of 120 patients … but by doing it in an uncontrolled fashion, they ended up with an impossible interpretation of whether the irinotecan added to the bevacizumab made any difference.”
Dr. van den Bent, professor of neuro-oncology at the cancer center of Erasmus University in Rotterdam, the Netherlands, charged that Duke has a history of doing uncontrolled trials, but he also criticized the field's eagerness to embrace bevacizumab based on such trials.
“The use of bevacizumab at present is based on uncontrolled studies; it's been FDA approved on a scientifically not valid end point,” said Dr. van den Bent, a past chair of the EORTC (European Organisation for Research and Treatment of Cancer) Brain Tumor Group.
Study design also triggered a complaint with the RTOG trial. Dr. Gilbert cautioned that randomization was not consistent because of safety concerns with the temozolomide regimen. Until these were resolved, the initial 90 patients were randomized 2:1 favoring irinotecan. Consequently, the final 30 temozolomide patients were assigned to that arm without randomization.
For these reasons, “we cannot on the basis of this study tell which of the two treatments” is better, “or in fact whether a combination of chemotherapy with bevacizumab is better than bevacizumab alone,” he said, stressing that the study was not powered for comparison.
A member of the audience asked whether this wasn't “kind of a charade,” since comparisons were being made anyway.
“It's not a charade,” Dr. Gilbert replied, reiterating that the investigators had two separate goals: safety with temozolomide and efficacy with irinotecan. “It is what it is,” he said. “We certainly weren't going to power it, because we weren't interested particularly in the question of which was the better regimen.”
Genentech sponsored the study from Duke University. Dr. Desjardins reported no conflicts of interest. Dr. Gilbert disclosed research support from Merck and Genentech, and honoraria/advisory board participation with Merck and Genentech. Dr. van den Bent said he is a consultant for eight companies, including F. Hoffmann-La Roche, parent company of Genentech.
Brief Decline in Quality of Life Seen After Brain Radiotherapy
MONTREAL – Adjuvant whole-brain radiotherapy following surgical or radiosurgical resection of brain metastases provided no survival benefit and modestly reduced health-related quality of life when compared with close MRI observation alone in a randomized study of 359 patients.
The worsening of health-related quality of life with whole-brain radiotherapy (WBRT) was slight and transient, first author Dr. Riccardo Soffietti said at the annual meeting of the Society for Neuro-Oncology. He noted that few scores "reached clinical or statistical significance, and most scores that differed significantly at the first time point had a tendency to recover over time."
The study was a secondary analysis of a phase III study in which patients were randomized to receive WBRT (180 patients) or undergo observation with MRI every 3 months (179) after undergoing surgery or radiosurgery for one to three brain metastases. The primary results of the study were published online Nov. 1 in the Journal of Clinical Oncology (doi:10.1200/JCO.2010.30.1655).
For the primary outcome of overall survival at 1 year, there was no difference in between the WBRT (median of 10.9 months) and observation groups (10.7 months). Both groups also had a similar degree of functional independence.
There was a modest increase in progression-free survival in the WBRT group "probably due to their significant reduction in intracranial progression," said Dr. Soffietti, professor of neuro-oncology at the University of Torino (Italy).
Intracranial progression causing death occurred in 28% of the WBRT group, compared with 44% of the observation group.
For the secondary end point of health-related quality of life (HRQOL), there was low patient compliance in completing the brain-specific questionnaires QLQ-C30 and BN-20. Only 315 (88%) of the participants completed baseline assessments, while many fewer completed 6-month (51%) or 1-year assessments (45%).
Among the patients for whom all assessments were available, Dr. Soffietti and his colleagues noted "a statistically significant and clinically relevant difference between the two arms at 9 months favoring patients who did not receive whole-brain radiation therapy." However, the difference in global HRQOL scores at that time (52.2 in the WBRT group and 63.2 in the observation group) was only transitory and was not maintained by the 1-year mark.
The European Organization for Research and Treatment of Cancer sponsored the trial. Dr. Soffietti did not offer disclosure information.
MONTREAL – Adjuvant whole-brain radiotherapy following surgical or radiosurgical resection of brain metastases provided no survival benefit and modestly reduced health-related quality of life when compared with close MRI observation alone in a randomized study of 359 patients.
The worsening of health-related quality of life with whole-brain radiotherapy (WBRT) was slight and transient, first author Dr. Riccardo Soffietti said at the annual meeting of the Society for Neuro-Oncology. He noted that few scores "reached clinical or statistical significance, and most scores that differed significantly at the first time point had a tendency to recover over time."
The study was a secondary analysis of a phase III study in which patients were randomized to receive WBRT (180 patients) or undergo observation with MRI every 3 months (179) after undergoing surgery or radiosurgery for one to three brain metastases. The primary results of the study were published online Nov. 1 in the Journal of Clinical Oncology (doi:10.1200/JCO.2010.30.1655).
For the primary outcome of overall survival at 1 year, there was no difference in between the WBRT (median of 10.9 months) and observation groups (10.7 months). Both groups also had a similar degree of functional independence.
There was a modest increase in progression-free survival in the WBRT group "probably due to their significant reduction in intracranial progression," said Dr. Soffietti, professor of neuro-oncology at the University of Torino (Italy).
Intracranial progression causing death occurred in 28% of the WBRT group, compared with 44% of the observation group.
For the secondary end point of health-related quality of life (HRQOL), there was low patient compliance in completing the brain-specific questionnaires QLQ-C30 and BN-20. Only 315 (88%) of the participants completed baseline assessments, while many fewer completed 6-month (51%) or 1-year assessments (45%).
Among the patients for whom all assessments were available, Dr. Soffietti and his colleagues noted "a statistically significant and clinically relevant difference between the two arms at 9 months favoring patients who did not receive whole-brain radiation therapy." However, the difference in global HRQOL scores at that time (52.2 in the WBRT group and 63.2 in the observation group) was only transitory and was not maintained by the 1-year mark.
The European Organization for Research and Treatment of Cancer sponsored the trial. Dr. Soffietti did not offer disclosure information.
MONTREAL – Adjuvant whole-brain radiotherapy following surgical or radiosurgical resection of brain metastases provided no survival benefit and modestly reduced health-related quality of life when compared with close MRI observation alone in a randomized study of 359 patients.
The worsening of health-related quality of life with whole-brain radiotherapy (WBRT) was slight and transient, first author Dr. Riccardo Soffietti said at the annual meeting of the Society for Neuro-Oncology. He noted that few scores "reached clinical or statistical significance, and most scores that differed significantly at the first time point had a tendency to recover over time."
The study was a secondary analysis of a phase III study in which patients were randomized to receive WBRT (180 patients) or undergo observation with MRI every 3 months (179) after undergoing surgery or radiosurgery for one to three brain metastases. The primary results of the study were published online Nov. 1 in the Journal of Clinical Oncology (doi:10.1200/JCO.2010.30.1655).
For the primary outcome of overall survival at 1 year, there was no difference in between the WBRT (median of 10.9 months) and observation groups (10.7 months). Both groups also had a similar degree of functional independence.
There was a modest increase in progression-free survival in the WBRT group "probably due to their significant reduction in intracranial progression," said Dr. Soffietti, professor of neuro-oncology at the University of Torino (Italy).
Intracranial progression causing death occurred in 28% of the WBRT group, compared with 44% of the observation group.
For the secondary end point of health-related quality of life (HRQOL), there was low patient compliance in completing the brain-specific questionnaires QLQ-C30 and BN-20. Only 315 (88%) of the participants completed baseline assessments, while many fewer completed 6-month (51%) or 1-year assessments (45%).
Among the patients for whom all assessments were available, Dr. Soffietti and his colleagues noted "a statistically significant and clinically relevant difference between the two arms at 9 months favoring patients who did not receive whole-brain radiation therapy." However, the difference in global HRQOL scores at that time (52.2 in the WBRT group and 63.2 in the observation group) was only transitory and was not maintained by the 1-year mark.
The European Organization for Research and Treatment of Cancer sponsored the trial. Dr. Soffietti did not offer disclosure information.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR NEURO-ONCOLOGY
Major Finding: Global HRQOL scores at 9 months were significantly worse in patients who received WBRT than in those who underwent observation alone (52.2 vs. 63.2, respectively).
Data Source: Randomized trial of 359 patients who underwent either observation or adjuvant whole-brain radiotherapy after surgical or radiosurgical resection of between one and three brain metastases.
Disclosures: The European Organization for Research and Treatment of Cancer sponsored the trial. Dr. Soffietti did not offer disclosure information.
Frail Elderly Benefit From Post-Op Temozolomide for Glioblastoma
MONTREAL – Elderly patients with newly diagnosed glioblastoma and poor Karnofsky performance scores can benefit from postsurgical chemotherapy with temozolomide, based on the results of a single-arm study in 70 patients.
Not only were most patients able to withstand treatment-related toxicity, but survival rates and performance status appeared to improve with treatment, Dr. Jamie Gállego Pérez-Larraya reported at the annual meeting of the Society for Neuro-Oncology.
Median overall survival, the primary end point, reached 25 weeks, with a 6-month overall survival rate of 44.3% and a 12-month rate of 11.4%, said Dr. Pérez-Larraya of the Hôpital Pitié-Salpêtrière in Paris. Median progression-free survival, a secondary end point, was 16 weeks, with a 6-month rate of 30%.
"These data compare favorably to the 17-week median survival reported in elderly patients with glioblastoma and good performance treated only with palliative care," he said.
The trial from ANOCEF (Association des Neuro-Oncologues d'Expression Française) enrolled 70 patients aged 70 and up (median age, 77 years), with newly diagnosed and histologically confirmed glioblastoma, a median Karnofsky performance score of 60, and no previous radiotherapy or chemotherapy for the brain tumor. Most (92%) had undergone biopsy of their tumor; five patients had received a partial resection, and one had a complete resection.
Temozolomide chemotherapy started at 150 mg/m2 for 5 consecutive days every 28 days, with dose escalation up to 200 mg/m2 in the absence of grade 3 or 4 toxicities for a maximum of 12 cycles, or until disease progression. Patients who progressed received supportive care; no radiotherapy was given.
Quality of life (QOL) and cognitive function outcomes were measured by EORTC (European Organisation for Research and Treatment of Cancer) questionnaires (QLQ-C30 and QLQ-BN20), and the Mini-Mental State Examination (MMSE). The study showed significant improvements on the global QOL scale and most functioning domains, with no decline in any domain, he said.
A third of the cohort improved their Karnofsky performance scores by at least 10 points, and 26% achieved a score of 70 or greater. "This is clinically significant because it means they became capable of self-care," he noted.
Treatment with temozolomide was "generally well tolerated," Dr. Pérez-Larraya reported. Twelve patients, (17%) had grade 3 or 4 thrombocytopenia or neutropenia, but these toxicities did not lead to dose delays or dose reductions.
All of the cohort had died by the time of presentation – 87% as a result of disease progression, and 13% from other causes, with no deaths due to toxicity.
"Until a few years ago the treatment of these patients received little attention mainly because of their poor expected survival, but also because of the fear of treatment-related toxicity," said Dr. Pérez-Larraya.
Now patients with good performance scores can be treated with postsurgical radiotherapy, which has been shown to prolong survival from 17 weeks to 29 weeks without causing deterioration in quality of life or cognitive function (N. Engl. J. Med. 2007;356:1527-35), he said. But the management of patients with poor scores has never been studied and remains uncertain.
"Radiotherapy requires many trips to the hospital, and increasing fatigue makes this difficult for these severely impaired patients with such a short expected survival. As a result, these patients frequently receive only supportive care," he explained.
"These results suggest that treatment with temozolomide in elderly patients with glioblastoma and poor performance score has an acceptable safety profile; is associated with an improvement in functional status in one-third of cases, as well as quality of life before progression; and seems to increase survival as compared to supportive care alone," Dr. Pérez-Larraya concluded.
He reported having no relevant financial disclosures.
MONTREAL – Elderly patients with newly diagnosed glioblastoma and poor Karnofsky performance scores can benefit from postsurgical chemotherapy with temozolomide, based on the results of a single-arm study in 70 patients.
Not only were most patients able to withstand treatment-related toxicity, but survival rates and performance status appeared to improve with treatment, Dr. Jamie Gállego Pérez-Larraya reported at the annual meeting of the Society for Neuro-Oncology.
Median overall survival, the primary end point, reached 25 weeks, with a 6-month overall survival rate of 44.3% and a 12-month rate of 11.4%, said Dr. Pérez-Larraya of the Hôpital Pitié-Salpêtrière in Paris. Median progression-free survival, a secondary end point, was 16 weeks, with a 6-month rate of 30%.
"These data compare favorably to the 17-week median survival reported in elderly patients with glioblastoma and good performance treated only with palliative care," he said.
The trial from ANOCEF (Association des Neuro-Oncologues d'Expression Française) enrolled 70 patients aged 70 and up (median age, 77 years), with newly diagnosed and histologically confirmed glioblastoma, a median Karnofsky performance score of 60, and no previous radiotherapy or chemotherapy for the brain tumor. Most (92%) had undergone biopsy of their tumor; five patients had received a partial resection, and one had a complete resection.
Temozolomide chemotherapy started at 150 mg/m2 for 5 consecutive days every 28 days, with dose escalation up to 200 mg/m2 in the absence of grade 3 or 4 toxicities for a maximum of 12 cycles, or until disease progression. Patients who progressed received supportive care; no radiotherapy was given.
Quality of life (QOL) and cognitive function outcomes were measured by EORTC (European Organisation for Research and Treatment of Cancer) questionnaires (QLQ-C30 and QLQ-BN20), and the Mini-Mental State Examination (MMSE). The study showed significant improvements on the global QOL scale and most functioning domains, with no decline in any domain, he said.
A third of the cohort improved their Karnofsky performance scores by at least 10 points, and 26% achieved a score of 70 or greater. "This is clinically significant because it means they became capable of self-care," he noted.
Treatment with temozolomide was "generally well tolerated," Dr. Pérez-Larraya reported. Twelve patients, (17%) had grade 3 or 4 thrombocytopenia or neutropenia, but these toxicities did not lead to dose delays or dose reductions.
All of the cohort had died by the time of presentation – 87% as a result of disease progression, and 13% from other causes, with no deaths due to toxicity.
"Until a few years ago the treatment of these patients received little attention mainly because of their poor expected survival, but also because of the fear of treatment-related toxicity," said Dr. Pérez-Larraya.
Now patients with good performance scores can be treated with postsurgical radiotherapy, which has been shown to prolong survival from 17 weeks to 29 weeks without causing deterioration in quality of life or cognitive function (N. Engl. J. Med. 2007;356:1527-35), he said. But the management of patients with poor scores has never been studied and remains uncertain.
"Radiotherapy requires many trips to the hospital, and increasing fatigue makes this difficult for these severely impaired patients with such a short expected survival. As a result, these patients frequently receive only supportive care," he explained.
"These results suggest that treatment with temozolomide in elderly patients with glioblastoma and poor performance score has an acceptable safety profile; is associated with an improvement in functional status in one-third of cases, as well as quality of life before progression; and seems to increase survival as compared to supportive care alone," Dr. Pérez-Larraya concluded.
He reported having no relevant financial disclosures.
MONTREAL – Elderly patients with newly diagnosed glioblastoma and poor Karnofsky performance scores can benefit from postsurgical chemotherapy with temozolomide, based on the results of a single-arm study in 70 patients.
Not only were most patients able to withstand treatment-related toxicity, but survival rates and performance status appeared to improve with treatment, Dr. Jamie Gállego Pérez-Larraya reported at the annual meeting of the Society for Neuro-Oncology.
Median overall survival, the primary end point, reached 25 weeks, with a 6-month overall survival rate of 44.3% and a 12-month rate of 11.4%, said Dr. Pérez-Larraya of the Hôpital Pitié-Salpêtrière in Paris. Median progression-free survival, a secondary end point, was 16 weeks, with a 6-month rate of 30%.
"These data compare favorably to the 17-week median survival reported in elderly patients with glioblastoma and good performance treated only with palliative care," he said.
The trial from ANOCEF (Association des Neuro-Oncologues d'Expression Française) enrolled 70 patients aged 70 and up (median age, 77 years), with newly diagnosed and histologically confirmed glioblastoma, a median Karnofsky performance score of 60, and no previous radiotherapy or chemotherapy for the brain tumor. Most (92%) had undergone biopsy of their tumor; five patients had received a partial resection, and one had a complete resection.
Temozolomide chemotherapy started at 150 mg/m2 for 5 consecutive days every 28 days, with dose escalation up to 200 mg/m2 in the absence of grade 3 or 4 toxicities for a maximum of 12 cycles, or until disease progression. Patients who progressed received supportive care; no radiotherapy was given.
Quality of life (QOL) and cognitive function outcomes were measured by EORTC (European Organisation for Research and Treatment of Cancer) questionnaires (QLQ-C30 and QLQ-BN20), and the Mini-Mental State Examination (MMSE). The study showed significant improvements on the global QOL scale and most functioning domains, with no decline in any domain, he said.
A third of the cohort improved their Karnofsky performance scores by at least 10 points, and 26% achieved a score of 70 or greater. "This is clinically significant because it means they became capable of self-care," he noted.
Treatment with temozolomide was "generally well tolerated," Dr. Pérez-Larraya reported. Twelve patients, (17%) had grade 3 or 4 thrombocytopenia or neutropenia, but these toxicities did not lead to dose delays or dose reductions.
All of the cohort had died by the time of presentation – 87% as a result of disease progression, and 13% from other causes, with no deaths due to toxicity.
"Until a few years ago the treatment of these patients received little attention mainly because of their poor expected survival, but also because of the fear of treatment-related toxicity," said Dr. Pérez-Larraya.
Now patients with good performance scores can be treated with postsurgical radiotherapy, which has been shown to prolong survival from 17 weeks to 29 weeks without causing deterioration in quality of life or cognitive function (N. Engl. J. Med. 2007;356:1527-35), he said. But the management of patients with poor scores has never been studied and remains uncertain.
"Radiotherapy requires many trips to the hospital, and increasing fatigue makes this difficult for these severely impaired patients with such a short expected survival. As a result, these patients frequently receive only supportive care," he explained.
"These results suggest that treatment with temozolomide in elderly patients with glioblastoma and poor performance score has an acceptable safety profile; is associated with an improvement in functional status in one-third of cases, as well as quality of life before progression; and seems to increase survival as compared to supportive care alone," Dr. Pérez-Larraya concluded.
He reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR NEURO-ONCOLOGY
Major Finding: More than half of patients with a median Karnofsky performance score of 60 improved their score by at least 10 points.
Data Source: A group of 70 elderly, poor-performance patients, treated with postsurgical temozolomide for newly diagnosed glioblastoma.
Disclosures: Dr. Pérez-Larraya reported having no relevant financial disclosures.
New Drugs Target Recurrent Meningioma
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
New Drugs Target Recurrent Meningioma
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Novartis-sponsored* study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Pfizer*, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Novartis-sponsored* study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Pfizer*, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Novartis-sponsored* study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Pfizer*, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
Major Finding: Two phase II studies of vatalanib and sunitinib showed 6-month progression-free survival rates of 57% and 36%, respectively.
Data Source: Two cohorts of 25 and 36 patients with recurrent grade II and III meningiomas.
Disclosures: Pfizer funded the study on its drug, sunitinib, and Novartis funded the study on its investigational drug, vatalanib. The investigators reported having no relevant conflicts of interest.*
Study Adds Uterine Fibroids to Meningioma Risk Factors
MONTREAL – Meningiomas in postmenopausal women are associated with an increased rate of uterine fibroids, low levels of physical activity, and greater height and body mass index, according to an analysis of the Iowa Women’s Health Study.
The link with uterine fibroids is a novel finding, "probably due to shared risk factors," commented Dr. Derek R. Johnson of the Mayo Clinic, Rochester, Minn. "I’m certainly not suggesting it’s causal," he said at the annual meeting of the Society for Neuro-Oncology.
The Iowa Women’s Health study is a prospective cohort of women followed since 1986. Dr. Johnson’s analysis included 27,791 of these women who had completed a follow-up self-report survey in 1993, had no history of cancer, and were enrolled in Medicare.
The mean age of the women was 70 years (in 1993), and their mean body mass index (BMI) at the time of first enrollment was 27 kg/m2.
The analysis found 125 incident meningiomas reported over 291,021 person-years of follow-up, for an overall incidence of 43/100,000 person-years.
BMI was the strongest of the self-reported risk factors for meningioma, with a relative risk (RR) of 2.14 for BMIs greater than 30 compared with BMIs in the normal range of 19.5-24.5 kg/m2. BMI at age 50 and age 40 was positively associated with the risk of meningioma, but BMI at younger ages was not.
Height was the second strongest risk factor for meningioma, with a relative risk of 2.04 for height above 66 inches compared with height of 62 inches or shorter.
Physical activity was protective against meningioma. Compared with a low rate of physical activity, medium and high levels were associated with decreased risk (RR, 0.57 and 0.61).
A history of uterine fibroids carried a relative risk of 1.78, but no other reproductive factors seemed to be correlated. "Fibrocystic breast disease, endometriosis, and some other reproductive covariates have not shown any association, so, with uterine fibroids being so strongly associated, I think it’s not simply a coincidence," Dr. Johnson said.
The associations were significant after adjustment for "current" BMI (1993).
The data raise the hypothesis that a metabolic environment associated with greater growth in adolescence, and greater weight later in life, may play a role in the etiology of meningiomas, he said.
"Potentially the key unifying factor in the things we found in meningioma risk is the influence of circulating sex hormones and insulin resistance," Dr. Johnson said.
Meningiomas occur at twice the rate in women as in men, and the incidence is increasing, he added.
When asked for his opinion on the findings, Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, said the association with uterine fibroids was intriguing. "It is biologically plausible that the same mechanism of exposure to hormones could explain the association, but it may also be some genetic predisposition, or it may be that women who seek out imaging have both of these things found with relatively minor symptoms."
Many meningiomas in elderly people are small and asymptomatic and are discovered only incidentally or on autopsy, he said in an interview. "As with fibroids, it could just be that certain patient behaviors lead to imaging being done."
Dr. Johnson said he had no relevant financial disclosures.
MONTREAL – Meningiomas in postmenopausal women are associated with an increased rate of uterine fibroids, low levels of physical activity, and greater height and body mass index, according to an analysis of the Iowa Women’s Health Study.
The link with uterine fibroids is a novel finding, "probably due to shared risk factors," commented Dr. Derek R. Johnson of the Mayo Clinic, Rochester, Minn. "I’m certainly not suggesting it’s causal," he said at the annual meeting of the Society for Neuro-Oncology.
The Iowa Women’s Health study is a prospective cohort of women followed since 1986. Dr. Johnson’s analysis included 27,791 of these women who had completed a follow-up self-report survey in 1993, had no history of cancer, and were enrolled in Medicare.
The mean age of the women was 70 years (in 1993), and their mean body mass index (BMI) at the time of first enrollment was 27 kg/m2.
The analysis found 125 incident meningiomas reported over 291,021 person-years of follow-up, for an overall incidence of 43/100,000 person-years.
BMI was the strongest of the self-reported risk factors for meningioma, with a relative risk (RR) of 2.14 for BMIs greater than 30 compared with BMIs in the normal range of 19.5-24.5 kg/m2. BMI at age 50 and age 40 was positively associated with the risk of meningioma, but BMI at younger ages was not.
Height was the second strongest risk factor for meningioma, with a relative risk of 2.04 for height above 66 inches compared with height of 62 inches or shorter.
Physical activity was protective against meningioma. Compared with a low rate of physical activity, medium and high levels were associated with decreased risk (RR, 0.57 and 0.61).
A history of uterine fibroids carried a relative risk of 1.78, but no other reproductive factors seemed to be correlated. "Fibrocystic breast disease, endometriosis, and some other reproductive covariates have not shown any association, so, with uterine fibroids being so strongly associated, I think it’s not simply a coincidence," Dr. Johnson said.
The associations were significant after adjustment for "current" BMI (1993).
The data raise the hypothesis that a metabolic environment associated with greater growth in adolescence, and greater weight later in life, may play a role in the etiology of meningiomas, he said.
"Potentially the key unifying factor in the things we found in meningioma risk is the influence of circulating sex hormones and insulin resistance," Dr. Johnson said.
Meningiomas occur at twice the rate in women as in men, and the incidence is increasing, he added.
When asked for his opinion on the findings, Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, said the association with uterine fibroids was intriguing. "It is biologically plausible that the same mechanism of exposure to hormones could explain the association, but it may also be some genetic predisposition, or it may be that women who seek out imaging have both of these things found with relatively minor symptoms."
Many meningiomas in elderly people are small and asymptomatic and are discovered only incidentally or on autopsy, he said in an interview. "As with fibroids, it could just be that certain patient behaviors lead to imaging being done."
Dr. Johnson said he had no relevant financial disclosures.
MONTREAL – Meningiomas in postmenopausal women are associated with an increased rate of uterine fibroids, low levels of physical activity, and greater height and body mass index, according to an analysis of the Iowa Women’s Health Study.
The link with uterine fibroids is a novel finding, "probably due to shared risk factors," commented Dr. Derek R. Johnson of the Mayo Clinic, Rochester, Minn. "I’m certainly not suggesting it’s causal," he said at the annual meeting of the Society for Neuro-Oncology.
The Iowa Women’s Health study is a prospective cohort of women followed since 1986. Dr. Johnson’s analysis included 27,791 of these women who had completed a follow-up self-report survey in 1993, had no history of cancer, and were enrolled in Medicare.
The mean age of the women was 70 years (in 1993), and their mean body mass index (BMI) at the time of first enrollment was 27 kg/m2.
The analysis found 125 incident meningiomas reported over 291,021 person-years of follow-up, for an overall incidence of 43/100,000 person-years.
BMI was the strongest of the self-reported risk factors for meningioma, with a relative risk (RR) of 2.14 for BMIs greater than 30 compared with BMIs in the normal range of 19.5-24.5 kg/m2. BMI at age 50 and age 40 was positively associated with the risk of meningioma, but BMI at younger ages was not.
Height was the second strongest risk factor for meningioma, with a relative risk of 2.04 for height above 66 inches compared with height of 62 inches or shorter.
Physical activity was protective against meningioma. Compared with a low rate of physical activity, medium and high levels were associated with decreased risk (RR, 0.57 and 0.61).
A history of uterine fibroids carried a relative risk of 1.78, but no other reproductive factors seemed to be correlated. "Fibrocystic breast disease, endometriosis, and some other reproductive covariates have not shown any association, so, with uterine fibroids being so strongly associated, I think it’s not simply a coincidence," Dr. Johnson said.
The associations were significant after adjustment for "current" BMI (1993).
The data raise the hypothesis that a metabolic environment associated with greater growth in adolescence, and greater weight later in life, may play a role in the etiology of meningiomas, he said.
"Potentially the key unifying factor in the things we found in meningioma risk is the influence of circulating sex hormones and insulin resistance," Dr. Johnson said.
Meningiomas occur at twice the rate in women as in men, and the incidence is increasing, he added.
When asked for his opinion on the findings, Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, said the association with uterine fibroids was intriguing. "It is biologically plausible that the same mechanism of exposure to hormones could explain the association, but it may also be some genetic predisposition, or it may be that women who seek out imaging have both of these things found with relatively minor symptoms."
Many meningiomas in elderly people are small and asymptomatic and are discovered only incidentally or on autopsy, he said in an interview. "As with fibroids, it could just be that certain patient behaviors lead to imaging being done."
Dr. Johnson said he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR NEURO-ONCOLOGY
Major Finding: Postmenopausal women with a history of uterine fibroids had a relative risk of 1.78 for meningioma, compared with women without such a history.
Data Source: A total of 27,791 elderly women in the prospective Iowa Women’s Health Study.
Disclosures: Dr. Johnson reported that he had no relevant financial disclosures.
Device for Glioblastoma May Offer Advantage Over Chemo
MONTREAL – An investigational treatment for recurrent glioblastoma that delivers alternating electric fields through scalp electrodes has shown signs of improved survival in a post hoc analysis of results in particular subgroups of patients enrolled in a phase III trial.
Quality of life outcomes also favored patients who used NovoTTF, compared with those who received chemotherapy.
To date, reports about the device have elicited both antagonistic and enthusiastic reaction from oncologists, with "neither the enthusiasts nor the antagonists having significant basis for either kind of acute reaction," Dr. Zvi Ram said in an interview after presenting the subgroup analyses at the annual meeting of the Society for Neuro-Oncology. "I think it is exciting that we’re getting something completely new – a different, noninvasive modality with no side-effects. I think we should be exhilarated."
The results obtained with a modified version of the device in a separate trial of patients with non–small cell lung cancer (NSCLC) suggest that the therapeutic effects of the device also may extend to other cancers, according to Dr. Ram, professor and chair of neurosurgery at Tel Aviv (Israel) Medical Center.
The device, known as NovoTTF-100A, delivers low-amplitude "tumor treatment fields" ranging from 100 to 300 kHz that have been shown in vitro to slow and reverse tumor cell proliferation by inhibiting mitosis, according to NovoCure Ltd., the manufacturer of the device and sponsor of the trial.
The portable device weighs about 6 pounds and connects to a battery pack. It is designed to be worn almost constantly, with a target of at least 20 hours of use each day.
In a phase III clinical trial presented earlier this year at the American Society of Clinical Oncology, an intent-to-treat analysis comparing NovoTTF vs. best-available chemotherapy found no statistical difference in 1-year overall survival (OS) among 237 recurrent glioblastoma patients randomized to either treatment.
However, a per-protocol analysis (which included only those patients who wore the device for at least 70% of the recommended time during the first month) showed a statistically significant benefit to NovoTTF in 1-year survival, compared with chemotherapy (29.5% vs. 19.1%, respectively; hazard ratio, 0.64; P = .01).
In the new post hoc analysis, a subgroup of 110 patients with a "good prognosis" (aged younger than 60 years, and with a Karnofsky performance status score greater than 80%) showed a "more robust" survival benefit than that seen in the overall intent-to-treat analysis, he said.
In this subgroup, patients who were treated with NovoTTF had a median survival of 9.2 months, compared with 6.6 months in those treated with chemotherapy (P less than .01). However, in the overall intent-to-treat group, median survival was 6.6 months and 6.0 months, respectively, he explained. Moreover, the 1-year OS in this subgroup was significantly higher in the NovoTTF group than in the chemotherapy group (35.2% vs. 20.8%, respectively; P less than .01), whereas the difference was nonsignificant in the larger analysis (23.6% vs. 20.7%).
Another subgroup analysis looked at patients who had previously failed treatment with bevacizumab (roughly 20% of the entire cohort). Both an intent-to-treat analysis and a per-protocol analysis showed significant OS advantages to NovoTTF, Dr. Ram said.
The median OS among 44 patients in the intent-to-treat group was 4 months with NovoTTF vs. 3.1 months with chemotherapy (HR, 0.43; P less than 0.02). NovoTTF also gave a significantly better median OS among 29 patients in the per-protocol analysis for this subgroup (6.3 months vs. 3.3 months; HR, 0.21; P = .02).
"You don’t see this anywhere," he said. "There’s no drug in the world that could produce such response in patients who had already failed" bevacizumab.
The investigators also analyzed a surgery-naive group. "You know these are going to be poor responders, almost identical to [those with] bevacizumab failure," Dr. Ram commented.
In this group of 38 patients, an intent-to-treat analysis showed that overall survival was 9.8 months with NovoTTF vs. 5.5 months with chemotherapy.
Patients also reported significantly better quality of life with NovoTTF than with chemotherapy. On the Quality of Life Symptom Scale, NovoTTF patients scored –34 and –35 on constipation and diarrhea, compared with scores of +77 and +50 for the chemotherapy group. Nausea and vomiting scores were 15 for the NovoTTF group and 61 for the chemotherapy group, and pain scores were –1 for the NovoTTF group and +63 for the chemotherapy group.
A quality of life analysis using the EORT (European Organization of Research and Treatment) QLQ-C30 instrument showed scores of 14 vs. –7 in favor of NovoTTF for cognitive functioning, and scores of 7 vs. 1 in favor of NovoTTF for emotional functioning.
"We do this to all our patients. We intoxicate them," Dr. Ram said. "So even if NovoTTF did not extend survival, if it was equivalent to chemotherapy [for survival], it may still improve quality of life."
Dr. Ram did not know the median length of time that the NovoTTF cohort wore the device, but an earlier phase II study followed some patients up to 59 months. He noted that "70% are still alive; that’s unheard of."
"There were concerns that patients might have more headaches or seizures, but there were none," he added.
In the current study, the rate of adverse events related to the central nervous system was similar (66% for NovoTTF and 67% for chemotherapy). Seizures occurred in 15% of the NovoTTF group and 12% of the chemotherapy group, and headaches occurred in 18% and 13%, respectively.
Dr. Ram also presented evidence to suggest that NovoTTF therapy may have benefits in other forms of cancer. A study reported by his colleagues earlier this year at the European Society for Medical Oncology Congress in Milan showed that NovoTTF therapy (combined with chemotherapy) resulted in significant prolongation of survival in patients with NSCLC, compared with historical controls, he said.
"If this kind of therapy acts against brain cancer cells, it should act also against other tumor types," he reasoned.
In the study, which included 42 NSCLC patients, NovoTTF was delivered with newly designed electrodes placed on the chest and neck of patients with locally advanced, metastatic, stage IIIb and IV disease, he explained. The combination of NovoTTF and pemetrexed increased overall survival to 13.8 months, compared with 8.3 months seen with pemetrexed alone; the 1-year survival rate for the combination was 57%, compared with 30% reported for pemetrexed alone.
"We’re talking about something that appears to be acting against cancer cells regardless of origin," Dr. Ram said. "Action in the lung seems similar to what has been seen in GBM over time: a slow resolution of malignant pleural effusion and masses within the chest."
"It’s very interesting and exciting, even if we do not yet have enough definitive data," commented Dr. Alba B. Brandes, moderator of the session and the chair of medical oncology at Azienda USL, a group of nine hospitals in and around Bologna, Italy.
The study investigators have been criticized for repackaging their nonsignificant intent-to-treat results into per-protocol results that show significance, she said. "An intention-to-treat population and per-protocol population are two different things, and from a statistical point of view, it is sometimes difficult for the oncologic community to accept."
Despite those reservations, she said that the per-protocol observations should not be dismissed, because when they are analyzed in this way the results are highly significant. "I was really surprised to see what happened in the lung cancer. I am a medical oncologist and I have never seen that complete a response. It’s surprising. We have to wonder if all that we know about the treatment of tumors is correct."
Dr. Ram acknowledged that per-protocol analysis of the findings is unconventional, but "there is no precedent for this kind of therapy and I think we may need to redesign the way we assess results in the future. We cannot use the same guidelines and definitions that we were traditionally using."
Dr. Ram disclosed that he is a consultant for NovoCure. Dr. Brandes did not disclose if she had any conflicts of interest at the meeting and did not respond to inquiries about disclosures.
MONTREAL – An investigational treatment for recurrent glioblastoma that delivers alternating electric fields through scalp electrodes has shown signs of improved survival in a post hoc analysis of results in particular subgroups of patients enrolled in a phase III trial.
Quality of life outcomes also favored patients who used NovoTTF, compared with those who received chemotherapy.
To date, reports about the device have elicited both antagonistic and enthusiastic reaction from oncologists, with "neither the enthusiasts nor the antagonists having significant basis for either kind of acute reaction," Dr. Zvi Ram said in an interview after presenting the subgroup analyses at the annual meeting of the Society for Neuro-Oncology. "I think it is exciting that we’re getting something completely new – a different, noninvasive modality with no side-effects. I think we should be exhilarated."
The results obtained with a modified version of the device in a separate trial of patients with non–small cell lung cancer (NSCLC) suggest that the therapeutic effects of the device also may extend to other cancers, according to Dr. Ram, professor and chair of neurosurgery at Tel Aviv (Israel) Medical Center.
The device, known as NovoTTF-100A, delivers low-amplitude "tumor treatment fields" ranging from 100 to 300 kHz that have been shown in vitro to slow and reverse tumor cell proliferation by inhibiting mitosis, according to NovoCure Ltd., the manufacturer of the device and sponsor of the trial.
The portable device weighs about 6 pounds and connects to a battery pack. It is designed to be worn almost constantly, with a target of at least 20 hours of use each day.
In a phase III clinical trial presented earlier this year at the American Society of Clinical Oncology, an intent-to-treat analysis comparing NovoTTF vs. best-available chemotherapy found no statistical difference in 1-year overall survival (OS) among 237 recurrent glioblastoma patients randomized to either treatment.
However, a per-protocol analysis (which included only those patients who wore the device for at least 70% of the recommended time during the first month) showed a statistically significant benefit to NovoTTF in 1-year survival, compared with chemotherapy (29.5% vs. 19.1%, respectively; hazard ratio, 0.64; P = .01).
In the new post hoc analysis, a subgroup of 110 patients with a "good prognosis" (aged younger than 60 years, and with a Karnofsky performance status score greater than 80%) showed a "more robust" survival benefit than that seen in the overall intent-to-treat analysis, he said.
In this subgroup, patients who were treated with NovoTTF had a median survival of 9.2 months, compared with 6.6 months in those treated with chemotherapy (P less than .01). However, in the overall intent-to-treat group, median survival was 6.6 months and 6.0 months, respectively, he explained. Moreover, the 1-year OS in this subgroup was significantly higher in the NovoTTF group than in the chemotherapy group (35.2% vs. 20.8%, respectively; P less than .01), whereas the difference was nonsignificant in the larger analysis (23.6% vs. 20.7%).
Another subgroup analysis looked at patients who had previously failed treatment with bevacizumab (roughly 20% of the entire cohort). Both an intent-to-treat analysis and a per-protocol analysis showed significant OS advantages to NovoTTF, Dr. Ram said.
The median OS among 44 patients in the intent-to-treat group was 4 months with NovoTTF vs. 3.1 months with chemotherapy (HR, 0.43; P less than 0.02). NovoTTF also gave a significantly better median OS among 29 patients in the per-protocol analysis for this subgroup (6.3 months vs. 3.3 months; HR, 0.21; P = .02).
"You don’t see this anywhere," he said. "There’s no drug in the world that could produce such response in patients who had already failed" bevacizumab.
The investigators also analyzed a surgery-naive group. "You know these are going to be poor responders, almost identical to [those with] bevacizumab failure," Dr. Ram commented.
In this group of 38 patients, an intent-to-treat analysis showed that overall survival was 9.8 months with NovoTTF vs. 5.5 months with chemotherapy.
Patients also reported significantly better quality of life with NovoTTF than with chemotherapy. On the Quality of Life Symptom Scale, NovoTTF patients scored –34 and –35 on constipation and diarrhea, compared with scores of +77 and +50 for the chemotherapy group. Nausea and vomiting scores were 15 for the NovoTTF group and 61 for the chemotherapy group, and pain scores were –1 for the NovoTTF group and +63 for the chemotherapy group.
A quality of life analysis using the EORT (European Organization of Research and Treatment) QLQ-C30 instrument showed scores of 14 vs. –7 in favor of NovoTTF for cognitive functioning, and scores of 7 vs. 1 in favor of NovoTTF for emotional functioning.
"We do this to all our patients. We intoxicate them," Dr. Ram said. "So even if NovoTTF did not extend survival, if it was equivalent to chemotherapy [for survival], it may still improve quality of life."
Dr. Ram did not know the median length of time that the NovoTTF cohort wore the device, but an earlier phase II study followed some patients up to 59 months. He noted that "70% are still alive; that’s unheard of."
"There were concerns that patients might have more headaches or seizures, but there were none," he added.
In the current study, the rate of adverse events related to the central nervous system was similar (66% for NovoTTF and 67% for chemotherapy). Seizures occurred in 15% of the NovoTTF group and 12% of the chemotherapy group, and headaches occurred in 18% and 13%, respectively.
Dr. Ram also presented evidence to suggest that NovoTTF therapy may have benefits in other forms of cancer. A study reported by his colleagues earlier this year at the European Society for Medical Oncology Congress in Milan showed that NovoTTF therapy (combined with chemotherapy) resulted in significant prolongation of survival in patients with NSCLC, compared with historical controls, he said.
"If this kind of therapy acts against brain cancer cells, it should act also against other tumor types," he reasoned.
In the study, which included 42 NSCLC patients, NovoTTF was delivered with newly designed electrodes placed on the chest and neck of patients with locally advanced, metastatic, stage IIIb and IV disease, he explained. The combination of NovoTTF and pemetrexed increased overall survival to 13.8 months, compared with 8.3 months seen with pemetrexed alone; the 1-year survival rate for the combination was 57%, compared with 30% reported for pemetrexed alone.
"We’re talking about something that appears to be acting against cancer cells regardless of origin," Dr. Ram said. "Action in the lung seems similar to what has been seen in GBM over time: a slow resolution of malignant pleural effusion and masses within the chest."
"It’s very interesting and exciting, even if we do not yet have enough definitive data," commented Dr. Alba B. Brandes, moderator of the session and the chair of medical oncology at Azienda USL, a group of nine hospitals in and around Bologna, Italy.
The study investigators have been criticized for repackaging their nonsignificant intent-to-treat results into per-protocol results that show significance, she said. "An intention-to-treat population and per-protocol population are two different things, and from a statistical point of view, it is sometimes difficult for the oncologic community to accept."
Despite those reservations, she said that the per-protocol observations should not be dismissed, because when they are analyzed in this way the results are highly significant. "I was really surprised to see what happened in the lung cancer. I am a medical oncologist and I have never seen that complete a response. It’s surprising. We have to wonder if all that we know about the treatment of tumors is correct."
Dr. Ram acknowledged that per-protocol analysis of the findings is unconventional, but "there is no precedent for this kind of therapy and I think we may need to redesign the way we assess results in the future. We cannot use the same guidelines and definitions that we were traditionally using."
Dr. Ram disclosed that he is a consultant for NovoCure. Dr. Brandes did not disclose if she had any conflicts of interest at the meeting and did not respond to inquiries about disclosures.
MONTREAL – An investigational treatment for recurrent glioblastoma that delivers alternating electric fields through scalp electrodes has shown signs of improved survival in a post hoc analysis of results in particular subgroups of patients enrolled in a phase III trial.
Quality of life outcomes also favored patients who used NovoTTF, compared with those who received chemotherapy.
To date, reports about the device have elicited both antagonistic and enthusiastic reaction from oncologists, with "neither the enthusiasts nor the antagonists having significant basis for either kind of acute reaction," Dr. Zvi Ram said in an interview after presenting the subgroup analyses at the annual meeting of the Society for Neuro-Oncology. "I think it is exciting that we’re getting something completely new – a different, noninvasive modality with no side-effects. I think we should be exhilarated."
The results obtained with a modified version of the device in a separate trial of patients with non–small cell lung cancer (NSCLC) suggest that the therapeutic effects of the device also may extend to other cancers, according to Dr. Ram, professor and chair of neurosurgery at Tel Aviv (Israel) Medical Center.
The device, known as NovoTTF-100A, delivers low-amplitude "tumor treatment fields" ranging from 100 to 300 kHz that have been shown in vitro to slow and reverse tumor cell proliferation by inhibiting mitosis, according to NovoCure Ltd., the manufacturer of the device and sponsor of the trial.
The portable device weighs about 6 pounds and connects to a battery pack. It is designed to be worn almost constantly, with a target of at least 20 hours of use each day.
In a phase III clinical trial presented earlier this year at the American Society of Clinical Oncology, an intent-to-treat analysis comparing NovoTTF vs. best-available chemotherapy found no statistical difference in 1-year overall survival (OS) among 237 recurrent glioblastoma patients randomized to either treatment.
However, a per-protocol analysis (which included only those patients who wore the device for at least 70% of the recommended time during the first month) showed a statistically significant benefit to NovoTTF in 1-year survival, compared with chemotherapy (29.5% vs. 19.1%, respectively; hazard ratio, 0.64; P = .01).
In the new post hoc analysis, a subgroup of 110 patients with a "good prognosis" (aged younger than 60 years, and with a Karnofsky performance status score greater than 80%) showed a "more robust" survival benefit than that seen in the overall intent-to-treat analysis, he said.
In this subgroup, patients who were treated with NovoTTF had a median survival of 9.2 months, compared with 6.6 months in those treated with chemotherapy (P less than .01). However, in the overall intent-to-treat group, median survival was 6.6 months and 6.0 months, respectively, he explained. Moreover, the 1-year OS in this subgroup was significantly higher in the NovoTTF group than in the chemotherapy group (35.2% vs. 20.8%, respectively; P less than .01), whereas the difference was nonsignificant in the larger analysis (23.6% vs. 20.7%).
Another subgroup analysis looked at patients who had previously failed treatment with bevacizumab (roughly 20% of the entire cohort). Both an intent-to-treat analysis and a per-protocol analysis showed significant OS advantages to NovoTTF, Dr. Ram said.
The median OS among 44 patients in the intent-to-treat group was 4 months with NovoTTF vs. 3.1 months with chemotherapy (HR, 0.43; P less than 0.02). NovoTTF also gave a significantly better median OS among 29 patients in the per-protocol analysis for this subgroup (6.3 months vs. 3.3 months; HR, 0.21; P = .02).
"You don’t see this anywhere," he said. "There’s no drug in the world that could produce such response in patients who had already failed" bevacizumab.
The investigators also analyzed a surgery-naive group. "You know these are going to be poor responders, almost identical to [those with] bevacizumab failure," Dr. Ram commented.
In this group of 38 patients, an intent-to-treat analysis showed that overall survival was 9.8 months with NovoTTF vs. 5.5 months with chemotherapy.
Patients also reported significantly better quality of life with NovoTTF than with chemotherapy. On the Quality of Life Symptom Scale, NovoTTF patients scored –34 and –35 on constipation and diarrhea, compared with scores of +77 and +50 for the chemotherapy group. Nausea and vomiting scores were 15 for the NovoTTF group and 61 for the chemotherapy group, and pain scores were –1 for the NovoTTF group and +63 for the chemotherapy group.
A quality of life analysis using the EORT (European Organization of Research and Treatment) QLQ-C30 instrument showed scores of 14 vs. –7 in favor of NovoTTF for cognitive functioning, and scores of 7 vs. 1 in favor of NovoTTF for emotional functioning.
"We do this to all our patients. We intoxicate them," Dr. Ram said. "So even if NovoTTF did not extend survival, if it was equivalent to chemotherapy [for survival], it may still improve quality of life."
Dr. Ram did not know the median length of time that the NovoTTF cohort wore the device, but an earlier phase II study followed some patients up to 59 months. He noted that "70% are still alive; that’s unheard of."
"There were concerns that patients might have more headaches or seizures, but there were none," he added.
In the current study, the rate of adverse events related to the central nervous system was similar (66% for NovoTTF and 67% for chemotherapy). Seizures occurred in 15% of the NovoTTF group and 12% of the chemotherapy group, and headaches occurred in 18% and 13%, respectively.
Dr. Ram also presented evidence to suggest that NovoTTF therapy may have benefits in other forms of cancer. A study reported by his colleagues earlier this year at the European Society for Medical Oncology Congress in Milan showed that NovoTTF therapy (combined with chemotherapy) resulted in significant prolongation of survival in patients with NSCLC, compared with historical controls, he said.
"If this kind of therapy acts against brain cancer cells, it should act also against other tumor types," he reasoned.
In the study, which included 42 NSCLC patients, NovoTTF was delivered with newly designed electrodes placed on the chest and neck of patients with locally advanced, metastatic, stage IIIb and IV disease, he explained. The combination of NovoTTF and pemetrexed increased overall survival to 13.8 months, compared with 8.3 months seen with pemetrexed alone; the 1-year survival rate for the combination was 57%, compared with 30% reported for pemetrexed alone.
"We’re talking about something that appears to be acting against cancer cells regardless of origin," Dr. Ram said. "Action in the lung seems similar to what has been seen in GBM over time: a slow resolution of malignant pleural effusion and masses within the chest."
"It’s very interesting and exciting, even if we do not yet have enough definitive data," commented Dr. Alba B. Brandes, moderator of the session and the chair of medical oncology at Azienda USL, a group of nine hospitals in and around Bologna, Italy.
The study investigators have been criticized for repackaging their nonsignificant intent-to-treat results into per-protocol results that show significance, she said. "An intention-to-treat population and per-protocol population are two different things, and from a statistical point of view, it is sometimes difficult for the oncologic community to accept."
Despite those reservations, she said that the per-protocol observations should not be dismissed, because when they are analyzed in this way the results are highly significant. "I was really surprised to see what happened in the lung cancer. I am a medical oncologist and I have never seen that complete a response. It’s surprising. We have to wonder if all that we know about the treatment of tumors is correct."
Dr. Ram acknowledged that per-protocol analysis of the findings is unconventional, but "there is no precedent for this kind of therapy and I think we may need to redesign the way we assess results in the future. We cannot use the same guidelines and definitions that we were traditionally using."
Dr. Ram disclosed that he is a consultant for NovoCure. Dr. Brandes did not disclose if she had any conflicts of interest at the meeting and did not respond to inquiries about disclosures.
Major Finding: In a subgroup of 110 patients aged younger than 60 years who had a Karnofsky score greater than 80%, the 1-year overall survival was significantly higher in the NovoTTF group than in the chemotherapy group (35.2% vs. 20.8%).
Data Source: Post hoc subgroup analysis of a trial comparing electric field treatment with chemotherapy in 237 patients with recurrent glioblastoma.
Disclosures: Dr. Zvi Ram disclosed that he is a consultant for NovoCure, which sponsored the trial and manufactures the device. Dr. Brandes did not disclose if she had any conflicts of interest at the meeting and did not respond to inquiries about disclosures.
PTSD Treatment Research Begins to Target Memory Reconsolidation
MONTREAL – The field of psychiatry is facing a paradigm shift with new research suggesting that medications and psychotherapy may be able to permanently erase the "trauma" from traumatic memories, according to several experts.
The experimental treatment, known as reconsolidation blockade, has been shown to interrupt the neurobiologic process of memory formation.
"We do not erase people’s memories," Alain Brunet, Ph.D., said at the annual meeting of the International Society for Traumatic Stress Studies. Dr. Brunet of the department of psychiatry at McGill University, Montreal, is one of the first researchers to report results of the treatment in patients with posttraumatic stress disorder (PTSD).
Rather than erasing an entire memory, reconsolidation blockade appears to erase the emotional reaction to the memory, explained Dr. Roger K. Pitman, director of the posttraumatic stress disorder and psychophysiology laboratory at Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, both in Boston.
The treatment, which normally involves two doses of the beta-blocker propranolol administered between 75 minutes and 2 hours apart, is "pioneering" in that it upends traditional theories about the permanence of memory, said Dr. Charles Marmar, professor and chair of the department of psychiatry and director of the Trauma Research Group at New York University.
Traditional cognitive-behavioral treatment for PTSD is based on the premise that traumatic memory is permanent, and therefore therapy should focus on learning a less emotional response to it, explained Gregory Quirk, Ph.D., professor of psychiatry and director of the laboratory of fear learning at the University of Puerto Rico, San Juan.
This learned response, known as "extinction," changes the body’s physiologic, amygdala-based memory, and teaches a cognitive, hippocampal response to the memory instead, he said. "With extinction, you reroute the stimulus so it does not go to the amygdala. You’re teaching the brain – it’s a learned thing – but the original memory is still in the amygdala somewhere. Extinction does not alter the original memory – we know that from Pavlov." Extinction works well in certain psychiatric conditions, such as phobia, but people with PTSD have hippocampal and prefrontal deficits that frequently cause extinction failure, he said.
In contrast, reconsolidation blockade does not recruit the hippocampus but instead targets the amygdala-dependent reaction. "As a prominent extinction researcher, I am very excited about reconsolidation blockade," Dr. Quirk said. "What’s new and exciting about this paradigm shift is that you don’t have to struggle your whole life with these terrible memories. You can alter them instead."
The theory behind reconsolidation blockade is that after a traumatic memory is consolidated in the brain, it can be reactivated and exist in a labile, modifiable state. During the labile window, which is believed to last up to 6 hours, propranolol can block protein synthesis involved in amygdala-dependent reconsolidation. "There’s no loss of the hippocampal-dependent declarative memory – what’s lost is the amygdala-dependent reaction that makes patients sick," Dr. Quirk said. "And that’s exactly the thing we want to erase – the part of the memory that makes them ill."
In one study conducted by Dr. Pitman and Dr. Brunet, PTSD patients treated with propranolol after memory reactivation showed a significantly decreased physiologic response when they engaged in script-driven mental imagery of their traumatic event 1 week later, compared with placebo-treated patients (J. Psychiatr. Res. 2008;42:503-6). But a more recent study by Dr. Pitman’s group found improvements in two separate propranolol-treated PTSD groups – one treated after memory reactivation, and one treated without reactivation. This suggests "there may be nonspecific effects of propranolol – which does not support the theory of reconsolidation blockade," Dr. Pitman commented.
Not everyone agrees on the mechanism through which propranolol impacts memory.
Earlier this year, at a meeting of the Canadian Psychiatric Association, Dr. Robert Menzies, a psychiatrist in private practice in Saskatoon, Sask., reported that his PTSD patients experience fragmented memories, emotional distance, and even amnesia after treatment with propranolol. In 31 patients (21 men) treated over a 2-year period, there was a 90% response rate, with the duration of effect continuing up to 2 years. The patients’ duration of traumatic memories ranged from 3 months to 38 years.
Dr. Menzies said he did not measure physiologic response after the treatment; he simply asked patients how their traumatic memories have changed. "My patients come back and say they can’t remember certain memories at all," he said in an interview.
A drug-free approach to reconsolidation blockade that uses psychotherapy during the labile window to "rewrite" fearful memories also has been reported by researchers at the center for neural science at New York University (Nature 2010;463:49-53). This approach is basically a variation of traditional extinction training, but because it is done during the window of biochemical lability, it permanently alters the amygdala-dependent memory.
The researchers wrote that they "provide evidence that old fear memories can be updated with nonfearful information provided during the reconsolidation window. As a consequence, fear responses are no longer expressed, an effect that lasted at least a year and was selective only to reactivated memories without affecting others."
Although targeting the traumatic memory is an important part of PTSD therapy, it is not the only part, noted several experts. "We might have to go beyond simply looking at the traumatic memory piece of things," said Rachel Yehuda, Ph.D., director of the traumatic stress studies division at Mount Sinai School of Medicine and director of mental health at the James J. Peters VA Medical Center, both in New York. "There are other components to PTSD – there’s loss, there’s grief, there’s sadness, there’s inability to experience pleasure, there’s anger and rage, there’s feelings of shame. Those are things that also have to be addressed," she said in an interview.
"This is important work – I am excited about it – but cognitive work also has to happen," added Dr. Thomas C. Neylan, professor of psychiatry (in residence) at the University of California, San Francisco, and director of the postttraumatic stress disorder program at the San Francisco VA Medical Center. "People who have been traumatized often have a whole new set of assumptions about their world and their place in the world that are sometimes erroneous – that the world is overly dangerous, or you can’t trust anybody," he said in an interview. "That’s cognitive work that has to be done, separate from reconsolidation or extinction work."
Dr. Menzies reported receiving honoraria and other fees from Wyeth. Dr. Brunet, Dr. Neylan, Dr. Pitman, Dr. Quirk, and Dr. Yehuda reported having no relevant financial disclosures. Disclosure information was not available for Dr. Marmar.
MONTREAL – The field of psychiatry is facing a paradigm shift with new research suggesting that medications and psychotherapy may be able to permanently erase the "trauma" from traumatic memories, according to several experts.
The experimental treatment, known as reconsolidation blockade, has been shown to interrupt the neurobiologic process of memory formation.
"We do not erase people’s memories," Alain Brunet, Ph.D., said at the annual meeting of the International Society for Traumatic Stress Studies. Dr. Brunet of the department of psychiatry at McGill University, Montreal, is one of the first researchers to report results of the treatment in patients with posttraumatic stress disorder (PTSD).
Rather than erasing an entire memory, reconsolidation blockade appears to erase the emotional reaction to the memory, explained Dr. Roger K. Pitman, director of the posttraumatic stress disorder and psychophysiology laboratory at Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, both in Boston.
The treatment, which normally involves two doses of the beta-blocker propranolol administered between 75 minutes and 2 hours apart, is "pioneering" in that it upends traditional theories about the permanence of memory, said Dr. Charles Marmar, professor and chair of the department of psychiatry and director of the Trauma Research Group at New York University.
Traditional cognitive-behavioral treatment for PTSD is based on the premise that traumatic memory is permanent, and therefore therapy should focus on learning a less emotional response to it, explained Gregory Quirk, Ph.D., professor of psychiatry and director of the laboratory of fear learning at the University of Puerto Rico, San Juan.
This learned response, known as "extinction," changes the body’s physiologic, amygdala-based memory, and teaches a cognitive, hippocampal response to the memory instead, he said. "With extinction, you reroute the stimulus so it does not go to the amygdala. You’re teaching the brain – it’s a learned thing – but the original memory is still in the amygdala somewhere. Extinction does not alter the original memory – we know that from Pavlov." Extinction works well in certain psychiatric conditions, such as phobia, but people with PTSD have hippocampal and prefrontal deficits that frequently cause extinction failure, he said.
In contrast, reconsolidation blockade does not recruit the hippocampus but instead targets the amygdala-dependent reaction. "As a prominent extinction researcher, I am very excited about reconsolidation blockade," Dr. Quirk said. "What’s new and exciting about this paradigm shift is that you don’t have to struggle your whole life with these terrible memories. You can alter them instead."
The theory behind reconsolidation blockade is that after a traumatic memory is consolidated in the brain, it can be reactivated and exist in a labile, modifiable state. During the labile window, which is believed to last up to 6 hours, propranolol can block protein synthesis involved in amygdala-dependent reconsolidation. "There’s no loss of the hippocampal-dependent declarative memory – what’s lost is the amygdala-dependent reaction that makes patients sick," Dr. Quirk said. "And that’s exactly the thing we want to erase – the part of the memory that makes them ill."
In one study conducted by Dr. Pitman and Dr. Brunet, PTSD patients treated with propranolol after memory reactivation showed a significantly decreased physiologic response when they engaged in script-driven mental imagery of their traumatic event 1 week later, compared with placebo-treated patients (J. Psychiatr. Res. 2008;42:503-6). But a more recent study by Dr. Pitman’s group found improvements in two separate propranolol-treated PTSD groups – one treated after memory reactivation, and one treated without reactivation. This suggests "there may be nonspecific effects of propranolol – which does not support the theory of reconsolidation blockade," Dr. Pitman commented.
Not everyone agrees on the mechanism through which propranolol impacts memory.
Earlier this year, at a meeting of the Canadian Psychiatric Association, Dr. Robert Menzies, a psychiatrist in private practice in Saskatoon, Sask., reported that his PTSD patients experience fragmented memories, emotional distance, and even amnesia after treatment with propranolol. In 31 patients (21 men) treated over a 2-year period, there was a 90% response rate, with the duration of effect continuing up to 2 years. The patients’ duration of traumatic memories ranged from 3 months to 38 years.
Dr. Menzies said he did not measure physiologic response after the treatment; he simply asked patients how their traumatic memories have changed. "My patients come back and say they can’t remember certain memories at all," he said in an interview.
A drug-free approach to reconsolidation blockade that uses psychotherapy during the labile window to "rewrite" fearful memories also has been reported by researchers at the center for neural science at New York University (Nature 2010;463:49-53). This approach is basically a variation of traditional extinction training, but because it is done during the window of biochemical lability, it permanently alters the amygdala-dependent memory.
The researchers wrote that they "provide evidence that old fear memories can be updated with nonfearful information provided during the reconsolidation window. As a consequence, fear responses are no longer expressed, an effect that lasted at least a year and was selective only to reactivated memories without affecting others."
Although targeting the traumatic memory is an important part of PTSD therapy, it is not the only part, noted several experts. "We might have to go beyond simply looking at the traumatic memory piece of things," said Rachel Yehuda, Ph.D., director of the traumatic stress studies division at Mount Sinai School of Medicine and director of mental health at the James J. Peters VA Medical Center, both in New York. "There are other components to PTSD – there’s loss, there’s grief, there’s sadness, there’s inability to experience pleasure, there’s anger and rage, there’s feelings of shame. Those are things that also have to be addressed," she said in an interview.
"This is important work – I am excited about it – but cognitive work also has to happen," added Dr. Thomas C. Neylan, professor of psychiatry (in residence) at the University of California, San Francisco, and director of the postttraumatic stress disorder program at the San Francisco VA Medical Center. "People who have been traumatized often have a whole new set of assumptions about their world and their place in the world that are sometimes erroneous – that the world is overly dangerous, or you can’t trust anybody," he said in an interview. "That’s cognitive work that has to be done, separate from reconsolidation or extinction work."
Dr. Menzies reported receiving honoraria and other fees from Wyeth. Dr. Brunet, Dr. Neylan, Dr. Pitman, Dr. Quirk, and Dr. Yehuda reported having no relevant financial disclosures. Disclosure information was not available for Dr. Marmar.
MONTREAL – The field of psychiatry is facing a paradigm shift with new research suggesting that medications and psychotherapy may be able to permanently erase the "trauma" from traumatic memories, according to several experts.
The experimental treatment, known as reconsolidation blockade, has been shown to interrupt the neurobiologic process of memory formation.
"We do not erase people’s memories," Alain Brunet, Ph.D., said at the annual meeting of the International Society for Traumatic Stress Studies. Dr. Brunet of the department of psychiatry at McGill University, Montreal, is one of the first researchers to report results of the treatment in patients with posttraumatic stress disorder (PTSD).
Rather than erasing an entire memory, reconsolidation blockade appears to erase the emotional reaction to the memory, explained Dr. Roger K. Pitman, director of the posttraumatic stress disorder and psychophysiology laboratory at Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, both in Boston.
The treatment, which normally involves two doses of the beta-blocker propranolol administered between 75 minutes and 2 hours apart, is "pioneering" in that it upends traditional theories about the permanence of memory, said Dr. Charles Marmar, professor and chair of the department of psychiatry and director of the Trauma Research Group at New York University.
Traditional cognitive-behavioral treatment for PTSD is based on the premise that traumatic memory is permanent, and therefore therapy should focus on learning a less emotional response to it, explained Gregory Quirk, Ph.D., professor of psychiatry and director of the laboratory of fear learning at the University of Puerto Rico, San Juan.
This learned response, known as "extinction," changes the body’s physiologic, amygdala-based memory, and teaches a cognitive, hippocampal response to the memory instead, he said. "With extinction, you reroute the stimulus so it does not go to the amygdala. You’re teaching the brain – it’s a learned thing – but the original memory is still in the amygdala somewhere. Extinction does not alter the original memory – we know that from Pavlov." Extinction works well in certain psychiatric conditions, such as phobia, but people with PTSD have hippocampal and prefrontal deficits that frequently cause extinction failure, he said.
In contrast, reconsolidation blockade does not recruit the hippocampus but instead targets the amygdala-dependent reaction. "As a prominent extinction researcher, I am very excited about reconsolidation blockade," Dr. Quirk said. "What’s new and exciting about this paradigm shift is that you don’t have to struggle your whole life with these terrible memories. You can alter them instead."
The theory behind reconsolidation blockade is that after a traumatic memory is consolidated in the brain, it can be reactivated and exist in a labile, modifiable state. During the labile window, which is believed to last up to 6 hours, propranolol can block protein synthesis involved in amygdala-dependent reconsolidation. "There’s no loss of the hippocampal-dependent declarative memory – what’s lost is the amygdala-dependent reaction that makes patients sick," Dr. Quirk said. "And that’s exactly the thing we want to erase – the part of the memory that makes them ill."
In one study conducted by Dr. Pitman and Dr. Brunet, PTSD patients treated with propranolol after memory reactivation showed a significantly decreased physiologic response when they engaged in script-driven mental imagery of their traumatic event 1 week later, compared with placebo-treated patients (J. Psychiatr. Res. 2008;42:503-6). But a more recent study by Dr. Pitman’s group found improvements in two separate propranolol-treated PTSD groups – one treated after memory reactivation, and one treated without reactivation. This suggests "there may be nonspecific effects of propranolol – which does not support the theory of reconsolidation blockade," Dr. Pitman commented.
Not everyone agrees on the mechanism through which propranolol impacts memory.
Earlier this year, at a meeting of the Canadian Psychiatric Association, Dr. Robert Menzies, a psychiatrist in private practice in Saskatoon, Sask., reported that his PTSD patients experience fragmented memories, emotional distance, and even amnesia after treatment with propranolol. In 31 patients (21 men) treated over a 2-year period, there was a 90% response rate, with the duration of effect continuing up to 2 years. The patients’ duration of traumatic memories ranged from 3 months to 38 years.
Dr. Menzies said he did not measure physiologic response after the treatment; he simply asked patients how their traumatic memories have changed. "My patients come back and say they can’t remember certain memories at all," he said in an interview.
A drug-free approach to reconsolidation blockade that uses psychotherapy during the labile window to "rewrite" fearful memories also has been reported by researchers at the center for neural science at New York University (Nature 2010;463:49-53). This approach is basically a variation of traditional extinction training, but because it is done during the window of biochemical lability, it permanently alters the amygdala-dependent memory.
The researchers wrote that they "provide evidence that old fear memories can be updated with nonfearful information provided during the reconsolidation window. As a consequence, fear responses are no longer expressed, an effect that lasted at least a year and was selective only to reactivated memories without affecting others."
Although targeting the traumatic memory is an important part of PTSD therapy, it is not the only part, noted several experts. "We might have to go beyond simply looking at the traumatic memory piece of things," said Rachel Yehuda, Ph.D., director of the traumatic stress studies division at Mount Sinai School of Medicine and director of mental health at the James J. Peters VA Medical Center, both in New York. "There are other components to PTSD – there’s loss, there’s grief, there’s sadness, there’s inability to experience pleasure, there’s anger and rage, there’s feelings of shame. Those are things that also have to be addressed," she said in an interview.
"This is important work – I am excited about it – but cognitive work also has to happen," added Dr. Thomas C. Neylan, professor of psychiatry (in residence) at the University of California, San Francisco, and director of the postttraumatic stress disorder program at the San Francisco VA Medical Center. "People who have been traumatized often have a whole new set of assumptions about their world and their place in the world that are sometimes erroneous – that the world is overly dangerous, or you can’t trust anybody," he said in an interview. "That’s cognitive work that has to be done, separate from reconsolidation or extinction work."
Dr. Menzies reported receiving honoraria and other fees from Wyeth. Dr. Brunet, Dr. Neylan, Dr. Pitman, Dr. Quirk, and Dr. Yehuda reported having no relevant financial disclosures. Disclosure information was not available for Dr. Marmar.
FROM THE ANNUAL MEETING OF THE INTERNATIONAL SOCIETY FOR TRAUMATIC STRESS STUDIES
Paternal Postpartum Depression High
A significant number of men experience prenatal and postpartum depression, and the rate is marginally higher in the United States than in other countries, according to a meta-analysis of 43 studies.
The overall rate of paternal depression was 10.4%, with a U.S. rate of 14.1% vs. 8.2% in other countries. The study also reported maternal depression at a rate of 23.8%, with a moderate positive correlation between maternal and paternal depression.
The findings suggest that “more efforts should be made to improve screening and referral, particularly in light of the mounting evidence that early paternal depression may have substantial emotional, behavioral, and developmental effects on children,” noted James F. Paulson, Ph.D., and his colleague Sharnail D. Bazemore of the department of pediatrics at Eastern Virginia Medical School in Norfolk (JAMA 2010;303:1961-9). The correlation between paternal and maternal depression “also suggests a screening rubric – depression in one patient should prompt clinical attention to the other,” the investigators wrote.
The meta-analysis included studies from 16 countries and involved 28,004 new and expectant fathers aged 18 years or older.
A significant number of men experience prenatal and postpartum depression, and the rate is marginally higher in the United States than in other countries, according to a meta-analysis of 43 studies.
The overall rate of paternal depression was 10.4%, with a U.S. rate of 14.1% vs. 8.2% in other countries. The study also reported maternal depression at a rate of 23.8%, with a moderate positive correlation between maternal and paternal depression.
The findings suggest that “more efforts should be made to improve screening and referral, particularly in light of the mounting evidence that early paternal depression may have substantial emotional, behavioral, and developmental effects on children,” noted James F. Paulson, Ph.D., and his colleague Sharnail D. Bazemore of the department of pediatrics at Eastern Virginia Medical School in Norfolk (JAMA 2010;303:1961-9). The correlation between paternal and maternal depression “also suggests a screening rubric – depression in one patient should prompt clinical attention to the other,” the investigators wrote.
The meta-analysis included studies from 16 countries and involved 28,004 new and expectant fathers aged 18 years or older.
A significant number of men experience prenatal and postpartum depression, and the rate is marginally higher in the United States than in other countries, according to a meta-analysis of 43 studies.
The overall rate of paternal depression was 10.4%, with a U.S. rate of 14.1% vs. 8.2% in other countries. The study also reported maternal depression at a rate of 23.8%, with a moderate positive correlation between maternal and paternal depression.
The findings suggest that “more efforts should be made to improve screening and referral, particularly in light of the mounting evidence that early paternal depression may have substantial emotional, behavioral, and developmental effects on children,” noted James F. Paulson, Ph.D., and his colleague Sharnail D. Bazemore of the department of pediatrics at Eastern Virginia Medical School in Norfolk (JAMA 2010;303:1961-9). The correlation between paternal and maternal depression “also suggests a screening rubric – depression in one patient should prompt clinical attention to the other,” the investigators wrote.
The meta-analysis included studies from 16 countries and involved 28,004 new and expectant fathers aged 18 years or older.
From JAMA
Induced Abortions Linked to Preterm Delivery
Major Finding: A history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks' gestation.
Data Source: A study of 3,138 women who had one or more therapeutic abortions who underwent a subsequent delivery of a live singleton or multiples and 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Disclosures: None was reported.
MONTREAL – Women with a history of one or more therapeutic abortions have double the risk of preterm delivery before 24 weeks' gestation in a subsequent pregnancy, compared with those with no abortion history, a study by researchers from McGill University in Montreal has shown.
“The implications are that abortions can lead to cervical insufficiency, and that public health efforts should aim at prevention through early counseling and provision of effective contraception for all women,” said the principal investigator, Dr. Haim Abenhaim, obstetrician/gynecologist at Montreal's SMBD Jewish General Hospital, which is part of McGill University.
The retrospective study, presented by Dr. Ghislain Hardy at the meeting, looked at all women who had undergone an induced abortion and subsequent delivery of a live singleton or multiples birth at a single, tertiary care institution between 2001 and 2006.
A total of 2,276 women had undergone one abortion, and 862 had undergone two or more. These women were compared with 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Most of the therapeutic abortions [TABs] were first-trimester dilation and curettage, Dr. Abenhaim said in an interview. “For a therapeutic abortion (voluntary, for a normal pregnancy), the most common approach is a D&C [that is, surgical], while a medical approach is the more common for an arrested pregnancy.”
After adjustment for age, smoking, alcohol consumption, body mass index, marital status, and education, the investigators found that a history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks. In addition, women with a history of abortion were more likely to require tocolysis – and this became statistically significant with two or more abortions (odds ratio, 1.42). Dr. Abenhaim did not present absolute numbers and did not respond to requests to acquire this information.
“The association between therapeutic abortions and prematurity has been seen before; however, our study was different in that it tried to examine the relationship between TABs and the timing of prematurity,” explained Dr. Abenhaim. “Prematurity caused by a cervical insufficiency is believed to occur earlier than prematurity which is considered idiopathic or infectious. Our results suggest that the effect of TABs is more consistent with early/cervical insufficiency–type preterm births.”
The findings are potentially important, said Dr. Anthony Armson, who chaired the session in which the study was presented. However, they should be interpreted with caution, especially since the study has not yet been published, said the professor and head of obstetrics and gynecology at Dalhousie University in Halifax, N.S.
“Certainly there has been evidence to support [the risk of] two or more therapeutic abortions in the first trimester, or any in the second-trimester – but that observation has not always been supported,” he said in an interview. “A number of investigators in the '80s and '90s, including myself, tried to come up with a risk-scoring algorithm to establish what factors were important in preterm birth. But in the vast majority, one therapeutic abortion did not come up as a risk factor.”
Major Finding: A history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks' gestation.
Data Source: A study of 3,138 women who had one or more therapeutic abortions who underwent a subsequent delivery of a live singleton or multiples and 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Disclosures: None was reported.
MONTREAL – Women with a history of one or more therapeutic abortions have double the risk of preterm delivery before 24 weeks' gestation in a subsequent pregnancy, compared with those with no abortion history, a study by researchers from McGill University in Montreal has shown.
“The implications are that abortions can lead to cervical insufficiency, and that public health efforts should aim at prevention through early counseling and provision of effective contraception for all women,” said the principal investigator, Dr. Haim Abenhaim, obstetrician/gynecologist at Montreal's SMBD Jewish General Hospital, which is part of McGill University.
The retrospective study, presented by Dr. Ghislain Hardy at the meeting, looked at all women who had undergone an induced abortion and subsequent delivery of a live singleton or multiples birth at a single, tertiary care institution between 2001 and 2006.
A total of 2,276 women had undergone one abortion, and 862 had undergone two or more. These women were compared with 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Most of the therapeutic abortions [TABs] were first-trimester dilation and curettage, Dr. Abenhaim said in an interview. “For a therapeutic abortion (voluntary, for a normal pregnancy), the most common approach is a D&C [that is, surgical], while a medical approach is the more common for an arrested pregnancy.”
After adjustment for age, smoking, alcohol consumption, body mass index, marital status, and education, the investigators found that a history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks. In addition, women with a history of abortion were more likely to require tocolysis – and this became statistically significant with two or more abortions (odds ratio, 1.42). Dr. Abenhaim did not present absolute numbers and did not respond to requests to acquire this information.
“The association between therapeutic abortions and prematurity has been seen before; however, our study was different in that it tried to examine the relationship between TABs and the timing of prematurity,” explained Dr. Abenhaim. “Prematurity caused by a cervical insufficiency is believed to occur earlier than prematurity which is considered idiopathic or infectious. Our results suggest that the effect of TABs is more consistent with early/cervical insufficiency–type preterm births.”
The findings are potentially important, said Dr. Anthony Armson, who chaired the session in which the study was presented. However, they should be interpreted with caution, especially since the study has not yet been published, said the professor and head of obstetrics and gynecology at Dalhousie University in Halifax, N.S.
“Certainly there has been evidence to support [the risk of] two or more therapeutic abortions in the first trimester, or any in the second-trimester – but that observation has not always been supported,” he said in an interview. “A number of investigators in the '80s and '90s, including myself, tried to come up with a risk-scoring algorithm to establish what factors were important in preterm birth. But in the vast majority, one therapeutic abortion did not come up as a risk factor.”
Major Finding: A history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks' gestation.
Data Source: A study of 3,138 women who had one or more therapeutic abortions who underwent a subsequent delivery of a live singleton or multiples and 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Disclosures: None was reported.
MONTREAL – Women with a history of one or more therapeutic abortions have double the risk of preterm delivery before 24 weeks' gestation in a subsequent pregnancy, compared with those with no abortion history, a study by researchers from McGill University in Montreal has shown.
“The implications are that abortions can lead to cervical insufficiency, and that public health efforts should aim at prevention through early counseling and provision of effective contraception for all women,” said the principal investigator, Dr. Haim Abenhaim, obstetrician/gynecologist at Montreal's SMBD Jewish General Hospital, which is part of McGill University.
The retrospective study, presented by Dr. Ghislain Hardy at the meeting, looked at all women who had undergone an induced abortion and subsequent delivery of a live singleton or multiples birth at a single, tertiary care institution between 2001 and 2006.
A total of 2,276 women had undergone one abortion, and 862 had undergone two or more. These women were compared with 14,778 women who had no history of abortion and who gave birth at the hospital during the same period.
Most of the therapeutic abortions [TABs] were first-trimester dilation and curettage, Dr. Abenhaim said in an interview. “For a therapeutic abortion (voluntary, for a normal pregnancy), the most common approach is a D&C [that is, surgical], while a medical approach is the more common for an arrested pregnancy.”
After adjustment for age, smoking, alcohol consumption, body mass index, marital status, and education, the investigators found that a history of one or more abortions was associated with a twofold increase in preterm delivery at less than 24 weeks. In addition, women with a history of abortion were more likely to require tocolysis – and this became statistically significant with two or more abortions (odds ratio, 1.42). Dr. Abenhaim did not present absolute numbers and did not respond to requests to acquire this information.
“The association between therapeutic abortions and prematurity has been seen before; however, our study was different in that it tried to examine the relationship between TABs and the timing of prematurity,” explained Dr. Abenhaim. “Prematurity caused by a cervical insufficiency is believed to occur earlier than prematurity which is considered idiopathic or infectious. Our results suggest that the effect of TABs is more consistent with early/cervical insufficiency–type preterm births.”
The findings are potentially important, said Dr. Anthony Armson, who chaired the session in which the study was presented. However, they should be interpreted with caution, especially since the study has not yet been published, said the professor and head of obstetrics and gynecology at Dalhousie University in Halifax, N.S.
“Certainly there has been evidence to support [the risk of] two or more therapeutic abortions in the first trimester, or any in the second-trimester – but that observation has not always been supported,” he said in an interview. “A number of investigators in the '80s and '90s, including myself, tried to come up with a risk-scoring algorithm to establish what factors were important in preterm birth. But in the vast majority, one therapeutic abortion did not come up as a risk factor.”
From the Annual Meeting of the Society of Obstetricians and Gynaecologists of Canada