User login
Antidepressants causing sexual problems? Give her Viagra
Tell women for whom you prescribe selective and nonselective serotonin reuptake inhibitors (SRIs) to let you know if they develop sexual dysfunction. Offer sildenafil (50 mg with the option to increase to 100 mg) to premenopausal women on stable, effective doses of SRIs who experience this common—and treatable—side effect.1
Strength of recommendation
B: One high-quality RCT that confirms smaller, open-label studies
Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
ILLUSTRATIVE CASE
A 34-year-old woman comes to your office and asks to be taken off the paroxetine you prescribed for her 4 months ago. The medication is working well; her depression has been in remission for at least 12 weeks. But she no longer enjoys sex. She used to have a healthy libido and satisfying arousal and orgasm, but since starting the antidepressant, her sexual interest and pleasure have been low.
Although she’s afraid of sinking back into a depression without the medication, she’s willing to take the risk. If she were your patient, what alternatives would you suggest?
Sexual dysfunction affects an estimated 30% to 50% of patients on selective and nonselective SRIs, and some studies report rates as high as 70% to 80%.2 Many patients stop taking these antidepressants prematurely, often because of sexual side effects.3,4
Phosphodiesterase type 5 (PDE-5) inhibitors are well established as an effective treatment for erectile dysfunction,5 and randomized controlled trials (RCTs) have shown sildenafil to be effective in treating male SRI-induced sexual impairment.6,7 For women, there has been no parallel evidence-based treatment.
Limited options, with little support
Typically, women who reported antidepressant-associated sexual disturbances have been offered options for which there was only weak evidence—dose changes or augmentation with another agent, switching to another antidepressant, or taking occasional drug holidays. A 2004 Cochrane review found that there were no RCTs involving dose changes or drug holidays.8 Among studies of the efficacy of switching to a different drug, nefazodone was the only agent whose use was supported by a double-blind RCT.9 Augmentation trials of a wide range of medications and supplements—including amantadine, bupropion, buspirone, granisetron, mirtazapine, olanzapine, ephedrine, ginkgo biloba, and yohimbine—yielded mixed results. Indeed, the research found that some were no better than placebo.
PDE-5 inhibitors for women? Inconclusive studies to date
Female sexual dysfunction is generally divided into 4 domains: disorders of desire, arousal, orgasm, or pain. Decreased desire and delayed or absent orgasm are the most common sexual side effects of SRI antidepressants in women.10 Several studies of PDE-5 inhibitors in this patient population have had positive results,11-15 so there has been good reason to think that they might help this subset of women. However, all the studies were small and nonblinded, and therefore inconclusive—until now.
STUDY SUMMARY: Finally, a well-done RCT provides some answers
Investigators enrolled 98 premenopausal women from 7 US research centers in a double-blind randomized trial. To qualify, participants had to be diagnosed with major depression in remission, be taking a selective or nonselective SRI for >8 weeks, and be on a stable dose for >4 weeks. They also had to meet Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for substance-induced sexual dysfunction lasting >4 weeks, but have no history of sexual impairment independent of antidepressants. Finally, participants had to engage in some form of regular sexual activity—intercourse, oral sex, and masturbation all qualified—at least twice a month, and be willing to continue efforts to have sex at least once a week during the study. Women with other medical, psychiatric, or sexual problems were excluded, as were those who were pregnant, breastfeeding, or able to become pregnant and not using reliable contraception.
Participants were randomized to receive 50 mg of sildenafil (n=49) or a matching placebo tablet (n=49), which they were instructed to take 1 to 2 hours before sexual activity. The dose could be adjusted to 2 tablets (100 mg sildenafil) based on investigator assessment of the patient’s response to the initial dose. Participants and all study personnel were blinded to group assignment.
The primary outcome was change from baseline to end-point in the Clinical Global Impression Scale, a clinician-rated scale based on review of patient symptoms that was adapted to evaluate sexual function. Secondary outcomes were changes in 3 other sexual function scales, the Hamilton Rating Scale for Depression, and measured hormone levels.
Investigators followed the women for 8 weeks, measuring outcomes at 2, 4, and 8 weeks.
Sildenafil is better than placebo
Using an intention-to-treat analysis with the last measurement (2, 4, or 8 weeks) as the end-point, both the treatment and placebo groups experienced improvement in sexual function. The sildenafil group improved more than the placebo group. On the Clinical Global Impression Scale (1 to 7, with higher scores indicating worse sexual function), sildenafil users went from a mean of 4.8 to 2.8, while placebo users went from a mean of 4.7 to 3.6. The difference in mean change from baseline was 0.8 (95% confidence interval [CI] 0.6-1.0; P=.001). Using a more conservative analysis in which participants who did not return for the 8-week follow-up visit were assumed to have returned to baseline, the difference in mean change from baseline was smaller (0.6, 95% CI, 0.3-0.8; P=0.03) but still statistically significant.
Orgasmic function shows significant improvement
The sexual function scales used as secondary outcomes provided more detail about which types of sexual dysfunction benefited from sildenafil. On all 3 scales, orgasmic function significantly favored sildenafil over placebo. In the domains of desire, arousal, and pain disorders, small to moderate improvements were seen in both groups, with no statistically significant differences. One potential confounder—a difference in the course of participants’ underlying depression— was ruled out because depression scale results remained unchanged from baseline to endpoint in both groups.
Baseline levels of cortisone, estradiol, follicle-stimulating hormone, leuteinizing hormone, progesterone, prolactin, sex hormone-binding globulin, testosterone, thyroid-stimulating hormone, and thyroxine, were normal, with no differences between the sildenafil and placebo groups.
WHAT’S NEW: Women have an evidence-based option
Like their male counterparts, we can now offer women whose depression is effectively treated by SRI antidepressants—and who are motivated to stay sexually active despite medication-associated side effects—an effective pharmacotherapeutic treatment.
CAVEATS: Side effects and study funding are worth noting
Side effects. Significantly more participants in the sildenafil group vs the placebo group experienced the following side effects: headache (43% vs 27%), visual disturbance (14% vs 2%), dyspepsia (12% vs 0%), flushing (24% vs 0%), nasal congestion (37% vs 6%), and palpitations (8% vs 2%). Nausea was the only side effect that was more common in the placebo group, reported by only 2% of those in the intervention group but 16% of those on placebo.
No serious adverse events occurred, however, and the medication appears to have been well tolerated overall, despite relatively high rates of side effects. Participants in the intervention group used an average of 5 doses of sildenafil per 2-week interval, the same number as those in the placebo group.
Small treatment effect. The difference in response between sildenafil and placebo was not large: 0.8 points on a 7-point scale. But this difference is likely a clinically meaningful effect to the women with this problem.
Drug company funding. Pfizer, the maker of Viagra, funded this study through an investigator-initiated grant. Some researchers argue that female sexual dysfunction has been defined, or even invented, by drug companies seeking to create new markets for their products.16 This concern, coupled with the fact that this is the only double-blind randomized trial to show that sildenafil benefits women with antidepressant-associated sexual impairment, raises the question of whether this finding will be replicated in future trials.
We were reassured by the authors’ statement that Pfizer had no role in the study design, implementation, analysis, or manuscript preparation. And we know from clinical practice that women do suffer from SRI-induced sexual side effects, and sometimes stop taking much-needed antidepressants because the medication interferes with their ability to have a satisfying sex life. We believe this study was well done and offers a promising new therapy that deserves consideration. We hope that additional trials will follow and that investigators and journals will not hesitate to publish negative results.
Not for all women with sexual dysfunction
It’s a safe bet that these findings will be used to market sildenafil to women. It is therefore important for physicians and patients to keep in mind that this trial focused on a well-defined subset of women with sexual dysfunction: those on a stable dose of an SRI, with depression in remission, who were otherwise healthy and not pregnant, breastfeeding, or planning pregnancy, and who were motivated to be sexually active. Although this study does support the use of sildenafil for women in this subset, it does not support the use of PDE-5 inhibitors such as sildenafil for all women with sexual difficulties.
CHALLENGES TO IMPLEMENTATION: You have to ask!
Studies have repeatedly found that many women who experience sexual problems do not broach the subject with their doctors.17 So don’t wait for your female patients to bring it up. Sexual side effects are common enough with SRI antidepressants that all prescribers should mention the possibility in advance. Tell patients to let you know if they develop medication-related sexual dysfunction, and reassure them that there are treatments that can help.
Acknowledgment
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. Spanish working group for the study of psychotropic-related sexual dysfunction. J Clin Psychiatry. 2001;62 (suppl 3):S10-S21.
3. Mitchell AJ. Depressed patients and treatment adherence. Lancet. 2006;367:2041-2043.
4. Mitchell AJ, Selmes T. Why don’t patients take their medicine? reasons and solutions in psychiatry. Adv Psychiatr Treat. 2007;13:336-346.
5. Fazio L, Brock G. Erectile dysfunction: Management update. CMAJ. 2004;170:1429-1437.
6. Nurnberg HG, Gelenberg A, Hargreave TB, Harrison WM, Siegel RL, Smith MD. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry. 2001;158:1926-1928.
7. Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S. Treatment of antidepressant-associated sexual dysfunction with sildenafil: A randomized controlled trial. JAMA. 2003;289:56-64.
8. Rudkin L, Taylor MJ, Hawton K. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2004;18(4):CD003382.
9. Ferguson JM, Shrivastava RK, Stahl SM, et al. Reemergence of sexual dysfunction in patients with major depressive disorder: Double-blind comparison of nefazodone and sertraline. J Clin Psychiatry. 2001;62:24-29.
10. Frank JE, Mistretta P, Will J. Diagnosis and treatment of female sexual dysfunction. Am Fam Physician. 2008;77:635-642.
11. Nurnberg HG, Hensley PL, Lauriello J, Parker LM, Keith SJ. Sildenafil for women patients with antidepressant-induced sexual dysfunction. Psychiatr Serv. 1999;50:1076-1078.
12. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for sexual dysfunction in women taking antidepressants. Am J Psychiatry. 1999;156:1664.
13. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for iatrogenic serotonergic antidepressant medication-induced sexual dysfunction in 4 patients. J Clin Psychiatry. 1999;60:33-35.
14. Ashton AK. Vardenafil reversal of female anorgasmia. Am J Psychiatry. 2004;161:2133.
15. Ashton AK, Weinstein W. Tadalafil reversal of sexual dysfunction caused by serotonin enhancing medications in women. J Sex Marital Ther. 2006;32:1-3.
16. Moynihan R. The making of a disease: Female sexual dysfunction. BMJ. 2003 Jan 4;326:45-47.
17. Rosenberg KP, Bleiberg KL, Koscis J, Gross C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: Impact on compliance. J Sex Marital Ther. 2003;29:289-296.
Tell women for whom you prescribe selective and nonselective serotonin reuptake inhibitors (SRIs) to let you know if they develop sexual dysfunction. Offer sildenafil (50 mg with the option to increase to 100 mg) to premenopausal women on stable, effective doses of SRIs who experience this common—and treatable—side effect.1
Strength of recommendation
B: One high-quality RCT that confirms smaller, open-label studies
Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
ILLUSTRATIVE CASE
A 34-year-old woman comes to your office and asks to be taken off the paroxetine you prescribed for her 4 months ago. The medication is working well; her depression has been in remission for at least 12 weeks. But she no longer enjoys sex. She used to have a healthy libido and satisfying arousal and orgasm, but since starting the antidepressant, her sexual interest and pleasure have been low.
Although she’s afraid of sinking back into a depression without the medication, she’s willing to take the risk. If she were your patient, what alternatives would you suggest?
Sexual dysfunction affects an estimated 30% to 50% of patients on selective and nonselective SRIs, and some studies report rates as high as 70% to 80%.2 Many patients stop taking these antidepressants prematurely, often because of sexual side effects.3,4
Phosphodiesterase type 5 (PDE-5) inhibitors are well established as an effective treatment for erectile dysfunction,5 and randomized controlled trials (RCTs) have shown sildenafil to be effective in treating male SRI-induced sexual impairment.6,7 For women, there has been no parallel evidence-based treatment.
Limited options, with little support
Typically, women who reported antidepressant-associated sexual disturbances have been offered options for which there was only weak evidence—dose changes or augmentation with another agent, switching to another antidepressant, or taking occasional drug holidays. A 2004 Cochrane review found that there were no RCTs involving dose changes or drug holidays.8 Among studies of the efficacy of switching to a different drug, nefazodone was the only agent whose use was supported by a double-blind RCT.9 Augmentation trials of a wide range of medications and supplements—including amantadine, bupropion, buspirone, granisetron, mirtazapine, olanzapine, ephedrine, ginkgo biloba, and yohimbine—yielded mixed results. Indeed, the research found that some were no better than placebo.
PDE-5 inhibitors for women? Inconclusive studies to date
Female sexual dysfunction is generally divided into 4 domains: disorders of desire, arousal, orgasm, or pain. Decreased desire and delayed or absent orgasm are the most common sexual side effects of SRI antidepressants in women.10 Several studies of PDE-5 inhibitors in this patient population have had positive results,11-15 so there has been good reason to think that they might help this subset of women. However, all the studies were small and nonblinded, and therefore inconclusive—until now.
STUDY SUMMARY: Finally, a well-done RCT provides some answers
Investigators enrolled 98 premenopausal women from 7 US research centers in a double-blind randomized trial. To qualify, participants had to be diagnosed with major depression in remission, be taking a selective or nonselective SRI for >8 weeks, and be on a stable dose for >4 weeks. They also had to meet Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for substance-induced sexual dysfunction lasting >4 weeks, but have no history of sexual impairment independent of antidepressants. Finally, participants had to engage in some form of regular sexual activity—intercourse, oral sex, and masturbation all qualified—at least twice a month, and be willing to continue efforts to have sex at least once a week during the study. Women with other medical, psychiatric, or sexual problems were excluded, as were those who were pregnant, breastfeeding, or able to become pregnant and not using reliable contraception.
Participants were randomized to receive 50 mg of sildenafil (n=49) or a matching placebo tablet (n=49), which they were instructed to take 1 to 2 hours before sexual activity. The dose could be adjusted to 2 tablets (100 mg sildenafil) based on investigator assessment of the patient’s response to the initial dose. Participants and all study personnel were blinded to group assignment.
The primary outcome was change from baseline to end-point in the Clinical Global Impression Scale, a clinician-rated scale based on review of patient symptoms that was adapted to evaluate sexual function. Secondary outcomes were changes in 3 other sexual function scales, the Hamilton Rating Scale for Depression, and measured hormone levels.
Investigators followed the women for 8 weeks, measuring outcomes at 2, 4, and 8 weeks.
Sildenafil is better than placebo
Using an intention-to-treat analysis with the last measurement (2, 4, or 8 weeks) as the end-point, both the treatment and placebo groups experienced improvement in sexual function. The sildenafil group improved more than the placebo group. On the Clinical Global Impression Scale (1 to 7, with higher scores indicating worse sexual function), sildenafil users went from a mean of 4.8 to 2.8, while placebo users went from a mean of 4.7 to 3.6. The difference in mean change from baseline was 0.8 (95% confidence interval [CI] 0.6-1.0; P=.001). Using a more conservative analysis in which participants who did not return for the 8-week follow-up visit were assumed to have returned to baseline, the difference in mean change from baseline was smaller (0.6, 95% CI, 0.3-0.8; P=0.03) but still statistically significant.
Orgasmic function shows significant improvement
The sexual function scales used as secondary outcomes provided more detail about which types of sexual dysfunction benefited from sildenafil. On all 3 scales, orgasmic function significantly favored sildenafil over placebo. In the domains of desire, arousal, and pain disorders, small to moderate improvements were seen in both groups, with no statistically significant differences. One potential confounder—a difference in the course of participants’ underlying depression— was ruled out because depression scale results remained unchanged from baseline to endpoint in both groups.
Baseline levels of cortisone, estradiol, follicle-stimulating hormone, leuteinizing hormone, progesterone, prolactin, sex hormone-binding globulin, testosterone, thyroid-stimulating hormone, and thyroxine, were normal, with no differences between the sildenafil and placebo groups.
WHAT’S NEW: Women have an evidence-based option
Like their male counterparts, we can now offer women whose depression is effectively treated by SRI antidepressants—and who are motivated to stay sexually active despite medication-associated side effects—an effective pharmacotherapeutic treatment.
CAVEATS: Side effects and study funding are worth noting
Side effects. Significantly more participants in the sildenafil group vs the placebo group experienced the following side effects: headache (43% vs 27%), visual disturbance (14% vs 2%), dyspepsia (12% vs 0%), flushing (24% vs 0%), nasal congestion (37% vs 6%), and palpitations (8% vs 2%). Nausea was the only side effect that was more common in the placebo group, reported by only 2% of those in the intervention group but 16% of those on placebo.
No serious adverse events occurred, however, and the medication appears to have been well tolerated overall, despite relatively high rates of side effects. Participants in the intervention group used an average of 5 doses of sildenafil per 2-week interval, the same number as those in the placebo group.
Small treatment effect. The difference in response between sildenafil and placebo was not large: 0.8 points on a 7-point scale. But this difference is likely a clinically meaningful effect to the women with this problem.
Drug company funding. Pfizer, the maker of Viagra, funded this study through an investigator-initiated grant. Some researchers argue that female sexual dysfunction has been defined, or even invented, by drug companies seeking to create new markets for their products.16 This concern, coupled with the fact that this is the only double-blind randomized trial to show that sildenafil benefits women with antidepressant-associated sexual impairment, raises the question of whether this finding will be replicated in future trials.
We were reassured by the authors’ statement that Pfizer had no role in the study design, implementation, analysis, or manuscript preparation. And we know from clinical practice that women do suffer from SRI-induced sexual side effects, and sometimes stop taking much-needed antidepressants because the medication interferes with their ability to have a satisfying sex life. We believe this study was well done and offers a promising new therapy that deserves consideration. We hope that additional trials will follow and that investigators and journals will not hesitate to publish negative results.
Not for all women with sexual dysfunction
It’s a safe bet that these findings will be used to market sildenafil to women. It is therefore important for physicians and patients to keep in mind that this trial focused on a well-defined subset of women with sexual dysfunction: those on a stable dose of an SRI, with depression in remission, who were otherwise healthy and not pregnant, breastfeeding, or planning pregnancy, and who were motivated to be sexually active. Although this study does support the use of sildenafil for women in this subset, it does not support the use of PDE-5 inhibitors such as sildenafil for all women with sexual difficulties.
CHALLENGES TO IMPLEMENTATION: You have to ask!
Studies have repeatedly found that many women who experience sexual problems do not broach the subject with their doctors.17 So don’t wait for your female patients to bring it up. Sexual side effects are common enough with SRI antidepressants that all prescribers should mention the possibility in advance. Tell patients to let you know if they develop medication-related sexual dysfunction, and reassure them that there are treatments that can help.
Acknowledgment
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Tell women for whom you prescribe selective and nonselective serotonin reuptake inhibitors (SRIs) to let you know if they develop sexual dysfunction. Offer sildenafil (50 mg with the option to increase to 100 mg) to premenopausal women on stable, effective doses of SRIs who experience this common—and treatable—side effect.1
Strength of recommendation
B: One high-quality RCT that confirms smaller, open-label studies
Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
ILLUSTRATIVE CASE
A 34-year-old woman comes to your office and asks to be taken off the paroxetine you prescribed for her 4 months ago. The medication is working well; her depression has been in remission for at least 12 weeks. But she no longer enjoys sex. She used to have a healthy libido and satisfying arousal and orgasm, but since starting the antidepressant, her sexual interest and pleasure have been low.
Although she’s afraid of sinking back into a depression without the medication, she’s willing to take the risk. If she were your patient, what alternatives would you suggest?
Sexual dysfunction affects an estimated 30% to 50% of patients on selective and nonselective SRIs, and some studies report rates as high as 70% to 80%.2 Many patients stop taking these antidepressants prematurely, often because of sexual side effects.3,4
Phosphodiesterase type 5 (PDE-5) inhibitors are well established as an effective treatment for erectile dysfunction,5 and randomized controlled trials (RCTs) have shown sildenafil to be effective in treating male SRI-induced sexual impairment.6,7 For women, there has been no parallel evidence-based treatment.
Limited options, with little support
Typically, women who reported antidepressant-associated sexual disturbances have been offered options for which there was only weak evidence—dose changes or augmentation with another agent, switching to another antidepressant, or taking occasional drug holidays. A 2004 Cochrane review found that there were no RCTs involving dose changes or drug holidays.8 Among studies of the efficacy of switching to a different drug, nefazodone was the only agent whose use was supported by a double-blind RCT.9 Augmentation trials of a wide range of medications and supplements—including amantadine, bupropion, buspirone, granisetron, mirtazapine, olanzapine, ephedrine, ginkgo biloba, and yohimbine—yielded mixed results. Indeed, the research found that some were no better than placebo.
PDE-5 inhibitors for women? Inconclusive studies to date
Female sexual dysfunction is generally divided into 4 domains: disorders of desire, arousal, orgasm, or pain. Decreased desire and delayed or absent orgasm are the most common sexual side effects of SRI antidepressants in women.10 Several studies of PDE-5 inhibitors in this patient population have had positive results,11-15 so there has been good reason to think that they might help this subset of women. However, all the studies were small and nonblinded, and therefore inconclusive—until now.
STUDY SUMMARY: Finally, a well-done RCT provides some answers
Investigators enrolled 98 premenopausal women from 7 US research centers in a double-blind randomized trial. To qualify, participants had to be diagnosed with major depression in remission, be taking a selective or nonselective SRI for >8 weeks, and be on a stable dose for >4 weeks. They also had to meet Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for substance-induced sexual dysfunction lasting >4 weeks, but have no history of sexual impairment independent of antidepressants. Finally, participants had to engage in some form of regular sexual activity—intercourse, oral sex, and masturbation all qualified—at least twice a month, and be willing to continue efforts to have sex at least once a week during the study. Women with other medical, psychiatric, or sexual problems were excluded, as were those who were pregnant, breastfeeding, or able to become pregnant and not using reliable contraception.
Participants were randomized to receive 50 mg of sildenafil (n=49) or a matching placebo tablet (n=49), which they were instructed to take 1 to 2 hours before sexual activity. The dose could be adjusted to 2 tablets (100 mg sildenafil) based on investigator assessment of the patient’s response to the initial dose. Participants and all study personnel were blinded to group assignment.
The primary outcome was change from baseline to end-point in the Clinical Global Impression Scale, a clinician-rated scale based on review of patient symptoms that was adapted to evaluate sexual function. Secondary outcomes were changes in 3 other sexual function scales, the Hamilton Rating Scale for Depression, and measured hormone levels.
Investigators followed the women for 8 weeks, measuring outcomes at 2, 4, and 8 weeks.
Sildenafil is better than placebo
Using an intention-to-treat analysis with the last measurement (2, 4, or 8 weeks) as the end-point, both the treatment and placebo groups experienced improvement in sexual function. The sildenafil group improved more than the placebo group. On the Clinical Global Impression Scale (1 to 7, with higher scores indicating worse sexual function), sildenafil users went from a mean of 4.8 to 2.8, while placebo users went from a mean of 4.7 to 3.6. The difference in mean change from baseline was 0.8 (95% confidence interval [CI] 0.6-1.0; P=.001). Using a more conservative analysis in which participants who did not return for the 8-week follow-up visit were assumed to have returned to baseline, the difference in mean change from baseline was smaller (0.6, 95% CI, 0.3-0.8; P=0.03) but still statistically significant.
Orgasmic function shows significant improvement
The sexual function scales used as secondary outcomes provided more detail about which types of sexual dysfunction benefited from sildenafil. On all 3 scales, orgasmic function significantly favored sildenafil over placebo. In the domains of desire, arousal, and pain disorders, small to moderate improvements were seen in both groups, with no statistically significant differences. One potential confounder—a difference in the course of participants’ underlying depression— was ruled out because depression scale results remained unchanged from baseline to endpoint in both groups.
Baseline levels of cortisone, estradiol, follicle-stimulating hormone, leuteinizing hormone, progesterone, prolactin, sex hormone-binding globulin, testosterone, thyroid-stimulating hormone, and thyroxine, were normal, with no differences between the sildenafil and placebo groups.
WHAT’S NEW: Women have an evidence-based option
Like their male counterparts, we can now offer women whose depression is effectively treated by SRI antidepressants—and who are motivated to stay sexually active despite medication-associated side effects—an effective pharmacotherapeutic treatment.
CAVEATS: Side effects and study funding are worth noting
Side effects. Significantly more participants in the sildenafil group vs the placebo group experienced the following side effects: headache (43% vs 27%), visual disturbance (14% vs 2%), dyspepsia (12% vs 0%), flushing (24% vs 0%), nasal congestion (37% vs 6%), and palpitations (8% vs 2%). Nausea was the only side effect that was more common in the placebo group, reported by only 2% of those in the intervention group but 16% of those on placebo.
No serious adverse events occurred, however, and the medication appears to have been well tolerated overall, despite relatively high rates of side effects. Participants in the intervention group used an average of 5 doses of sildenafil per 2-week interval, the same number as those in the placebo group.
Small treatment effect. The difference in response between sildenafil and placebo was not large: 0.8 points on a 7-point scale. But this difference is likely a clinically meaningful effect to the women with this problem.
Drug company funding. Pfizer, the maker of Viagra, funded this study through an investigator-initiated grant. Some researchers argue that female sexual dysfunction has been defined, or even invented, by drug companies seeking to create new markets for their products.16 This concern, coupled with the fact that this is the only double-blind randomized trial to show that sildenafil benefits women with antidepressant-associated sexual impairment, raises the question of whether this finding will be replicated in future trials.
We were reassured by the authors’ statement that Pfizer had no role in the study design, implementation, analysis, or manuscript preparation. And we know from clinical practice that women do suffer from SRI-induced sexual side effects, and sometimes stop taking much-needed antidepressants because the medication interferes with their ability to have a satisfying sex life. We believe this study was well done and offers a promising new therapy that deserves consideration. We hope that additional trials will follow and that investigators and journals will not hesitate to publish negative results.
Not for all women with sexual dysfunction
It’s a safe bet that these findings will be used to market sildenafil to women. It is therefore important for physicians and patients to keep in mind that this trial focused on a well-defined subset of women with sexual dysfunction: those on a stable dose of an SRI, with depression in remission, who were otherwise healthy and not pregnant, breastfeeding, or planning pregnancy, and who were motivated to be sexually active. Although this study does support the use of sildenafil for women in this subset, it does not support the use of PDE-5 inhibitors such as sildenafil for all women with sexual difficulties.
CHALLENGES TO IMPLEMENTATION: You have to ask!
Studies have repeatedly found that many women who experience sexual problems do not broach the subject with their doctors.17 So don’t wait for your female patients to bring it up. Sexual side effects are common enough with SRI antidepressants that all prescribers should mention the possibility in advance. Tell patients to let you know if they develop medication-related sexual dysfunction, and reassure them that there are treatments that can help.
Acknowledgment
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. Spanish working group for the study of psychotropic-related sexual dysfunction. J Clin Psychiatry. 2001;62 (suppl 3):S10-S21.
3. Mitchell AJ. Depressed patients and treatment adherence. Lancet. 2006;367:2041-2043.
4. Mitchell AJ, Selmes T. Why don’t patients take their medicine? reasons and solutions in psychiatry. Adv Psychiatr Treat. 2007;13:336-346.
5. Fazio L, Brock G. Erectile dysfunction: Management update. CMAJ. 2004;170:1429-1437.
6. Nurnberg HG, Gelenberg A, Hargreave TB, Harrison WM, Siegel RL, Smith MD. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry. 2001;158:1926-1928.
7. Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S. Treatment of antidepressant-associated sexual dysfunction with sildenafil: A randomized controlled trial. JAMA. 2003;289:56-64.
8. Rudkin L, Taylor MJ, Hawton K. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2004;18(4):CD003382.
9. Ferguson JM, Shrivastava RK, Stahl SM, et al. Reemergence of sexual dysfunction in patients with major depressive disorder: Double-blind comparison of nefazodone and sertraline. J Clin Psychiatry. 2001;62:24-29.
10. Frank JE, Mistretta P, Will J. Diagnosis and treatment of female sexual dysfunction. Am Fam Physician. 2008;77:635-642.
11. Nurnberg HG, Hensley PL, Lauriello J, Parker LM, Keith SJ. Sildenafil for women patients with antidepressant-induced sexual dysfunction. Psychiatr Serv. 1999;50:1076-1078.
12. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for sexual dysfunction in women taking antidepressants. Am J Psychiatry. 1999;156:1664.
13. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for iatrogenic serotonergic antidepressant medication-induced sexual dysfunction in 4 patients. J Clin Psychiatry. 1999;60:33-35.
14. Ashton AK. Vardenafil reversal of female anorgasmia. Am J Psychiatry. 2004;161:2133.
15. Ashton AK, Weinstein W. Tadalafil reversal of sexual dysfunction caused by serotonin enhancing medications in women. J Sex Marital Ther. 2006;32:1-3.
16. Moynihan R. The making of a disease: Female sexual dysfunction. BMJ. 2003 Jan 4;326:45-47.
17. Rosenberg KP, Bleiberg KL, Koscis J, Gross C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: Impact on compliance. J Sex Marital Ther. 2003;29:289-296.
1. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. Spanish working group for the study of psychotropic-related sexual dysfunction. J Clin Psychiatry. 2001;62 (suppl 3):S10-S21.
3. Mitchell AJ. Depressed patients and treatment adherence. Lancet. 2006;367:2041-2043.
4. Mitchell AJ, Selmes T. Why don’t patients take their medicine? reasons and solutions in psychiatry. Adv Psychiatr Treat. 2007;13:336-346.
5. Fazio L, Brock G. Erectile dysfunction: Management update. CMAJ. 2004;170:1429-1437.
6. Nurnberg HG, Gelenberg A, Hargreave TB, Harrison WM, Siegel RL, Smith MD. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry. 2001;158:1926-1928.
7. Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S. Treatment of antidepressant-associated sexual dysfunction with sildenafil: A randomized controlled trial. JAMA. 2003;289:56-64.
8. Rudkin L, Taylor MJ, Hawton K. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2004;18(4):CD003382.
9. Ferguson JM, Shrivastava RK, Stahl SM, et al. Reemergence of sexual dysfunction in patients with major depressive disorder: Double-blind comparison of nefazodone and sertraline. J Clin Psychiatry. 2001;62:24-29.
10. Frank JE, Mistretta P, Will J. Diagnosis and treatment of female sexual dysfunction. Am Fam Physician. 2008;77:635-642.
11. Nurnberg HG, Hensley PL, Lauriello J, Parker LM, Keith SJ. Sildenafil for women patients with antidepressant-induced sexual dysfunction. Psychiatr Serv. 1999;50:1076-1078.
12. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for sexual dysfunction in women taking antidepressants. Am J Psychiatry. 1999;156:1664.
13. Nurnberg HG, Lauriello J, Hensley PL, Parker LM, Keith SJ. Sildenafil for iatrogenic serotonergic antidepressant medication-induced sexual dysfunction in 4 patients. J Clin Psychiatry. 1999;60:33-35.
14. Ashton AK. Vardenafil reversal of female anorgasmia. Am J Psychiatry. 2004;161:2133.
15. Ashton AK, Weinstein W. Tadalafil reversal of sexual dysfunction caused by serotonin enhancing medications in women. J Sex Marital Ther. 2006;32:1-3.
16. Moynihan R. The making of a disease: Female sexual dysfunction. BMJ. 2003 Jan 4;326:45-47.
17. Rosenberg KP, Bleiberg KL, Koscis J, Gross C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: Impact on compliance. J Sex Marital Ther. 2003;29:289-296.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Dust mite control measures don’t help asthma patients
ILLUSTRATIVE CASE
The parents of a 10-year-old patient whom you recently diagnosed with asthma want to do everything they can to reduce his asthma symptoms. They are considering buying hypoallergenic mattress covers and an expensive air filtration system to decrease the levels of dust mite allergens in their home and want to know if you think that will help their son. What do you tell them?
We want to do everything we can to help our patients control their asthma symptoms, but when it comes to household dust mite control measures, this extensive Cochrane review confirms that interventions like mattress covers and air filtration don’t work, despite recent reviews and guidelines recommending them.
Dust mites (Dermatophagoides pteronyssinus) are one of the most common allergens that provoke asthma symptoms in children and adults.2 Dust mites live in warm, humid places and feed on human skin scales. The areas with the highest levels of household infestation are carpets, mattresses, pillows, drapes, upholstered furniture, and clothing.
Guidelines still encourage mattress cover use
The National Asthma Education and Prevention Program (NAEPP) 2007 guidelines recommend using allergen-impermeable mattress and pillow covers and washing sheets and blankets in hot water. They also recommend “considering” reducing indoor humidity, removing bedroom carpets, and washing stuffed toys weekly. The NAEPP Expert Panel cites many studies to support these recommendations.3
The National Environmental Education and Training Foundation (NEETF) 2005 guidelines recommend additional measures to reduce dust mite exposure including vacuuming using a high-efficiency particulate air (HEPA) filter, removing draperies, and considering using a portable air cleaner with a HEPA filter.4
STUDY SUMMARY: 54 trials, but no support for dust mite measures
This Cochrane systematic review included 54 randomized trials that assessed the effects of physical and/or chemical interventions to reduce exposure to house dust mite antigens in the homes of patients with mite-sensitive asthma. These studies included a total of 3002 pediatric and adult asthma patients (9 - 628 patients analyzed per trial) with mite sensitization confirmed by skin testing or IgE serum assays.
Thirty-six studies tested physical interventions, including mattress covers, vacuum cleaning, heating, ventilation, freezing, washing, air filtration, and ionizers. Ten used chemical interventions to kill dust mites; 8 used a combination of physical and chemical methods. Control groups received either placebo or no treatment.
Outcomes studied. The authors extracted data for the following outcomes: subjective well-being, asthma symptom scores, use of medication, days of sick leave from school or work, number of unscheduled visits to a physician or hospital, forced expiratory volume in 1 second (FEV1), peak expiratory flow rate (PEFR), and provocative concentration that causes a 20% fall in FEV1 (PC20). Length of the intervention and follow-up ranged from 2 weeks to 2 years.
Quality of studies. According to modern standards for randomized trials, the quality of many of the 54 studies was not optimal, especially in the descriptions of randomization and the reporting of outcomes. The method of randomization and concealment of allocation was rarely described. Eleven trial reports did not contain any usable data for the meta-analysis because of the way data were reported, and there was significant potential for reporting bias in favor of a treatment effect in the studies included. Mite reduction was successful in 17 trials, unsuccessful in 24 trials, and not reported in 13 trials.
Interventions didn’t help. There were no differences between the intervention and control groups for any of the outcomes. The percentage of patients who improved after the experimental interventions was not significantly different from the percentage of patients in the control groups (relative risk [RR]=1.01; 95% confidence interval [CI], 0.80-1.27; data based on 7 trials). There was no difference in medication usage (data from 10 trials), FEV1 (data from 14 trials), morning PEFR (data from 23 trials), or PC 20 (data from 14 trials) between the intervention and control groups ( TABLE ).1
TABLE
Dust mite control measures didn’t improve these outcomes
| OUTCOME | STANDARDIZED MEAN DIFFERENCE* (95% CI) |
|---|---|
| Medication usage | -0.06 (-0.18 to 0.07) |
| FEV1 | 0.11 (-0.05 to 0.28) |
| Morning PEFR | 0.00 (-1.0 to 0.10) |
| PC 20 | 0.05 (-0.13 to 0.22) |
| CI, confidence interval; FEV1, forced expiratory volume in 1 second; PC20, provocative concentration that causes a 20% fall in FEV1; PEFR, peak expiratory flow rate. | |
| *Standardized mean difference is a common way to combine results of different studies for comparison purposes. If the 95% CI crosses 0, there is no effect of the intervention compared with the control. | |
WHAT’S NEW?: Nothing is new, yet this will be “news” to many
This Cochrane review includes 5 additional trials that have been conducted since the last Cochrane review of this topic in 2004. However, the 2004 review reported the same conclusion—that interventions to reduce house dust mite exposure in asthma patients are ineffective—as did 3 other Cochrane reviews on the same topic beginning in 1998.5-8
So why are the guidelines out of step? Schmidt and Gøtzsche (one of the authors of the Cochrane review) conducted a systematic review of narrative review articles in 2005 to answer this question. They found 70 review articles, 90% of which recommended physical methods to reduce exposure to house dust mites. They discovered that although these review articles included references to support their recommendations of dust mite control measures, the reviews showed significant bias in favor of positive studies and highlighted the results of low-quality studies, including non-randomized studies that had been excluded from the Cochrane reviews.9
CAVEATS: Duration of studies not long enough?
We know that extreme measures to reduce exposure to dust mite allergen, such as relocating to a high altitude or prolonged hospitalization, can reduce asthma symptoms,10,11 but these are clearly not practical solutions for most patients with dust mite-sensitive asthma. When it comes to this Cochrane review, some might argue that many of the interventions included were not of sufficient duration and did not sufficiently reduce the level of house mite allergen to improve asthma symptoms.
However, the subgroups of trials with long treatment duration (1-2 years) and successful mite reduction (determined by different methods, including mite counts and measured antigen levels in dust samples) also failed to show a significant difference between intervention and control groups.1
Tweak the approach? Most dust mite-sensitive asthma patients are sensitive to other allergens, so perhaps multifaceted interventions that target multiple allergens would be more effective.12 But until these potential interventions are supported by stronger evidence, we should not recommend them to our patients.
CHALLENGES TO IMPLEMENTATION: Swimming against the tide is never easy
Although the evidence to date indicates that interventions to reduce home dust mite exposure are ineffective, there are hundreds of products—including mattress and pillow covers ($10-$100), ionizers ($100-$200), and air filtration systems ($500-$800)—that are being marketed to patients with asthma. In addition, patient education handouts from sources such as the American Academy of Family Physicians, the American Academy of Pediatrics, and UpToDate recommend implementing dust mite control measures to reduce dust mite allergen exposure.13-15
We need to start educating our asthma patients properly so they can spend their time, energy, and money on interventions, such as medications, that work—and not on interventions that make no difference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Gotzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;(2):CD001187.-
2. German JA, Harper MB. Environmental control of allergic diseases. Am Fam Physician. 2002;66:421-426.
3. National Asthma Education and Prevention Program (NAEPP). Control of environmental factors and comorbid conditions that affect asthma. In: Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007.
4. National Environmental Education & Training Foundation (NEETF). Environmental management of pediatric asthma. Guidelines for health care providers. Washington, DC: National Environmental Education & Training Foundation (NEETF); 2005.
5. Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ. 1998;317:1105-1110.
6. Hammarquist C, Burr ML, Gotzsche PC. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2000;(2):CD001187.-
7. Gøtzsche PC, Johansen HK, Burr ML, Hammarquist C. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2001;(3):CD001187.-
8. Gøtzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2004;(4):CD001187.-
9. Schmidt LM, Gøtzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract. 2005;54:334-338.
10. Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet 1982;2:675-678.
11. Grootendorst DC, Dahlen SE. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. 2001;31:400-408.
12. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068-1080.
13. American Academy of Family Physicians. Dust mites in the home [patient handout]. Available at: http://familydoctor.org/online/famdocen/home/common/asthma/triggers/683.html. Accessed October 23, 2008.
14. American Academy of Pediatrics. Non-pharmacologic approaches to asthma management [patient handout]. Available at: http://www.aap.org/sections/allergy/nonrxchild.pdf. Accessed October 23, 2008.
15. Bailey W. Patient information: Trigger avoidance in asthma. UpToDate [online database]. Version 16.2. Waltham, Mass: UpToDate; 2008.
ILLUSTRATIVE CASE
The parents of a 10-year-old patient whom you recently diagnosed with asthma want to do everything they can to reduce his asthma symptoms. They are considering buying hypoallergenic mattress covers and an expensive air filtration system to decrease the levels of dust mite allergens in their home and want to know if you think that will help their son. What do you tell them?
We want to do everything we can to help our patients control their asthma symptoms, but when it comes to household dust mite control measures, this extensive Cochrane review confirms that interventions like mattress covers and air filtration don’t work, despite recent reviews and guidelines recommending them.
Dust mites (Dermatophagoides pteronyssinus) are one of the most common allergens that provoke asthma symptoms in children and adults.2 Dust mites live in warm, humid places and feed on human skin scales. The areas with the highest levels of household infestation are carpets, mattresses, pillows, drapes, upholstered furniture, and clothing.
Guidelines still encourage mattress cover use
The National Asthma Education and Prevention Program (NAEPP) 2007 guidelines recommend using allergen-impermeable mattress and pillow covers and washing sheets and blankets in hot water. They also recommend “considering” reducing indoor humidity, removing bedroom carpets, and washing stuffed toys weekly. The NAEPP Expert Panel cites many studies to support these recommendations.3
The National Environmental Education and Training Foundation (NEETF) 2005 guidelines recommend additional measures to reduce dust mite exposure including vacuuming using a high-efficiency particulate air (HEPA) filter, removing draperies, and considering using a portable air cleaner with a HEPA filter.4
STUDY SUMMARY: 54 trials, but no support for dust mite measures
This Cochrane systematic review included 54 randomized trials that assessed the effects of physical and/or chemical interventions to reduce exposure to house dust mite antigens in the homes of patients with mite-sensitive asthma. These studies included a total of 3002 pediatric and adult asthma patients (9 - 628 patients analyzed per trial) with mite sensitization confirmed by skin testing or IgE serum assays.
Thirty-six studies tested physical interventions, including mattress covers, vacuum cleaning, heating, ventilation, freezing, washing, air filtration, and ionizers. Ten used chemical interventions to kill dust mites; 8 used a combination of physical and chemical methods. Control groups received either placebo or no treatment.
Outcomes studied. The authors extracted data for the following outcomes: subjective well-being, asthma symptom scores, use of medication, days of sick leave from school or work, number of unscheduled visits to a physician or hospital, forced expiratory volume in 1 second (FEV1), peak expiratory flow rate (PEFR), and provocative concentration that causes a 20% fall in FEV1 (PC20). Length of the intervention and follow-up ranged from 2 weeks to 2 years.
Quality of studies. According to modern standards for randomized trials, the quality of many of the 54 studies was not optimal, especially in the descriptions of randomization and the reporting of outcomes. The method of randomization and concealment of allocation was rarely described. Eleven trial reports did not contain any usable data for the meta-analysis because of the way data were reported, and there was significant potential for reporting bias in favor of a treatment effect in the studies included. Mite reduction was successful in 17 trials, unsuccessful in 24 trials, and not reported in 13 trials.
Interventions didn’t help. There were no differences between the intervention and control groups for any of the outcomes. The percentage of patients who improved after the experimental interventions was not significantly different from the percentage of patients in the control groups (relative risk [RR]=1.01; 95% confidence interval [CI], 0.80-1.27; data based on 7 trials). There was no difference in medication usage (data from 10 trials), FEV1 (data from 14 trials), morning PEFR (data from 23 trials), or PC 20 (data from 14 trials) between the intervention and control groups ( TABLE ).1
TABLE
Dust mite control measures didn’t improve these outcomes
| OUTCOME | STANDARDIZED MEAN DIFFERENCE* (95% CI) |
|---|---|
| Medication usage | -0.06 (-0.18 to 0.07) |
| FEV1 | 0.11 (-0.05 to 0.28) |
| Morning PEFR | 0.00 (-1.0 to 0.10) |
| PC 20 | 0.05 (-0.13 to 0.22) |
| CI, confidence interval; FEV1, forced expiratory volume in 1 second; PC20, provocative concentration that causes a 20% fall in FEV1; PEFR, peak expiratory flow rate. | |
| *Standardized mean difference is a common way to combine results of different studies for comparison purposes. If the 95% CI crosses 0, there is no effect of the intervention compared with the control. | |
WHAT’S NEW?: Nothing is new, yet this will be “news” to many
This Cochrane review includes 5 additional trials that have been conducted since the last Cochrane review of this topic in 2004. However, the 2004 review reported the same conclusion—that interventions to reduce house dust mite exposure in asthma patients are ineffective—as did 3 other Cochrane reviews on the same topic beginning in 1998.5-8
So why are the guidelines out of step? Schmidt and Gøtzsche (one of the authors of the Cochrane review) conducted a systematic review of narrative review articles in 2005 to answer this question. They found 70 review articles, 90% of which recommended physical methods to reduce exposure to house dust mites. They discovered that although these review articles included references to support their recommendations of dust mite control measures, the reviews showed significant bias in favor of positive studies and highlighted the results of low-quality studies, including non-randomized studies that had been excluded from the Cochrane reviews.9
CAVEATS: Duration of studies not long enough?
We know that extreme measures to reduce exposure to dust mite allergen, such as relocating to a high altitude or prolonged hospitalization, can reduce asthma symptoms,10,11 but these are clearly not practical solutions for most patients with dust mite-sensitive asthma. When it comes to this Cochrane review, some might argue that many of the interventions included were not of sufficient duration and did not sufficiently reduce the level of house mite allergen to improve asthma symptoms.
However, the subgroups of trials with long treatment duration (1-2 years) and successful mite reduction (determined by different methods, including mite counts and measured antigen levels in dust samples) also failed to show a significant difference between intervention and control groups.1
Tweak the approach? Most dust mite-sensitive asthma patients are sensitive to other allergens, so perhaps multifaceted interventions that target multiple allergens would be more effective.12 But until these potential interventions are supported by stronger evidence, we should not recommend them to our patients.
CHALLENGES TO IMPLEMENTATION: Swimming against the tide is never easy
Although the evidence to date indicates that interventions to reduce home dust mite exposure are ineffective, there are hundreds of products—including mattress and pillow covers ($10-$100), ionizers ($100-$200), and air filtration systems ($500-$800)—that are being marketed to patients with asthma. In addition, patient education handouts from sources such as the American Academy of Family Physicians, the American Academy of Pediatrics, and UpToDate recommend implementing dust mite control measures to reduce dust mite allergen exposure.13-15
We need to start educating our asthma patients properly so they can spend their time, energy, and money on interventions, such as medications, that work—and not on interventions that make no difference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
ILLUSTRATIVE CASE
The parents of a 10-year-old patient whom you recently diagnosed with asthma want to do everything they can to reduce his asthma symptoms. They are considering buying hypoallergenic mattress covers and an expensive air filtration system to decrease the levels of dust mite allergens in their home and want to know if you think that will help their son. What do you tell them?
We want to do everything we can to help our patients control their asthma symptoms, but when it comes to household dust mite control measures, this extensive Cochrane review confirms that interventions like mattress covers and air filtration don’t work, despite recent reviews and guidelines recommending them.
Dust mites (Dermatophagoides pteronyssinus) are one of the most common allergens that provoke asthma symptoms in children and adults.2 Dust mites live in warm, humid places and feed on human skin scales. The areas with the highest levels of household infestation are carpets, mattresses, pillows, drapes, upholstered furniture, and clothing.
Guidelines still encourage mattress cover use
The National Asthma Education and Prevention Program (NAEPP) 2007 guidelines recommend using allergen-impermeable mattress and pillow covers and washing sheets and blankets in hot water. They also recommend “considering” reducing indoor humidity, removing bedroom carpets, and washing stuffed toys weekly. The NAEPP Expert Panel cites many studies to support these recommendations.3
The National Environmental Education and Training Foundation (NEETF) 2005 guidelines recommend additional measures to reduce dust mite exposure including vacuuming using a high-efficiency particulate air (HEPA) filter, removing draperies, and considering using a portable air cleaner with a HEPA filter.4
STUDY SUMMARY: 54 trials, but no support for dust mite measures
This Cochrane systematic review included 54 randomized trials that assessed the effects of physical and/or chemical interventions to reduce exposure to house dust mite antigens in the homes of patients with mite-sensitive asthma. These studies included a total of 3002 pediatric and adult asthma patients (9 - 628 patients analyzed per trial) with mite sensitization confirmed by skin testing or IgE serum assays.
Thirty-six studies tested physical interventions, including mattress covers, vacuum cleaning, heating, ventilation, freezing, washing, air filtration, and ionizers. Ten used chemical interventions to kill dust mites; 8 used a combination of physical and chemical methods. Control groups received either placebo or no treatment.
Outcomes studied. The authors extracted data for the following outcomes: subjective well-being, asthma symptom scores, use of medication, days of sick leave from school or work, number of unscheduled visits to a physician or hospital, forced expiratory volume in 1 second (FEV1), peak expiratory flow rate (PEFR), and provocative concentration that causes a 20% fall in FEV1 (PC20). Length of the intervention and follow-up ranged from 2 weeks to 2 years.
Quality of studies. According to modern standards for randomized trials, the quality of many of the 54 studies was not optimal, especially in the descriptions of randomization and the reporting of outcomes. The method of randomization and concealment of allocation was rarely described. Eleven trial reports did not contain any usable data for the meta-analysis because of the way data were reported, and there was significant potential for reporting bias in favor of a treatment effect in the studies included. Mite reduction was successful in 17 trials, unsuccessful in 24 trials, and not reported in 13 trials.
Interventions didn’t help. There were no differences between the intervention and control groups for any of the outcomes. The percentage of patients who improved after the experimental interventions was not significantly different from the percentage of patients in the control groups (relative risk [RR]=1.01; 95% confidence interval [CI], 0.80-1.27; data based on 7 trials). There was no difference in medication usage (data from 10 trials), FEV1 (data from 14 trials), morning PEFR (data from 23 trials), or PC 20 (data from 14 trials) between the intervention and control groups ( TABLE ).1
TABLE
Dust mite control measures didn’t improve these outcomes
| OUTCOME | STANDARDIZED MEAN DIFFERENCE* (95% CI) |
|---|---|
| Medication usage | -0.06 (-0.18 to 0.07) |
| FEV1 | 0.11 (-0.05 to 0.28) |
| Morning PEFR | 0.00 (-1.0 to 0.10) |
| PC 20 | 0.05 (-0.13 to 0.22) |
| CI, confidence interval; FEV1, forced expiratory volume in 1 second; PC20, provocative concentration that causes a 20% fall in FEV1; PEFR, peak expiratory flow rate. | |
| *Standardized mean difference is a common way to combine results of different studies for comparison purposes. If the 95% CI crosses 0, there is no effect of the intervention compared with the control. | |
WHAT’S NEW?: Nothing is new, yet this will be “news” to many
This Cochrane review includes 5 additional trials that have been conducted since the last Cochrane review of this topic in 2004. However, the 2004 review reported the same conclusion—that interventions to reduce house dust mite exposure in asthma patients are ineffective—as did 3 other Cochrane reviews on the same topic beginning in 1998.5-8
So why are the guidelines out of step? Schmidt and Gøtzsche (one of the authors of the Cochrane review) conducted a systematic review of narrative review articles in 2005 to answer this question. They found 70 review articles, 90% of which recommended physical methods to reduce exposure to house dust mites. They discovered that although these review articles included references to support their recommendations of dust mite control measures, the reviews showed significant bias in favor of positive studies and highlighted the results of low-quality studies, including non-randomized studies that had been excluded from the Cochrane reviews.9
CAVEATS: Duration of studies not long enough?
We know that extreme measures to reduce exposure to dust mite allergen, such as relocating to a high altitude or prolonged hospitalization, can reduce asthma symptoms,10,11 but these are clearly not practical solutions for most patients with dust mite-sensitive asthma. When it comes to this Cochrane review, some might argue that many of the interventions included were not of sufficient duration and did not sufficiently reduce the level of house mite allergen to improve asthma symptoms.
However, the subgroups of trials with long treatment duration (1-2 years) and successful mite reduction (determined by different methods, including mite counts and measured antigen levels in dust samples) also failed to show a significant difference between intervention and control groups.1
Tweak the approach? Most dust mite-sensitive asthma patients are sensitive to other allergens, so perhaps multifaceted interventions that target multiple allergens would be more effective.12 But until these potential interventions are supported by stronger evidence, we should not recommend them to our patients.
CHALLENGES TO IMPLEMENTATION: Swimming against the tide is never easy
Although the evidence to date indicates that interventions to reduce home dust mite exposure are ineffective, there are hundreds of products—including mattress and pillow covers ($10-$100), ionizers ($100-$200), and air filtration systems ($500-$800)—that are being marketed to patients with asthma. In addition, patient education handouts from sources such as the American Academy of Family Physicians, the American Academy of Pediatrics, and UpToDate recommend implementing dust mite control measures to reduce dust mite allergen exposure.13-15
We need to start educating our asthma patients properly so they can spend their time, energy, and money on interventions, such as medications, that work—and not on interventions that make no difference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Gotzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;(2):CD001187.-
2. German JA, Harper MB. Environmental control of allergic diseases. Am Fam Physician. 2002;66:421-426.
3. National Asthma Education and Prevention Program (NAEPP). Control of environmental factors and comorbid conditions that affect asthma. In: Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007.
4. National Environmental Education & Training Foundation (NEETF). Environmental management of pediatric asthma. Guidelines for health care providers. Washington, DC: National Environmental Education & Training Foundation (NEETF); 2005.
5. Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ. 1998;317:1105-1110.
6. Hammarquist C, Burr ML, Gotzsche PC. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2000;(2):CD001187.-
7. Gøtzsche PC, Johansen HK, Burr ML, Hammarquist C. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2001;(3):CD001187.-
8. Gøtzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2004;(4):CD001187.-
9. Schmidt LM, Gøtzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract. 2005;54:334-338.
10. Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet 1982;2:675-678.
11. Grootendorst DC, Dahlen SE. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. 2001;31:400-408.
12. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068-1080.
13. American Academy of Family Physicians. Dust mites in the home [patient handout]. Available at: http://familydoctor.org/online/famdocen/home/common/asthma/triggers/683.html. Accessed October 23, 2008.
14. American Academy of Pediatrics. Non-pharmacologic approaches to asthma management [patient handout]. Available at: http://www.aap.org/sections/allergy/nonrxchild.pdf. Accessed October 23, 2008.
15. Bailey W. Patient information: Trigger avoidance in asthma. UpToDate [online database]. Version 16.2. Waltham, Mass: UpToDate; 2008.
1. Gotzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;(2):CD001187.-
2. German JA, Harper MB. Environmental control of allergic diseases. Am Fam Physician. 2002;66:421-426.
3. National Asthma Education and Prevention Program (NAEPP). Control of environmental factors and comorbid conditions that affect asthma. In: Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007.
4. National Environmental Education & Training Foundation (NEETF). Environmental management of pediatric asthma. Guidelines for health care providers. Washington, DC: National Environmental Education & Training Foundation (NEETF); 2005.
5. Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ. 1998;317:1105-1110.
6. Hammarquist C, Burr ML, Gotzsche PC. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2000;(2):CD001187.-
7. Gøtzsche PC, Johansen HK, Burr ML, Hammarquist C. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2001;(3):CD001187.-
8. Gøtzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2004;(4):CD001187.-
9. Schmidt LM, Gøtzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract. 2005;54:334-338.
10. Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet 1982;2:675-678.
11. Grootendorst DC, Dahlen SE. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. 2001;31:400-408.
12. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068-1080.
13. American Academy of Family Physicians. Dust mites in the home [patient handout]. Available at: http://familydoctor.org/online/famdocen/home/common/asthma/triggers/683.html. Accessed October 23, 2008.
14. American Academy of Pediatrics. Non-pharmacologic approaches to asthma management [patient handout]. Available at: http://www.aap.org/sections/allergy/nonrxchild.pdf. Accessed October 23, 2008.
15. Bailey W. Patient information: Trigger avoidance in asthma. UpToDate [online database]. Version 16.2. Waltham, Mass: UpToDate; 2008.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
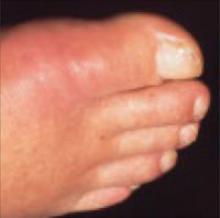
Acute gout: Oral steroids work as well as NSAIDs
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Help smokers quit: Tell them their “lung age”
ILLUSTRATIVE CASE
A 48-year-old man comes to your office for a routine physical. He has a 30 pack-year smoking history. When you talk to him about smoking cessation, he tells you he’s tried to stop more than once, but he can’t seem to stay motivated. You find no evidence of chronic lung disease and do not perform spirometry screening. (The US Preventive Services Task Force does not recommend spirometry for asymptomatic patients.) But could spirometry have therapeutic value in this case?
Smoking is the leading modifiable risk factor for mortality in the United States,2 and smoking cessation is the most effective intervention. Nortriptyline, bupropion, nicotine replacement agents, and varenicline are effective pharmacological treatments.3 Adding counseling to medication significantly improves quit rates (estimated odds ratio [OR]=1.4; 95% confidence interval [CI], 1.2-1.6).3 Nonetheless, physicians’ efforts to help patients stop smoking frequently fail.
But another option has caught—and held—the attention of researchers.
The promise of biomarkers
It has long been suspected that presenting smokers with evidence of tobacco’s harmful effect on their bodies—biomarkers—might encourage them to stop. Biomarkers that have been tested in randomized controlled trials (RCTs) include spirometry, exhaled carbon monoxide measurement, ultrasonography of carotid and femoral arteries, and genetic susceptibility to lung cancer, as well as combinations of these markers. But the results of most biomarker studies have been disappointing. A 2005 Cochrane Database review found insufficient evidence of the effectiveness of these markers in boosting quit rates.4
Lung age, a biomarker that’s easily understood
Lung age, a clever presentation of spirometry results, had not been tested in an RCT prior to the study we summarize below. Defined in 1985, lung age refers to the average age of a nonsmoker with a forced expiratory volume at 1 second (FEV1) equal to that of the person being tested ( FIGURE 1 ). The primary purpose was to make spirometry results easier for patients to understand, but researchers also envisioned it as a way to demonstrate the premature lung damage suffered as a consequence of smoking.5
FIGURE 1
Translating FEV1 into lung age1
STUDY SUMMARY: Graphic display more effective than FEV1 results
This study was a well-done, multicenter RCT evaluating the effect on tobacco quit rates of informing adult smokers of their lung age.1 Smokers ages 35 and older from 5 general practices in England were invited to participate. The authors excluded patients using oxygen and those with a history of tuberculosis, lung cancer, asbestosis, bronchiectasis, silicosis, or pneumonectomy. The study included 561 participants with an average of 33 pack-years of smoking, who underwent spirometry before being divided into an intervention or a control group. The researchers used standardized instruments to confirm the baseline comparability of the 2 groups.
Subjects in both groups were given information about local smoking cessation clinics and strongly encouraged to quit. All were told that their lung function would be retested in 12 months.
The controls received letters with their spirometry results presented as FEV1. In contrast, participants in the intervention group received the results in the form of a computer-generated graphic display of lung age ( FIGURE 2 ), which was further explained by a health care worker. They also received a letter within 1 month containing the same data. Participants were evaluated for smoking cessation at 12 months, and those who reported quitting received confirmatory carbon monoxide breath testing and salivary cotinine testing. Eleven percent of the subjects were lost to follow-up.
FIGURE 2
Lung age helps spirometry pack a bigger punch
Drawing a vertical line from the patient’s age (on the horizontal axis) to reach the solid curve representing the lung function of the “susceptible smoker” and extending the line horizontally to reach the curve with the broken lines representing “never smokers” graphically shows the patient’s lung age and the accelerated decline in lung function associated with smoking. The patient shown here is a 52-year-old smoker with FEV1 equivalent to a 75-year-old nonsmoker.
Source: Parkes G et al. BMJ. 2008;336:598-600. Reproduced with permission from the BMJ Publishing Group.
Quit rates higher when patients know lung age
At 1 year, verified quit rates were 13.6% in the intervention group and 6.4% in the control group (a difference of 7.2%, 95% CI, 2.2%-12.1%; P=.005). This means that for every 14 smokers who are told their lung age and shown a graphic display of this biomarker, 1 additional smoker will quit after 1 year.
Contrary to what might be expected, the investigators found that quitting did not depend on the degree of lung damage. Patients with both normal and abnormal lung age quit smoking at similar rates.
WHAT’S NEW: Lung age resonates more than spirometry alone
This is the first RCT demonstrating that informing smokers of their lung age can help them quit, and the first well-designed study to demonstrate improved cessation rates using a physiological biomarker. The research also suggests that successful quitting may have less to do with spirometry results—the level of severity of lung damage it shows—than with the way the results are presented. Giving patients information about their lung function in an easily understandable format, the authors observe, appears to result in higher quit rates.
CAVEATS: Young smokers weren’t studied
The study did not test to see if this intervention would work in younger adults, as only those 35 years of age and older were enrolled. This is a single study, and it is possible that the findings cannot be generalized to other groups or are due to unmeasured confounding factors. However, the intervention is unlikely to cause any significant harm, so we see no risks associated with it other than the cost of spirometry.
CHALLENGES TO IMPLEMENTATION: Time and expense of spirometry
We suspect the biggest challenges to implementing this recommendation in clinical practice are the expense of obtaining a spirometer ( TABLE ), staff training for those practices without one, and the time needed for the intervention. The average time to perform spirometry on study participants was 30 minutes; a health care worker spent, on average, another 15 minutes reviewing results with each member of the intervention group.
Another challenge: Not all spirometers calculate lung age or can create a graphic similar to FIGURE 2 . However, any FEV1 measurement, whether it is generated by formal pulmonary function testing or by an inexpensive hand-held meter, can easily be converted to lung age using the formula shown in FIGURE 1 . If desired, the same elements—the patient’s age, height, and gender as well as FEV1—could also be used to create a computer-generated graphic display.
TABLE
Spirometry: equipment costs
| The initial cost of a spirometer varies widely, depending on the sophistication of the equipment and the available options and features. Additional costs—for disposable mouthpieces, line filters, nose clips, and hoses, for example—are low. A sampling of reasonably priced models well suited for office use is shown below. All of these models meet American Thoracic Society criteria for spirometry, and all calculate lung age. | ||
|---|---|---|
| SPIROMETER MANUFACTURER/MODEL | PRICE | SUPPLIER |
| Futuremed Discovery-2 | $2,125 | medsupplier.com |
| Micro Medical MicroLoop | $1,780 | Miami-med.com |
| Micro Medical SpiroUSB | $1,580 | Miami-med.com |
| NDD EasyOne Frontline | $1,000 | medsupplier.com |
| SDI Diagnostics Spirolab II | $2,600 | med-electronics.com |
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
2. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
3. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed July 6, 2008.
4. Bize R, Burnand B, Mueller Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2005;(4):CD004705.-
5. Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985;14:655-662.
ILLUSTRATIVE CASE
A 48-year-old man comes to your office for a routine physical. He has a 30 pack-year smoking history. When you talk to him about smoking cessation, he tells you he’s tried to stop more than once, but he can’t seem to stay motivated. You find no evidence of chronic lung disease and do not perform spirometry screening. (The US Preventive Services Task Force does not recommend spirometry for asymptomatic patients.) But could spirometry have therapeutic value in this case?
Smoking is the leading modifiable risk factor for mortality in the United States,2 and smoking cessation is the most effective intervention. Nortriptyline, bupropion, nicotine replacement agents, and varenicline are effective pharmacological treatments.3 Adding counseling to medication significantly improves quit rates (estimated odds ratio [OR]=1.4; 95% confidence interval [CI], 1.2-1.6).3 Nonetheless, physicians’ efforts to help patients stop smoking frequently fail.
But another option has caught—and held—the attention of researchers.
The promise of biomarkers
It has long been suspected that presenting smokers with evidence of tobacco’s harmful effect on their bodies—biomarkers—might encourage them to stop. Biomarkers that have been tested in randomized controlled trials (RCTs) include spirometry, exhaled carbon monoxide measurement, ultrasonography of carotid and femoral arteries, and genetic susceptibility to lung cancer, as well as combinations of these markers. But the results of most biomarker studies have been disappointing. A 2005 Cochrane Database review found insufficient evidence of the effectiveness of these markers in boosting quit rates.4
Lung age, a biomarker that’s easily understood
Lung age, a clever presentation of spirometry results, had not been tested in an RCT prior to the study we summarize below. Defined in 1985, lung age refers to the average age of a nonsmoker with a forced expiratory volume at 1 second (FEV1) equal to that of the person being tested ( FIGURE 1 ). The primary purpose was to make spirometry results easier for patients to understand, but researchers also envisioned it as a way to demonstrate the premature lung damage suffered as a consequence of smoking.5
FIGURE 1
Translating FEV1 into lung age1
STUDY SUMMARY: Graphic display more effective than FEV1 results
This study was a well-done, multicenter RCT evaluating the effect on tobacco quit rates of informing adult smokers of their lung age.1 Smokers ages 35 and older from 5 general practices in England were invited to participate. The authors excluded patients using oxygen and those with a history of tuberculosis, lung cancer, asbestosis, bronchiectasis, silicosis, or pneumonectomy. The study included 561 participants with an average of 33 pack-years of smoking, who underwent spirometry before being divided into an intervention or a control group. The researchers used standardized instruments to confirm the baseline comparability of the 2 groups.
Subjects in both groups were given information about local smoking cessation clinics and strongly encouraged to quit. All were told that their lung function would be retested in 12 months.
The controls received letters with their spirometry results presented as FEV1. In contrast, participants in the intervention group received the results in the form of a computer-generated graphic display of lung age ( FIGURE 2 ), which was further explained by a health care worker. They also received a letter within 1 month containing the same data. Participants were evaluated for smoking cessation at 12 months, and those who reported quitting received confirmatory carbon monoxide breath testing and salivary cotinine testing. Eleven percent of the subjects were lost to follow-up.
FIGURE 2
Lung age helps spirometry pack a bigger punch
Drawing a vertical line from the patient’s age (on the horizontal axis) to reach the solid curve representing the lung function of the “susceptible smoker” and extending the line horizontally to reach the curve with the broken lines representing “never smokers” graphically shows the patient’s lung age and the accelerated decline in lung function associated with smoking. The patient shown here is a 52-year-old smoker with FEV1 equivalent to a 75-year-old nonsmoker.
Source: Parkes G et al. BMJ. 2008;336:598-600. Reproduced with permission from the BMJ Publishing Group.
Quit rates higher when patients know lung age
At 1 year, verified quit rates were 13.6% in the intervention group and 6.4% in the control group (a difference of 7.2%, 95% CI, 2.2%-12.1%; P=.005). This means that for every 14 smokers who are told their lung age and shown a graphic display of this biomarker, 1 additional smoker will quit after 1 year.
Contrary to what might be expected, the investigators found that quitting did not depend on the degree of lung damage. Patients with both normal and abnormal lung age quit smoking at similar rates.
WHAT’S NEW: Lung age resonates more than spirometry alone
This is the first RCT demonstrating that informing smokers of their lung age can help them quit, and the first well-designed study to demonstrate improved cessation rates using a physiological biomarker. The research also suggests that successful quitting may have less to do with spirometry results—the level of severity of lung damage it shows—than with the way the results are presented. Giving patients information about their lung function in an easily understandable format, the authors observe, appears to result in higher quit rates.
CAVEATS: Young smokers weren’t studied
The study did not test to see if this intervention would work in younger adults, as only those 35 years of age and older were enrolled. This is a single study, and it is possible that the findings cannot be generalized to other groups or are due to unmeasured confounding factors. However, the intervention is unlikely to cause any significant harm, so we see no risks associated with it other than the cost of spirometry.
CHALLENGES TO IMPLEMENTATION: Time and expense of spirometry
We suspect the biggest challenges to implementing this recommendation in clinical practice are the expense of obtaining a spirometer ( TABLE ), staff training for those practices without one, and the time needed for the intervention. The average time to perform spirometry on study participants was 30 minutes; a health care worker spent, on average, another 15 minutes reviewing results with each member of the intervention group.
Another challenge: Not all spirometers calculate lung age or can create a graphic similar to FIGURE 2 . However, any FEV1 measurement, whether it is generated by formal pulmonary function testing or by an inexpensive hand-held meter, can easily be converted to lung age using the formula shown in FIGURE 1 . If desired, the same elements—the patient’s age, height, and gender as well as FEV1—could also be used to create a computer-generated graphic display.
TABLE
Spirometry: equipment costs
| The initial cost of a spirometer varies widely, depending on the sophistication of the equipment and the available options and features. Additional costs—for disposable mouthpieces, line filters, nose clips, and hoses, for example—are low. A sampling of reasonably priced models well suited for office use is shown below. All of these models meet American Thoracic Society criteria for spirometry, and all calculate lung age. | ||
|---|---|---|
| SPIROMETER MANUFACTURER/MODEL | PRICE | SUPPLIER |
| Futuremed Discovery-2 | $2,125 | medsupplier.com |
| Micro Medical MicroLoop | $1,780 | Miami-med.com |
| Micro Medical SpiroUSB | $1,580 | Miami-med.com |
| NDD EasyOne Frontline | $1,000 | medsupplier.com |
| SDI Diagnostics Spirolab II | $2,600 | med-electronics.com |
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 48-year-old man comes to your office for a routine physical. He has a 30 pack-year smoking history. When you talk to him about smoking cessation, he tells you he’s tried to stop more than once, but he can’t seem to stay motivated. You find no evidence of chronic lung disease and do not perform spirometry screening. (The US Preventive Services Task Force does not recommend spirometry for asymptomatic patients.) But could spirometry have therapeutic value in this case?
Smoking is the leading modifiable risk factor for mortality in the United States,2 and smoking cessation is the most effective intervention. Nortriptyline, bupropion, nicotine replacement agents, and varenicline are effective pharmacological treatments.3 Adding counseling to medication significantly improves quit rates (estimated odds ratio [OR]=1.4; 95% confidence interval [CI], 1.2-1.6).3 Nonetheless, physicians’ efforts to help patients stop smoking frequently fail.
But another option has caught—and held—the attention of researchers.
The promise of biomarkers
It has long been suspected that presenting smokers with evidence of tobacco’s harmful effect on their bodies—biomarkers—might encourage them to stop. Biomarkers that have been tested in randomized controlled trials (RCTs) include spirometry, exhaled carbon monoxide measurement, ultrasonography of carotid and femoral arteries, and genetic susceptibility to lung cancer, as well as combinations of these markers. But the results of most biomarker studies have been disappointing. A 2005 Cochrane Database review found insufficient evidence of the effectiveness of these markers in boosting quit rates.4
Lung age, a biomarker that’s easily understood
Lung age, a clever presentation of spirometry results, had not been tested in an RCT prior to the study we summarize below. Defined in 1985, lung age refers to the average age of a nonsmoker with a forced expiratory volume at 1 second (FEV1) equal to that of the person being tested ( FIGURE 1 ). The primary purpose was to make spirometry results easier for patients to understand, but researchers also envisioned it as a way to demonstrate the premature lung damage suffered as a consequence of smoking.5
FIGURE 1
Translating FEV1 into lung age1
STUDY SUMMARY: Graphic display more effective than FEV1 results
This study was a well-done, multicenter RCT evaluating the effect on tobacco quit rates of informing adult smokers of their lung age.1 Smokers ages 35 and older from 5 general practices in England were invited to participate. The authors excluded patients using oxygen and those with a history of tuberculosis, lung cancer, asbestosis, bronchiectasis, silicosis, or pneumonectomy. The study included 561 participants with an average of 33 pack-years of smoking, who underwent spirometry before being divided into an intervention or a control group. The researchers used standardized instruments to confirm the baseline comparability of the 2 groups.
Subjects in both groups were given information about local smoking cessation clinics and strongly encouraged to quit. All were told that their lung function would be retested in 12 months.
The controls received letters with their spirometry results presented as FEV1. In contrast, participants in the intervention group received the results in the form of a computer-generated graphic display of lung age ( FIGURE 2 ), which was further explained by a health care worker. They also received a letter within 1 month containing the same data. Participants were evaluated for smoking cessation at 12 months, and those who reported quitting received confirmatory carbon monoxide breath testing and salivary cotinine testing. Eleven percent of the subjects were lost to follow-up.
FIGURE 2
Lung age helps spirometry pack a bigger punch
Drawing a vertical line from the patient’s age (on the horizontal axis) to reach the solid curve representing the lung function of the “susceptible smoker” and extending the line horizontally to reach the curve with the broken lines representing “never smokers” graphically shows the patient’s lung age and the accelerated decline in lung function associated with smoking. The patient shown here is a 52-year-old smoker with FEV1 equivalent to a 75-year-old nonsmoker.
Source: Parkes G et al. BMJ. 2008;336:598-600. Reproduced with permission from the BMJ Publishing Group.
Quit rates higher when patients know lung age
At 1 year, verified quit rates were 13.6% in the intervention group and 6.4% in the control group (a difference of 7.2%, 95% CI, 2.2%-12.1%; P=.005). This means that for every 14 smokers who are told their lung age and shown a graphic display of this biomarker, 1 additional smoker will quit after 1 year.
Contrary to what might be expected, the investigators found that quitting did not depend on the degree of lung damage. Patients with both normal and abnormal lung age quit smoking at similar rates.
WHAT’S NEW: Lung age resonates more than spirometry alone
This is the first RCT demonstrating that informing smokers of their lung age can help them quit, and the first well-designed study to demonstrate improved cessation rates using a physiological biomarker. The research also suggests that successful quitting may have less to do with spirometry results—the level of severity of lung damage it shows—than with the way the results are presented. Giving patients information about their lung function in an easily understandable format, the authors observe, appears to result in higher quit rates.
CAVEATS: Young smokers weren’t studied
The study did not test to see if this intervention would work in younger adults, as only those 35 years of age and older were enrolled. This is a single study, and it is possible that the findings cannot be generalized to other groups or are due to unmeasured confounding factors. However, the intervention is unlikely to cause any significant harm, so we see no risks associated with it other than the cost of spirometry.
CHALLENGES TO IMPLEMENTATION: Time and expense of spirometry
We suspect the biggest challenges to implementing this recommendation in clinical practice are the expense of obtaining a spirometer ( TABLE ), staff training for those practices without one, and the time needed for the intervention. The average time to perform spirometry on study participants was 30 minutes; a health care worker spent, on average, another 15 minutes reviewing results with each member of the intervention group.
Another challenge: Not all spirometers calculate lung age or can create a graphic similar to FIGURE 2 . However, any FEV1 measurement, whether it is generated by formal pulmonary function testing or by an inexpensive hand-held meter, can easily be converted to lung age using the formula shown in FIGURE 1 . If desired, the same elements—the patient’s age, height, and gender as well as FEV1—could also be used to create a computer-generated graphic display.
TABLE
Spirometry: equipment costs
| The initial cost of a spirometer varies widely, depending on the sophistication of the equipment and the available options and features. Additional costs—for disposable mouthpieces, line filters, nose clips, and hoses, for example—are low. A sampling of reasonably priced models well suited for office use is shown below. All of these models meet American Thoracic Society criteria for spirometry, and all calculate lung age. | ||
|---|---|---|
| SPIROMETER MANUFACTURER/MODEL | PRICE | SUPPLIER |
| Futuremed Discovery-2 | $2,125 | medsupplier.com |
| Micro Medical MicroLoop | $1,780 | Miami-med.com |
| Micro Medical SpiroUSB | $1,580 | Miami-med.com |
| NDD EasyOne Frontline | $1,000 | medsupplier.com |
| SDI Diagnostics Spirolab II | $2,600 | med-electronics.com |
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
2. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
3. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed July 6, 2008.
4. Bize R, Burnand B, Mueller Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2005;(4):CD004705.-
5. Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985;14:655-662.
1. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
2. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
3. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed July 6, 2008.
4. Bize R, Burnand B, Mueller Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2005;(4):CD004705.-
5. Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985;14:655-662.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Patients insist on antibiotics for sinusitis? Here is a good reason to say “no”
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Use anesthetic drops to relieve acute otitis media pain
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
This obscure herb works for the common cold
Offer patients Pelargonium sidoides (30 drops 3 times a day) to reduce the severity and duration of common cold symptoms and to get patients back to work sooner.1
Strength of recommendation
B: A single well-designed randomized controlled trial
Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007; 3:573–584.
ILLUSTRATIVE CASE
A 39-year-old, otherwise healthy woman presents to your clinic with a sore throat, nasal congestion, and dry cough she’s had since yesterday. She wants an antibiotic, but your evaluation reveals an uncomplicated viral upper respiratory infection—a common cold. You would like to provide her with an alternative treatment, but you are aware of the lack of evidence for clear benefit of zinc lozenges, echinacea, and vitamin C. Is there any other medication that might benefit this patient?
Yes. Pelargonium sidoides, a species of South African geranium used for centuries in Zulu medicine,2 shows promise as an herbal remedy for respiratory infections. Two randomized trials show that extracts of P sidoides improve symptoms of acute bronchitis which, like the common cold, is usually caused by a virus.3-5
There is a plausible biological mechanism of action. In vitro studies show that Pelargonium extract induces the interferon system and up-regulates cytokines important in protecting host cells from viral infection.6
BACKGROUND: $17 billion dollar cold
Our patients want more relief from cold symptoms and are clearly willing to pay for it. Americans spend approximately $2.9 billion annually on over-the-counter (OTC) cold preparations and $1.1 billion on unnecessary antibiotics.7 The term “common cold” refers to a collection of symptoms, including sore throat, rhinorrhea, nasal congestion, cough, low-grade fever, and malaise, usually self-limited and lasting 10 to 14 days, caused by a number of viruses, most commonly by a rhinovirus.8 According to the 2005 National Ambulatory Medical Care Survey, the common cold is the third most common diagnosis in physicians’ offices behind only hypertension and well-infant/child visits.9 A 2001 US telephone survey determined that approximately 500 million episodes of non-influenza viral infection occur annually, resulting in direct costs of $17 billion for physician services and medications and approximately 200 million missed days of work.7
CLINICAL CONTEXT: Evidence proves most cold remedies don’t work
Although colds are common and result in annoying symptoms and missed work, much of the money spent on remedies is wasted. A truly effective treatment would be valuable to our patients.
Despite brisk sales, evidence for the efficacy of various cold remedies is inconclusive and contradictory. We found 6 Cochrane reviews of cold treatments, including antitussives, antihistamines, decongestants, vitamin C, echinacea, and zinc lozenges. With the exception of pseudoephedrine for nasal symptoms, the evidence that any product improves symptoms or decreases the duration of the cold is not encouraging.
Cough medications. The 2004 Cochrane Review of OTC medications for cough10 found no consistent evidence that any of them work. Codeine was no more effective than placebo for reducing cough symptoms. Three efficacy studies of dextromethorphan for cough showed either no difference or small but possibly clinically insignificant improvement in cough over placebo. One study of guaifenesin showed benefit over placebo in reducing cough frequency; another one showed no benefit over placebo.
Vitamin C, echinacea. Three Cochrane reviews found no conclusive evidence of benefit over placebo for either vitamin C11 or echinacea12 in treating the common cold.
Zinc. A new panel has been convened by the Cochrane group to reassess the effectiveness of zinc, but a 1999 Cochrane review13 found no benefit for zinc over placebo.
Antihistamines are not effective for relieving cold symptoms.14
Pseudoephedrine is the only medication with good-quality evidence for effectiveness, but only for reducing nasal symptoms.15 The authors concluded that patients may be encouraged to continue pseudoephedrine for up to 5 days if found to be effective with the first dose. Nasal congestion and discharge, however, are only 2 of the many irritating symptoms of a cold.

Bernard Ewigman, MD, MSPH
If you are in full-time clinical practice, a medical director of a practice, or otherwise directly involved in decision-making about adopting new practices, join our team of “reality checkers.”
Just email me at [email protected]
STUDY SUMMARY: Duration and severity of symptoms are reduced
This was a multicenter, prospective, double-blind, placebo-controlled randomized trial to evaluate the effectiveness of a liquid herbal preparation from the roots of Pelargonium sidoides for decreasing the duration and severity of symptoms of the common cold.
Patient characteristics. Patients were recruited from 8 outpatient departments in Ukraine between December 2003 and May 2004. Two hundred and seven (207) patients were eligible. The number of ineligible and excluded patients was not stated. These 207 patients were randomized into 1 of 4 groups:
- 52 received 30 drops 3 times daily vs 51 patients who received placebo
- 52 patients received 60 drops 3 times daily vs 52 patients who received a higher-dose placebo.
The report gives the outcomes of the low-dose arm only. Two-thirds of the participants were women; all were Caucasian. Patients in the treatment and placebo groups were similar in terms of recurrent disease, prior use of medication for the common cold, smoking, and alcohol and caffeine consumption. All had a negative Group A beta-hemolytic strep test.
Inclusion criteria. Patients included men and women 18 to 55 years of age; able to provide written informed consent; with 2 major cold symptoms (nasal discharge, sore throat) and at least 1 minor cold symptom (nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, or fever) or presence of 1 major cold symptom and at least 3 minor cold symptoms; duration of symptoms 24 to 48 hours.
Exclusion criteria were any acute ear, nose, throat and respiratory tract disease other than the common cold; positive rapid strep test; 6 or more episodes of recurrent tonsillitis, sinusitis, or otitis within the past 12 months or any chronic ear, nose, throat or respiratory tract disease; treatment with antibiotics, glucocorticoids, or antihistamine drugs during the 4 weeks prior to enrollment in the trial; treatment with cold medications that might impair the trial results (eg, decongestants, local anesthetics); and use of cough or pain relief medications, or any other treatment for the common cold within 7 days prior to enrollment in the trial.
Treatment regimen. Patients were assigned to take 30 drops of either the study herbal preparation or 30 drops of placebo 3 times daily, at least 30 minutes before or after a meal, from day 1 continuing to day 10. The investigational drug and placebo were supplied by Dr. Willmar Schwabe GmbH & Co. (Karlsruhe, Germany). The investigational medication is a preparation of the roots of P sidoides, extraction solution: ethanol 11% (1:8-10) (wt/wt). The placebo was matched for color, smell, taste, and viscosity. Paracetamol (acetaminophen) tablets were allowed for all patients for fever greater than 39°C.
Primary endpoint. Severity of cold symptoms was evaluated using the Cold Intensity Score (CIS), a validated scale derived from the sum of scores for 10 cold-related symptoms (nasal drainage, sore throat, nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, and fever) on a scale of 0 to 4, where 0=not present and 4=very severe, to a maximum of 40 points. At baseline, the mean total CIS was comparable in both treatment and placebo groups (17.8±4.0 vs 16.9±3.4). From baseline to day 5, the mean total CIS decreased by 10.4±3.0 in the treatment group vs 5.6±4.3 in the placebo group (P<.0001).
Secondary endpoints. The number of patients achieving clinical cure (defined by CIS ≤1) by day 10 was significantly higher in the treatment group (78.8% vs 31.4%, P<.0001). The mean duration of days absent from work was significantly lower in the treatment group (6.9±1.8 vs 8.2±2.1, P<.0003), as was number of days with less than 100% usual activity level (7.1±1.5 vs 8.7±1.3, P<.0001). Data for both the primary and secondary endpoints were evaluated according to an intention-to-treat analysis. Both the intervention and placebo sides each had 4 patients that became ineligible after initial randomization. No patients were lost to follow-up.
Safety and tolerability. Patients in the low-dose arm experienced 3 nonserious adverse events, and 1 experienced mild epistaxis. Two additional patients (1 in the treatment and 1 in the placebo group) experienced moderate to severe tracheitis, not attributable to the study medication. Tolerability was rated slightly better in the treatment than placebo group on day 5. Forty-nine of 52 patients (94%) in the treatment group rated the preparation as good or very good tolerability vs 42 of 51 patients (82%) in the placebo group.
WHAT’S NEW?: A first
This is the first study that demonstrates the efficacy and safety of P sidoides in the treatment of the common cold. More importantly, this degree of improvement in cold symptoms is dramatically better than other common OTC treatments, including vitamin C, echinacea, and zinc preparations.
CAVEATS: How is this different from other cold remedies?
Patients are already spending a lot on cold remedies; this study suggests money would be better spent on having a ready supply of Pelargonium in the medicine cabinet, and it appears to be safe.
Other initially promising complementary and alternative therapies, such as zinc, echinacea, and vitamin C, have not been shown to be effective with more vigorous evaluation. We recognize that this is only 1 clinical trial, and the results may not be replicated in future trials. However, we are impressed by the effect size—twice the size as that seen for placebo, with a reduction in half of total cold symptom severity over 5 days and a reduction of missed time from work by more than a full day on average over placebo.
In vitro studies suggest a physiologic mechanism that is consistent with the study outcomes.
Similar findings are reported for symptom reduction in acute bronchitis.
Safety
There were no significant adverse events in this study, which is consistent with the findings of the studies of acute bronchitis.12-14P sidoides has been widely used in Germany since the 1980s, with an annual sale value in 2002 of $55 million or 4.1 million packages.
The Uppsala Monitoring Centre, in conjunction with the World Health Organization international pharmacovigilance program, received 34 case reports between 2002 and 2006 of allergic reactions to ethanolic herbal extract of Pelargonium root, 2 of which involved life-threatening circulatory collapse requiring emergency medical attention. Given the extremely rare occurrence of these events we believe the minimal risk is acceptable. The others involved rash and pruritus.
Also of note: contact dermatitis to Pelargonium houseplants has been reported. As a result, product information will be added to product packaging, warning of common reactions of gastrointestinal complaints (gastric pain, heartburn, nausea, and diarrhea) as well as the potential for serious allergic reaction. In addition, since some of the active compounds are plant coumarins, there is a theoretical risk of interaction with warfarin and aspirin but no serious bleeding events have been reported.16
It is also recommended that individuals with renal or hepatic disease or women who are pregnant or breastfeeding avoid use of this preparation, as safety studies have not been performed.
Other study design issues
A few other issues struck us as important when assessing the validity of this study. For example, 1 of the authors appears to be an employee of the pharmaceutical company that manufactures the preparation, raising the conflict of interest issue.
We were also curious about why the results of the high-dose arm were not reported in this manuscript. Could there have been a higher rate of adverse events in the high-dose arm? Knowing how many patients were ineligible or excluded, and the efficacy or safety in the high-dose arm would give us more confidence in the findings, but we decided that these were not necessarily fatal flaws.
Bottom line
Despite the above caveats, this was a well-designed randomized controlled trial that suggests that P sidoides is impressively efficacious in decreasing the duration and severity of the common cold. In the final analysis, we think that these findings justify recommending this to our patients.
CHALLENGES TO IMPLEMENTATION: 2-day window
The medication was started within 48 hours of the onset of symptoms. We generally see patients seeking treatment for the common cold well after the first 2 days. The efficacy of Pelargonium is no doubt less when started later in the course of the illness. Colds resolve spontaneously, so to get the benefit of this treatment, it likely must be started early.
Our conclusion is that patients could be advised to purchase the medication to have on hand at home at the start of the cold season.
Availability of the drug
P sidoides is available in the US under the brand name Umcka Coldcare.
The preparation used in the study is marketed in Europe by ISO-Arzneimittel17 under the name Umckaloabo, which is a combination of the Zulu words for lung symptoms and breast pain.18
Our Internet search on the term “Pelargonium sidoides” failed to yield a distributor of the German preparation used in the study that would be available in the United States. However, a different manufacturer, Nature’s Way, distributes a number of similar preparations containing extract of the root of P sidoides under the name Umcka Coldcare. Umcka Cold-care appears to be readily available for purchase, both on-line and through local health food stores, for less than $20 per 4-oz (120-mL) bottle. A different retailer, African Red Tea, offers syrup, 1:10 ethanolic extract for $29.95 for 100-mL bottle.19 Like the German preparation, these formulations are delivered by dropper.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007;3:573-584.
2. Bladt S, Wagner H. From Zulu medicine to the European phytomedicine Umckaloabo. Phytomedicine 2007;14 (suppl 1):2-4.
3. Matthys H, Eisebitt R, Seith B, Heger M. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2003;10(Suppl 4):7-17.
4. Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs 7630): A randomized, double-blind, placebo-controlled trial. Explore (NY) 2005;1:437-445.
5. Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomized, double-blind, placebo-controlled multicentre study. Curr Med Res Opinion 2007;23:323-331.
6. Kolodziej H, Kiderlen AF. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs 7630. Phytomedicine 2007;14 (suppl 1):18-26.
7. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med 2003;163:487-494.
8. Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361:51-59.
9. Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 Summary. Adv Data 2007;387:1-39.
10. Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2004;(4):cD001831.-
11. Douglas RM, Hemila H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004;(4):cD000980.-
12. Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2006;(1):cD000530.-
13. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 1999;(2):cD001364.-(With-drawn 2006, Issue 3).
14. Sutter AI, Lemiengre M, Campbell H, Mackinnon HF. Anithistamines for the common cold. Cochrane Database Syst Rev 2003;(3):cD001267.-
15. Taverner D, Latte J, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2004;(3):cD001953.-
16. De Boer HJ, Hagemann U, Bate J, Meyboom RHB. Allergic reactions to medicines derived from Pelargonium species. Drug Saf 2007;30:677-680.
17. ISO-Arzneimittel. Distributor of Umckaloabo. Available at: umckaloabo.com. Accessed January 7, 2008.
18. Taylor PW, Maalim S, Coleman S. The strange story of umckaloabo. Pharm J 2005;275:790-792.
19. African Red Tea Imports. Available at: www.africanredtea.com/pelargonium-syrup.html. Accessed January 7, 2008.
Offer patients Pelargonium sidoides (30 drops 3 times a day) to reduce the severity and duration of common cold symptoms and to get patients back to work sooner.1
Strength of recommendation
B: A single well-designed randomized controlled trial
Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007; 3:573–584.
ILLUSTRATIVE CASE
A 39-year-old, otherwise healthy woman presents to your clinic with a sore throat, nasal congestion, and dry cough she’s had since yesterday. She wants an antibiotic, but your evaluation reveals an uncomplicated viral upper respiratory infection—a common cold. You would like to provide her with an alternative treatment, but you are aware of the lack of evidence for clear benefit of zinc lozenges, echinacea, and vitamin C. Is there any other medication that might benefit this patient?
Yes. Pelargonium sidoides, a species of South African geranium used for centuries in Zulu medicine,2 shows promise as an herbal remedy for respiratory infections. Two randomized trials show that extracts of P sidoides improve symptoms of acute bronchitis which, like the common cold, is usually caused by a virus.3-5
There is a plausible biological mechanism of action. In vitro studies show that Pelargonium extract induces the interferon system and up-regulates cytokines important in protecting host cells from viral infection.6
BACKGROUND: $17 billion dollar cold
Our patients want more relief from cold symptoms and are clearly willing to pay for it. Americans spend approximately $2.9 billion annually on over-the-counter (OTC) cold preparations and $1.1 billion on unnecessary antibiotics.7 The term “common cold” refers to a collection of symptoms, including sore throat, rhinorrhea, nasal congestion, cough, low-grade fever, and malaise, usually self-limited and lasting 10 to 14 days, caused by a number of viruses, most commonly by a rhinovirus.8 According to the 2005 National Ambulatory Medical Care Survey, the common cold is the third most common diagnosis in physicians’ offices behind only hypertension and well-infant/child visits.9 A 2001 US telephone survey determined that approximately 500 million episodes of non-influenza viral infection occur annually, resulting in direct costs of $17 billion for physician services and medications and approximately 200 million missed days of work.7
CLINICAL CONTEXT: Evidence proves most cold remedies don’t work
Although colds are common and result in annoying symptoms and missed work, much of the money spent on remedies is wasted. A truly effective treatment would be valuable to our patients.
Despite brisk sales, evidence for the efficacy of various cold remedies is inconclusive and contradictory. We found 6 Cochrane reviews of cold treatments, including antitussives, antihistamines, decongestants, vitamin C, echinacea, and zinc lozenges. With the exception of pseudoephedrine for nasal symptoms, the evidence that any product improves symptoms or decreases the duration of the cold is not encouraging.
Cough medications. The 2004 Cochrane Review of OTC medications for cough10 found no consistent evidence that any of them work. Codeine was no more effective than placebo for reducing cough symptoms. Three efficacy studies of dextromethorphan for cough showed either no difference or small but possibly clinically insignificant improvement in cough over placebo. One study of guaifenesin showed benefit over placebo in reducing cough frequency; another one showed no benefit over placebo.
Vitamin C, echinacea. Three Cochrane reviews found no conclusive evidence of benefit over placebo for either vitamin C11 or echinacea12 in treating the common cold.
Zinc. A new panel has been convened by the Cochrane group to reassess the effectiveness of zinc, but a 1999 Cochrane review13 found no benefit for zinc over placebo.
Antihistamines are not effective for relieving cold symptoms.14
Pseudoephedrine is the only medication with good-quality evidence for effectiveness, but only for reducing nasal symptoms.15 The authors concluded that patients may be encouraged to continue pseudoephedrine for up to 5 days if found to be effective with the first dose. Nasal congestion and discharge, however, are only 2 of the many irritating symptoms of a cold.

Bernard Ewigman, MD, MSPH
If you are in full-time clinical practice, a medical director of a practice, or otherwise directly involved in decision-making about adopting new practices, join our team of “reality checkers.”
Just email me at [email protected]
STUDY SUMMARY: Duration and severity of symptoms are reduced
This was a multicenter, prospective, double-blind, placebo-controlled randomized trial to evaluate the effectiveness of a liquid herbal preparation from the roots of Pelargonium sidoides for decreasing the duration and severity of symptoms of the common cold.
Patient characteristics. Patients were recruited from 8 outpatient departments in Ukraine between December 2003 and May 2004. Two hundred and seven (207) patients were eligible. The number of ineligible and excluded patients was not stated. These 207 patients were randomized into 1 of 4 groups:
- 52 received 30 drops 3 times daily vs 51 patients who received placebo
- 52 patients received 60 drops 3 times daily vs 52 patients who received a higher-dose placebo.
The report gives the outcomes of the low-dose arm only. Two-thirds of the participants were women; all were Caucasian. Patients in the treatment and placebo groups were similar in terms of recurrent disease, prior use of medication for the common cold, smoking, and alcohol and caffeine consumption. All had a negative Group A beta-hemolytic strep test.
Inclusion criteria. Patients included men and women 18 to 55 years of age; able to provide written informed consent; with 2 major cold symptoms (nasal discharge, sore throat) and at least 1 minor cold symptom (nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, or fever) or presence of 1 major cold symptom and at least 3 minor cold symptoms; duration of symptoms 24 to 48 hours.
Exclusion criteria were any acute ear, nose, throat and respiratory tract disease other than the common cold; positive rapid strep test; 6 or more episodes of recurrent tonsillitis, sinusitis, or otitis within the past 12 months or any chronic ear, nose, throat or respiratory tract disease; treatment with antibiotics, glucocorticoids, or antihistamine drugs during the 4 weeks prior to enrollment in the trial; treatment with cold medications that might impair the trial results (eg, decongestants, local anesthetics); and use of cough or pain relief medications, or any other treatment for the common cold within 7 days prior to enrollment in the trial.
Treatment regimen. Patients were assigned to take 30 drops of either the study herbal preparation or 30 drops of placebo 3 times daily, at least 30 minutes before or after a meal, from day 1 continuing to day 10. The investigational drug and placebo were supplied by Dr. Willmar Schwabe GmbH & Co. (Karlsruhe, Germany). The investigational medication is a preparation of the roots of P sidoides, extraction solution: ethanol 11% (1:8-10) (wt/wt). The placebo was matched for color, smell, taste, and viscosity. Paracetamol (acetaminophen) tablets were allowed for all patients for fever greater than 39°C.
Primary endpoint. Severity of cold symptoms was evaluated using the Cold Intensity Score (CIS), a validated scale derived from the sum of scores for 10 cold-related symptoms (nasal drainage, sore throat, nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, and fever) on a scale of 0 to 4, where 0=not present and 4=very severe, to a maximum of 40 points. At baseline, the mean total CIS was comparable in both treatment and placebo groups (17.8±4.0 vs 16.9±3.4). From baseline to day 5, the mean total CIS decreased by 10.4±3.0 in the treatment group vs 5.6±4.3 in the placebo group (P<.0001).
Secondary endpoints. The number of patients achieving clinical cure (defined by CIS ≤1) by day 10 was significantly higher in the treatment group (78.8% vs 31.4%, P<.0001). The mean duration of days absent from work was significantly lower in the treatment group (6.9±1.8 vs 8.2±2.1, P<.0003), as was number of days with less than 100% usual activity level (7.1±1.5 vs 8.7±1.3, P<.0001). Data for both the primary and secondary endpoints were evaluated according to an intention-to-treat analysis. Both the intervention and placebo sides each had 4 patients that became ineligible after initial randomization. No patients were lost to follow-up.
Safety and tolerability. Patients in the low-dose arm experienced 3 nonserious adverse events, and 1 experienced mild epistaxis. Two additional patients (1 in the treatment and 1 in the placebo group) experienced moderate to severe tracheitis, not attributable to the study medication. Tolerability was rated slightly better in the treatment than placebo group on day 5. Forty-nine of 52 patients (94%) in the treatment group rated the preparation as good or very good tolerability vs 42 of 51 patients (82%) in the placebo group.
WHAT’S NEW?: A first
This is the first study that demonstrates the efficacy and safety of P sidoides in the treatment of the common cold. More importantly, this degree of improvement in cold symptoms is dramatically better than other common OTC treatments, including vitamin C, echinacea, and zinc preparations.
CAVEATS: How is this different from other cold remedies?
Patients are already spending a lot on cold remedies; this study suggests money would be better spent on having a ready supply of Pelargonium in the medicine cabinet, and it appears to be safe.
Other initially promising complementary and alternative therapies, such as zinc, echinacea, and vitamin C, have not been shown to be effective with more vigorous evaluation. We recognize that this is only 1 clinical trial, and the results may not be replicated in future trials. However, we are impressed by the effect size—twice the size as that seen for placebo, with a reduction in half of total cold symptom severity over 5 days and a reduction of missed time from work by more than a full day on average over placebo.
In vitro studies suggest a physiologic mechanism that is consistent with the study outcomes.
Similar findings are reported for symptom reduction in acute bronchitis.
Safety
There were no significant adverse events in this study, which is consistent with the findings of the studies of acute bronchitis.12-14P sidoides has been widely used in Germany since the 1980s, with an annual sale value in 2002 of $55 million or 4.1 million packages.
The Uppsala Monitoring Centre, in conjunction with the World Health Organization international pharmacovigilance program, received 34 case reports between 2002 and 2006 of allergic reactions to ethanolic herbal extract of Pelargonium root, 2 of which involved life-threatening circulatory collapse requiring emergency medical attention. Given the extremely rare occurrence of these events we believe the minimal risk is acceptable. The others involved rash and pruritus.
Also of note: contact dermatitis to Pelargonium houseplants has been reported. As a result, product information will be added to product packaging, warning of common reactions of gastrointestinal complaints (gastric pain, heartburn, nausea, and diarrhea) as well as the potential for serious allergic reaction. In addition, since some of the active compounds are plant coumarins, there is a theoretical risk of interaction with warfarin and aspirin but no serious bleeding events have been reported.16
It is also recommended that individuals with renal or hepatic disease or women who are pregnant or breastfeeding avoid use of this preparation, as safety studies have not been performed.
Other study design issues
A few other issues struck us as important when assessing the validity of this study. For example, 1 of the authors appears to be an employee of the pharmaceutical company that manufactures the preparation, raising the conflict of interest issue.
We were also curious about why the results of the high-dose arm were not reported in this manuscript. Could there have been a higher rate of adverse events in the high-dose arm? Knowing how many patients were ineligible or excluded, and the efficacy or safety in the high-dose arm would give us more confidence in the findings, but we decided that these were not necessarily fatal flaws.
Bottom line
Despite the above caveats, this was a well-designed randomized controlled trial that suggests that P sidoides is impressively efficacious in decreasing the duration and severity of the common cold. In the final analysis, we think that these findings justify recommending this to our patients.
CHALLENGES TO IMPLEMENTATION: 2-day window
The medication was started within 48 hours of the onset of symptoms. We generally see patients seeking treatment for the common cold well after the first 2 days. The efficacy of Pelargonium is no doubt less when started later in the course of the illness. Colds resolve spontaneously, so to get the benefit of this treatment, it likely must be started early.
Our conclusion is that patients could be advised to purchase the medication to have on hand at home at the start of the cold season.
Availability of the drug
P sidoides is available in the US under the brand name Umcka Coldcare.
The preparation used in the study is marketed in Europe by ISO-Arzneimittel17 under the name Umckaloabo, which is a combination of the Zulu words for lung symptoms and breast pain.18
Our Internet search on the term “Pelargonium sidoides” failed to yield a distributor of the German preparation used in the study that would be available in the United States. However, a different manufacturer, Nature’s Way, distributes a number of similar preparations containing extract of the root of P sidoides under the name Umcka Coldcare. Umcka Cold-care appears to be readily available for purchase, both on-line and through local health food stores, for less than $20 per 4-oz (120-mL) bottle. A different retailer, African Red Tea, offers syrup, 1:10 ethanolic extract for $29.95 for 100-mL bottle.19 Like the German preparation, these formulations are delivered by dropper.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Offer patients Pelargonium sidoides (30 drops 3 times a day) to reduce the severity and duration of common cold symptoms and to get patients back to work sooner.1
Strength of recommendation
B: A single well-designed randomized controlled trial
Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007; 3:573–584.
ILLUSTRATIVE CASE
A 39-year-old, otherwise healthy woman presents to your clinic with a sore throat, nasal congestion, and dry cough she’s had since yesterday. She wants an antibiotic, but your evaluation reveals an uncomplicated viral upper respiratory infection—a common cold. You would like to provide her with an alternative treatment, but you are aware of the lack of evidence for clear benefit of zinc lozenges, echinacea, and vitamin C. Is there any other medication that might benefit this patient?
Yes. Pelargonium sidoides, a species of South African geranium used for centuries in Zulu medicine,2 shows promise as an herbal remedy for respiratory infections. Two randomized trials show that extracts of P sidoides improve symptoms of acute bronchitis which, like the common cold, is usually caused by a virus.3-5
There is a plausible biological mechanism of action. In vitro studies show that Pelargonium extract induces the interferon system and up-regulates cytokines important in protecting host cells from viral infection.6
BACKGROUND: $17 billion dollar cold
Our patients want more relief from cold symptoms and are clearly willing to pay for it. Americans spend approximately $2.9 billion annually on over-the-counter (OTC) cold preparations and $1.1 billion on unnecessary antibiotics.7 The term “common cold” refers to a collection of symptoms, including sore throat, rhinorrhea, nasal congestion, cough, low-grade fever, and malaise, usually self-limited and lasting 10 to 14 days, caused by a number of viruses, most commonly by a rhinovirus.8 According to the 2005 National Ambulatory Medical Care Survey, the common cold is the third most common diagnosis in physicians’ offices behind only hypertension and well-infant/child visits.9 A 2001 US telephone survey determined that approximately 500 million episodes of non-influenza viral infection occur annually, resulting in direct costs of $17 billion for physician services and medications and approximately 200 million missed days of work.7
CLINICAL CONTEXT: Evidence proves most cold remedies don’t work
Although colds are common and result in annoying symptoms and missed work, much of the money spent on remedies is wasted. A truly effective treatment would be valuable to our patients.
Despite brisk sales, evidence for the efficacy of various cold remedies is inconclusive and contradictory. We found 6 Cochrane reviews of cold treatments, including antitussives, antihistamines, decongestants, vitamin C, echinacea, and zinc lozenges. With the exception of pseudoephedrine for nasal symptoms, the evidence that any product improves symptoms or decreases the duration of the cold is not encouraging.
Cough medications. The 2004 Cochrane Review of OTC medications for cough10 found no consistent evidence that any of them work. Codeine was no more effective than placebo for reducing cough symptoms. Three efficacy studies of dextromethorphan for cough showed either no difference or small but possibly clinically insignificant improvement in cough over placebo. One study of guaifenesin showed benefit over placebo in reducing cough frequency; another one showed no benefit over placebo.
Vitamin C, echinacea. Three Cochrane reviews found no conclusive evidence of benefit over placebo for either vitamin C11 or echinacea12 in treating the common cold.
Zinc. A new panel has been convened by the Cochrane group to reassess the effectiveness of zinc, but a 1999 Cochrane review13 found no benefit for zinc over placebo.
Antihistamines are not effective for relieving cold symptoms.14
Pseudoephedrine is the only medication with good-quality evidence for effectiveness, but only for reducing nasal symptoms.15 The authors concluded that patients may be encouraged to continue pseudoephedrine for up to 5 days if found to be effective with the first dose. Nasal congestion and discharge, however, are only 2 of the many irritating symptoms of a cold.

Bernard Ewigman, MD, MSPH
If you are in full-time clinical practice, a medical director of a practice, or otherwise directly involved in decision-making about adopting new practices, join our team of “reality checkers.”
Just email me at [email protected]
STUDY SUMMARY: Duration and severity of symptoms are reduced
This was a multicenter, prospective, double-blind, placebo-controlled randomized trial to evaluate the effectiveness of a liquid herbal preparation from the roots of Pelargonium sidoides for decreasing the duration and severity of symptoms of the common cold.
Patient characteristics. Patients were recruited from 8 outpatient departments in Ukraine between December 2003 and May 2004. Two hundred and seven (207) patients were eligible. The number of ineligible and excluded patients was not stated. These 207 patients were randomized into 1 of 4 groups:
- 52 received 30 drops 3 times daily vs 51 patients who received placebo
- 52 patients received 60 drops 3 times daily vs 52 patients who received a higher-dose placebo.
The report gives the outcomes of the low-dose arm only. Two-thirds of the participants were women; all were Caucasian. Patients in the treatment and placebo groups were similar in terms of recurrent disease, prior use of medication for the common cold, smoking, and alcohol and caffeine consumption. All had a negative Group A beta-hemolytic strep test.
Inclusion criteria. Patients included men and women 18 to 55 years of age; able to provide written informed consent; with 2 major cold symptoms (nasal discharge, sore throat) and at least 1 minor cold symptom (nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, or fever) or presence of 1 major cold symptom and at least 3 minor cold symptoms; duration of symptoms 24 to 48 hours.
Exclusion criteria were any acute ear, nose, throat and respiratory tract disease other than the common cold; positive rapid strep test; 6 or more episodes of recurrent tonsillitis, sinusitis, or otitis within the past 12 months or any chronic ear, nose, throat or respiratory tract disease; treatment with antibiotics, glucocorticoids, or antihistamine drugs during the 4 weeks prior to enrollment in the trial; treatment with cold medications that might impair the trial results (eg, decongestants, local anesthetics); and use of cough or pain relief medications, or any other treatment for the common cold within 7 days prior to enrollment in the trial.
Treatment regimen. Patients were assigned to take 30 drops of either the study herbal preparation or 30 drops of placebo 3 times daily, at least 30 minutes before or after a meal, from day 1 continuing to day 10. The investigational drug and placebo were supplied by Dr. Willmar Schwabe GmbH & Co. (Karlsruhe, Germany). The investigational medication is a preparation of the roots of P sidoides, extraction solution: ethanol 11% (1:8-10) (wt/wt). The placebo was matched for color, smell, taste, and viscosity. Paracetamol (acetaminophen) tablets were allowed for all patients for fever greater than 39°C.
Primary endpoint. Severity of cold symptoms was evaluated using the Cold Intensity Score (CIS), a validated scale derived from the sum of scores for 10 cold-related symptoms (nasal drainage, sore throat, nasal congestion, sneezing, scratchy throat, hoarseness, cough, headaches, muscle aches, and fever) on a scale of 0 to 4, where 0=not present and 4=very severe, to a maximum of 40 points. At baseline, the mean total CIS was comparable in both treatment and placebo groups (17.8±4.0 vs 16.9±3.4). From baseline to day 5, the mean total CIS decreased by 10.4±3.0 in the treatment group vs 5.6±4.3 in the placebo group (P<.0001).
Secondary endpoints. The number of patients achieving clinical cure (defined by CIS ≤1) by day 10 was significantly higher in the treatment group (78.8% vs 31.4%, P<.0001). The mean duration of days absent from work was significantly lower in the treatment group (6.9±1.8 vs 8.2±2.1, P<.0003), as was number of days with less than 100% usual activity level (7.1±1.5 vs 8.7±1.3, P<.0001). Data for both the primary and secondary endpoints were evaluated according to an intention-to-treat analysis. Both the intervention and placebo sides each had 4 patients that became ineligible after initial randomization. No patients were lost to follow-up.
Safety and tolerability. Patients in the low-dose arm experienced 3 nonserious adverse events, and 1 experienced mild epistaxis. Two additional patients (1 in the treatment and 1 in the placebo group) experienced moderate to severe tracheitis, not attributable to the study medication. Tolerability was rated slightly better in the treatment than placebo group on day 5. Forty-nine of 52 patients (94%) in the treatment group rated the preparation as good or very good tolerability vs 42 of 51 patients (82%) in the placebo group.
WHAT’S NEW?: A first
This is the first study that demonstrates the efficacy and safety of P sidoides in the treatment of the common cold. More importantly, this degree of improvement in cold symptoms is dramatically better than other common OTC treatments, including vitamin C, echinacea, and zinc preparations.
CAVEATS: How is this different from other cold remedies?
Patients are already spending a lot on cold remedies; this study suggests money would be better spent on having a ready supply of Pelargonium in the medicine cabinet, and it appears to be safe.
Other initially promising complementary and alternative therapies, such as zinc, echinacea, and vitamin C, have not been shown to be effective with more vigorous evaluation. We recognize that this is only 1 clinical trial, and the results may not be replicated in future trials. However, we are impressed by the effect size—twice the size as that seen for placebo, with a reduction in half of total cold symptom severity over 5 days and a reduction of missed time from work by more than a full day on average over placebo.
In vitro studies suggest a physiologic mechanism that is consistent with the study outcomes.
Similar findings are reported for symptom reduction in acute bronchitis.
Safety
There were no significant adverse events in this study, which is consistent with the findings of the studies of acute bronchitis.12-14P sidoides has been widely used in Germany since the 1980s, with an annual sale value in 2002 of $55 million or 4.1 million packages.
The Uppsala Monitoring Centre, in conjunction with the World Health Organization international pharmacovigilance program, received 34 case reports between 2002 and 2006 of allergic reactions to ethanolic herbal extract of Pelargonium root, 2 of which involved life-threatening circulatory collapse requiring emergency medical attention. Given the extremely rare occurrence of these events we believe the minimal risk is acceptable. The others involved rash and pruritus.
Also of note: contact dermatitis to Pelargonium houseplants has been reported. As a result, product information will be added to product packaging, warning of common reactions of gastrointestinal complaints (gastric pain, heartburn, nausea, and diarrhea) as well as the potential for serious allergic reaction. In addition, since some of the active compounds are plant coumarins, there is a theoretical risk of interaction with warfarin and aspirin but no serious bleeding events have been reported.16
It is also recommended that individuals with renal or hepatic disease or women who are pregnant or breastfeeding avoid use of this preparation, as safety studies have not been performed.
Other study design issues
A few other issues struck us as important when assessing the validity of this study. For example, 1 of the authors appears to be an employee of the pharmaceutical company that manufactures the preparation, raising the conflict of interest issue.
We were also curious about why the results of the high-dose arm were not reported in this manuscript. Could there have been a higher rate of adverse events in the high-dose arm? Knowing how many patients were ineligible or excluded, and the efficacy or safety in the high-dose arm would give us more confidence in the findings, but we decided that these were not necessarily fatal flaws.
Bottom line
Despite the above caveats, this was a well-designed randomized controlled trial that suggests that P sidoides is impressively efficacious in decreasing the duration and severity of the common cold. In the final analysis, we think that these findings justify recommending this to our patients.
CHALLENGES TO IMPLEMENTATION: 2-day window
The medication was started within 48 hours of the onset of symptoms. We generally see patients seeking treatment for the common cold well after the first 2 days. The efficacy of Pelargonium is no doubt less when started later in the course of the illness. Colds resolve spontaneously, so to get the benefit of this treatment, it likely must be started early.
Our conclusion is that patients could be advised to purchase the medication to have on hand at home at the start of the cold season.
Availability of the drug
P sidoides is available in the US under the brand name Umcka Coldcare.
The preparation used in the study is marketed in Europe by ISO-Arzneimittel17 under the name Umckaloabo, which is a combination of the Zulu words for lung symptoms and breast pain.18
Our Internet search on the term “Pelargonium sidoides” failed to yield a distributor of the German preparation used in the study that would be available in the United States. However, a different manufacturer, Nature’s Way, distributes a number of similar preparations containing extract of the root of P sidoides under the name Umcka Coldcare. Umcka Cold-care appears to be readily available for purchase, both on-line and through local health food stores, for less than $20 per 4-oz (120-mL) bottle. A different retailer, African Red Tea, offers syrup, 1:10 ethanolic extract for $29.95 for 100-mL bottle.19 Like the German preparation, these formulations are delivered by dropper.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007;3:573-584.
2. Bladt S, Wagner H. From Zulu medicine to the European phytomedicine Umckaloabo. Phytomedicine 2007;14 (suppl 1):2-4.
3. Matthys H, Eisebitt R, Seith B, Heger M. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2003;10(Suppl 4):7-17.
4. Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs 7630): A randomized, double-blind, placebo-controlled trial. Explore (NY) 2005;1:437-445.
5. Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomized, double-blind, placebo-controlled multicentre study. Curr Med Res Opinion 2007;23:323-331.
6. Kolodziej H, Kiderlen AF. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs 7630. Phytomedicine 2007;14 (suppl 1):18-26.
7. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med 2003;163:487-494.
8. Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361:51-59.
9. Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 Summary. Adv Data 2007;387:1-39.
10. Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2004;(4):cD001831.-
11. Douglas RM, Hemila H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004;(4):cD000980.-
12. Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2006;(1):cD000530.-
13. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 1999;(2):cD001364.-(With-drawn 2006, Issue 3).
14. Sutter AI, Lemiengre M, Campbell H, Mackinnon HF. Anithistamines for the common cold. Cochrane Database Syst Rev 2003;(3):cD001267.-
15. Taverner D, Latte J, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2004;(3):cD001953.-
16. De Boer HJ, Hagemann U, Bate J, Meyboom RHB. Allergic reactions to medicines derived from Pelargonium species. Drug Saf 2007;30:677-680.
17. ISO-Arzneimittel. Distributor of Umckaloabo. Available at: umckaloabo.com. Accessed January 7, 2008.
18. Taylor PW, Maalim S, Coleman S. The strange story of umckaloabo. Pharm J 2005;275:790-792.
19. African Red Tea Imports. Available at: www.africanredtea.com/pelargonium-syrup.html. Accessed January 7, 2008.
1. Lizogub VG, Riley DS, Heger M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 2007;3:573-584.
2. Bladt S, Wagner H. From Zulu medicine to the European phytomedicine Umckaloabo. Phytomedicine 2007;14 (suppl 1):2-4.
3. Matthys H, Eisebitt R, Seith B, Heger M. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2003;10(Suppl 4):7-17.
4. Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs 7630): A randomized, double-blind, placebo-controlled trial. Explore (NY) 2005;1:437-445.
5. Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomized, double-blind, placebo-controlled multicentre study. Curr Med Res Opinion 2007;23:323-331.
6. Kolodziej H, Kiderlen AF. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs 7630. Phytomedicine 2007;14 (suppl 1):18-26.
7. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med 2003;163:487-494.
8. Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361:51-59.
9. Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 Summary. Adv Data 2007;387:1-39.
10. Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2004;(4):cD001831.-
11. Douglas RM, Hemila H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004;(4):cD000980.-
12. Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2006;(1):cD000530.-
13. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 1999;(2):cD001364.-(With-drawn 2006, Issue 3).
14. Sutter AI, Lemiengre M, Campbell H, Mackinnon HF. Anithistamines for the common cold. Cochrane Database Syst Rev 2003;(3):cD001267.-
15. Taverner D, Latte J, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2004;(3):cD001953.-
16. De Boer HJ, Hagemann U, Bate J, Meyboom RHB. Allergic reactions to medicines derived from Pelargonium species. Drug Saf 2007;30:677-680.
17. ISO-Arzneimittel. Distributor of Umckaloabo. Available at: umckaloabo.com. Accessed January 7, 2008.
18. Taylor PW, Maalim S, Coleman S. The strange story of umckaloabo. Pharm J 2005;275:790-792.
19. African Red Tea Imports. Available at: www.africanredtea.com/pelargonium-syrup.html. Accessed January 7, 2008.
Annual zoledronic acid infusion lowers risk of fracture, death
For patients with a recent hip fracture, intravenous zoledronic acid annually is an option for reducing the risk of new fractures and death.1
Strength of recommendation (SOR)
B: based on one well-designed randomized controlled trial
Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357: 1799–1809. Epub Sep 17.
Illustrative Case
A 75-year-old woman comes to see you 1 month after she had surgery to repair a hip fracture. She was diagnosed with osteoporosis 3 years prior to the hip fracture and is currently taking calcium and vitamin D. She tried taking an oral bisphosphonate but couldn’t tolerate the gastrointestinal side effects. What treatment can you recommend to reduce her risk of sustaining another fracture?
Background: First fracture heightens risk
Patients with a prior hip fracture have 2.5 times the risk of a new fracture compared to age-matched persons without a previous hip fracture.2 Women who have hip fractures are 3 times more likely to die in the first 6 months after the fracture than women of the same age and health status without fractures.3 Ten million people in the US have osteoporosis and 300,000 per year suffer hip fracture.4
Guidelines from the National Osteoporosis Foundation (NOF) and the Institute for Clinical Systems Improvement (ICSI) include these recommendations for hip fracture patients: discuss adequacy of total calcium and vitamin D intake; address home safety and falls prevention; and encourage specific exercises for muscle strength. They also recommend treating all patients with a prior hip or vertebral fracture with an antiresorptive agent. Options include oral bisphosphonates (alendronate, ibandronate, or risedronate), calcitonin intranasal spray or subcutaneous calcitonin, hormone therapy, parathyroid hormone, and raloxifene.5,6
Clinical context: Are we doing our best?
Most patients with hip fracture are not properly evaluated or treated for osteoporosis. A 2002 study of 500 hip fracture patients treated at 4 Midwestern health systems found that only 12% to 24% of patients had a DXA (dual-energy x-ray absorptiometry) scan either before or after hip fracture, 5% to 27% of the patients received documented advice to take adequate calcium and vitamin D, and 5% to 37% received a prescription for any antiresorptive medication (bisphosphonate [2% to 10%], estrogen, calcitonin, or raloxifene).7
Bisphosphonates are effective but compliance is poor
Bisphosphonates are effective in preventing recurrence of hip fracture. One cohort study that included over 35,000 women over age 45 who had received a bisphosphonate prescription showed that patients who are adherent to treatment have a 44.5% relative risk reduction over 2 years and an absolute risk reduction of 0.8%, for an NNT of 125.
However, compliance with oral bisphosphonate therapy is poor; only 20% of the women in this study persisted with the therapy for 24 months.8 Patients must take these medications first thing in the morning with 8 ounces of water and then remain upright for 30 to 60 minutes before eating or drinking. Gastrointestinal side effects, including dyspepsia, nausea, and reflux disease, occur in about 25% of patients, and there is a small risk of developing gastric or duodenal ulcers.
Study summary
The HORIZON Recurrent Fracture Trial was an international, randomized, double blind, placebo-controlled trial of 2127 patients with a recent hip fracture.
- The primary endpoint was a new clinical fracture.
- Secondary endpoints included the change in bone mineral density in the non-fractured hip, new vertebral and hip fractures, and pre-specified safety endpoints, including death.
Patients. Women and men age 50 or older who had undergone a surgical repair of a minimal trauma hip fracture in the previous 90 days were eligible for the study. Ninety-one percent of the patients were white, 76% were female, and the mean age was 74.5 years. Forty-one percent of patients had a T score at the femoral neck of –2.5 or less at baseline (meeting diagnostic criteria for osteoporosis).
Method. Patients were randomized to receive either intravenous zoledronic acid 5 mg or placebo within 90 days of surgical repair of a hip fracture and yearly thereafter for the duration of the study. Patients with serum 25-hydroxyvitamin D levels <15 ng/mL received a loading dose of vitamin D (50,000–125,000 IU) 14 days before the first dose of the study drug. All patients were given daily calcium (1000–1500 mg) and vitamin D (800–1200 IU) supplements. Simultaneous treatment with nasal calcitonin, selective estrogen-receptor modulators, hormone replacement, tobolone, and external hip protectors was permitted “at the discretion of the investigators,” and 10.5% of the study patients did receive one of these other osteoporosis therapies.
Patients were followed for up to 5 years. Bone mineral density was tested by DXA at the hip and femoral neck at baseline and then annually. The median follow-up time was 1.9 years; 71.3% of patients completing the trial, and 3% of patients were lost to follow-up.
Results. The patients assigned to zoledronic acid had a 5.3% absolute risk reduction for new clinical fractures, yielding an NNT of 19 over 2 years to prevent one new clinical fracture ( TABLE 1 ). Bone mineral density of the contralateral hip increased in the zoledronic acid group by 2.6% after 1 year, 4.7% after 2 years, and 5.5% after 3 years and declined in the placebo group by 1.0%, 0.7%, and 0.9% respectively.
There was a 3.7% absolute risk reduction of death, with an NNT of 27 for 2 years to prevent one death.
Adverse events that were more common in the zoledronic acid group included fever (8.7% vs 3.1%) and musculoskeletal pain (3.1% vs. 1.2%). There were no reported cases of jaw osteonecrosis in either group and no statistically significant delay in the healing of fractures with zoledronic acid. Both groups had similar rates of renal and cardiovascular events, including atrial fibrillation and stroke.
Novartis provided funding for the study. An independent data and safety monitoring board oversaw the conduct and safety of the study and recommended that it be stopped early after having surpassed the pre-specified efficacy boundaries. Independent statisticians confirmed the data analysis that was performed by the sponsor.1
TABLE 1
The HORIZON study: Rates of fracture and death were lower in the zoledronic group compared to placebo1
| OUTCOME | PLACEBO (N=1062) | ZOLEDRONIC ACID (N=1065) | HAZARD RATIO (95% CI) | P VALUE |
|---|---|---|---|---|
| Any fracture | 139 (13.9%) | 92 (8.6%) | 0.65 (0.50-0.84) | .001 |
| Hip fracture | 33 (3.5%) | 23 (2.0%) | 0.70 (0.41-1.19) | .18 |
| Vertebral fracture | 39 (3.8%) | 21 (1.7%) | 0.54 (0.32–0.92) | .02 |
| Death | 141 (13.3%) | 101 (9.6%) | 0.72 (0.56-0.93) | .01 |
| Rates of fracture were calculated by Kaplan-Meier methods at 24 months and are not simple percentages | ||||
What’s new: The adherence advantage
The obvious advantage of zoledronic acid over other bisphosphonates is the high level of adherence that is possible under the controlled environment of a once yearly infusion administered under medical supervision. Considering the low rates of adherence to oral bisphosphonates, this is a significant medical advance.
This study shows that a yearly infusion of zoledronic acid is highly effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture. It is the first randomized-controlled trial of a bisphosphonate for secondary prevention in patients with recent hip fracture, regardless of their bone mineral density status.
In a previous 3-year randomized controlled trial of 5mg zoledronic acid once yearly vs placebo for postmenopausal women with osteoporosis, the risk of vertebral fractures was reduced by 70% (3.3% vs 10.9% placebo) and the risk of hip fracture was reduced by 41% (1.4% vs 2.5% placebo).9
The NNT of 19 for 2 years to prevent one clinical fracture and NNT of 27 for 2 years to prevent one death strikes us as a good bargain compared to many modern medical interventions. Are these results too good to be true? We don’t think so.
No serious design flaws
This was a well done trial with no serious flaws in design. The number of deaths, however, was relatively small, so the NNT may be as high as 50 by our rough calculations. As a “worst case” for the benefit, however, this still seems worthwhile.
Caveats
Patients with uncorrected hypocalcemia or creatinine clearance <35 mL/min should not take bisphosphonates, including zoledronic acid. Although this study did not show any evidence of an increased risk of atrial fibrillation or report any cases of osteonecrosis of the jaw, providers should monitor patients for these potential adverse events.
Challenges to implementation: $1200 per dose
The FDA approved Reclast (zoledronic acid 5 mg) as a once-yearly treatment for postmenopausal osteoporosis and Paget’s disease in August 2007. (Zometa is the brand name for zoledronic acid 4 mg that is indicated for multiple myeloma, bone metastasis, and hypercalcemia of malignancy.) Medicare and most insurance plans will reimburse Reclast infusion for these FDA-approved indications when billed by a provider using a CPT code. It is administered intravenously over 15-minutes and there are no risks beyond those associated with local infiltration.
The greatest barrier to implementing this practice for solo physicians or small group practices will likely be the up front expense of buying the drug; one dose is approximately $1200. Patients can be referred to larger practices or hospitals with the capital to have zoledronic acid on hand and the capability of providing the infusion.
The cost is comparable to the annual cost of oral bisphosphonates and less than the cost of the other IV bisphosphonate (ibandronate), which is administered every 3 months ( TABLE 2 ).
TABLE 2
Bisphosphonates for osteoporosis: Routes, dosage, and cost
| GENERIC NAME | BRAND NAME | ROUTE OF ADMINISTRATION | DOSE AND FREQUENCY | APPROXIMATE ANNUAL COST |
|---|---|---|---|---|
| Alendronate | Fosamax | Oral | 10 mg/d or 70 mg/week | $960–$1120 |
| Ibandronate | Boniva | Oral or IV | 2.5 mg/d, 150 mg monthly (PO) or 3 mg IV every 3 months | $1140–$1200 PO, $1980 IV |
| Risedronate | Actonel | Oral | 5 mg/d or 35 mg/week | $1000–$1100 |
| Zoledronic acid | Reclast | IV | 5 mg once a year | $1200 |
PURLS methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at here.
1. Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799-1809.Epub Sept 17.
2. Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporosis Int 2003;14:879-883.
3. Empana JP, Dargent-Molina P, Breart G, et al. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc 2004;52:685-690.
4. National Osteoporosis Foundation. About osteoporosis: fast facts. Available at: www.nof.org/osteoporosis/diseasefacts.htm. Accessed on October 4, 2007.
5. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2005.
6. Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of osteoporosis. Bloomington (MN): Institute for Clinical Systems Improvement; 2006.
7. Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum 2002;47:651-654.
8. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 2006;81:1013-1022.
9. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-1822.
For patients with a recent hip fracture, intravenous zoledronic acid annually is an option for reducing the risk of new fractures and death.1
Strength of recommendation (SOR)
B: based on one well-designed randomized controlled trial
Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357: 1799–1809. Epub Sep 17.
Illustrative Case
A 75-year-old woman comes to see you 1 month after she had surgery to repair a hip fracture. She was diagnosed with osteoporosis 3 years prior to the hip fracture and is currently taking calcium and vitamin D. She tried taking an oral bisphosphonate but couldn’t tolerate the gastrointestinal side effects. What treatment can you recommend to reduce her risk of sustaining another fracture?
Background: First fracture heightens risk
Patients with a prior hip fracture have 2.5 times the risk of a new fracture compared to age-matched persons without a previous hip fracture.2 Women who have hip fractures are 3 times more likely to die in the first 6 months after the fracture than women of the same age and health status without fractures.3 Ten million people in the US have osteoporosis and 300,000 per year suffer hip fracture.4
Guidelines from the National Osteoporosis Foundation (NOF) and the Institute for Clinical Systems Improvement (ICSI) include these recommendations for hip fracture patients: discuss adequacy of total calcium and vitamin D intake; address home safety and falls prevention; and encourage specific exercises for muscle strength. They also recommend treating all patients with a prior hip or vertebral fracture with an antiresorptive agent. Options include oral bisphosphonates (alendronate, ibandronate, or risedronate), calcitonin intranasal spray or subcutaneous calcitonin, hormone therapy, parathyroid hormone, and raloxifene.5,6
Clinical context: Are we doing our best?
Most patients with hip fracture are not properly evaluated or treated for osteoporosis. A 2002 study of 500 hip fracture patients treated at 4 Midwestern health systems found that only 12% to 24% of patients had a DXA (dual-energy x-ray absorptiometry) scan either before or after hip fracture, 5% to 27% of the patients received documented advice to take adequate calcium and vitamin D, and 5% to 37% received a prescription for any antiresorptive medication (bisphosphonate [2% to 10%], estrogen, calcitonin, or raloxifene).7
Bisphosphonates are effective but compliance is poor
Bisphosphonates are effective in preventing recurrence of hip fracture. One cohort study that included over 35,000 women over age 45 who had received a bisphosphonate prescription showed that patients who are adherent to treatment have a 44.5% relative risk reduction over 2 years and an absolute risk reduction of 0.8%, for an NNT of 125.
However, compliance with oral bisphosphonate therapy is poor; only 20% of the women in this study persisted with the therapy for 24 months.8 Patients must take these medications first thing in the morning with 8 ounces of water and then remain upright for 30 to 60 minutes before eating or drinking. Gastrointestinal side effects, including dyspepsia, nausea, and reflux disease, occur in about 25% of patients, and there is a small risk of developing gastric or duodenal ulcers.
Study summary
The HORIZON Recurrent Fracture Trial was an international, randomized, double blind, placebo-controlled trial of 2127 patients with a recent hip fracture.
- The primary endpoint was a new clinical fracture.
- Secondary endpoints included the change in bone mineral density in the non-fractured hip, new vertebral and hip fractures, and pre-specified safety endpoints, including death.
Patients. Women and men age 50 or older who had undergone a surgical repair of a minimal trauma hip fracture in the previous 90 days were eligible for the study. Ninety-one percent of the patients were white, 76% were female, and the mean age was 74.5 years. Forty-one percent of patients had a T score at the femoral neck of –2.5 or less at baseline (meeting diagnostic criteria for osteoporosis).
Method. Patients were randomized to receive either intravenous zoledronic acid 5 mg or placebo within 90 days of surgical repair of a hip fracture and yearly thereafter for the duration of the study. Patients with serum 25-hydroxyvitamin D levels <15 ng/mL received a loading dose of vitamin D (50,000–125,000 IU) 14 days before the first dose of the study drug. All patients were given daily calcium (1000–1500 mg) and vitamin D (800–1200 IU) supplements. Simultaneous treatment with nasal calcitonin, selective estrogen-receptor modulators, hormone replacement, tobolone, and external hip protectors was permitted “at the discretion of the investigators,” and 10.5% of the study patients did receive one of these other osteoporosis therapies.
Patients were followed for up to 5 years. Bone mineral density was tested by DXA at the hip and femoral neck at baseline and then annually. The median follow-up time was 1.9 years; 71.3% of patients completing the trial, and 3% of patients were lost to follow-up.
Results. The patients assigned to zoledronic acid had a 5.3% absolute risk reduction for new clinical fractures, yielding an NNT of 19 over 2 years to prevent one new clinical fracture ( TABLE 1 ). Bone mineral density of the contralateral hip increased in the zoledronic acid group by 2.6% after 1 year, 4.7% after 2 years, and 5.5% after 3 years and declined in the placebo group by 1.0%, 0.7%, and 0.9% respectively.
There was a 3.7% absolute risk reduction of death, with an NNT of 27 for 2 years to prevent one death.
Adverse events that were more common in the zoledronic acid group included fever (8.7% vs 3.1%) and musculoskeletal pain (3.1% vs. 1.2%). There were no reported cases of jaw osteonecrosis in either group and no statistically significant delay in the healing of fractures with zoledronic acid. Both groups had similar rates of renal and cardiovascular events, including atrial fibrillation and stroke.
Novartis provided funding for the study. An independent data and safety monitoring board oversaw the conduct and safety of the study and recommended that it be stopped early after having surpassed the pre-specified efficacy boundaries. Independent statisticians confirmed the data analysis that was performed by the sponsor.1
TABLE 1
The HORIZON study: Rates of fracture and death were lower in the zoledronic group compared to placebo1
| OUTCOME | PLACEBO (N=1062) | ZOLEDRONIC ACID (N=1065) | HAZARD RATIO (95% CI) | P VALUE |
|---|---|---|---|---|
| Any fracture | 139 (13.9%) | 92 (8.6%) | 0.65 (0.50-0.84) | .001 |
| Hip fracture | 33 (3.5%) | 23 (2.0%) | 0.70 (0.41-1.19) | .18 |
| Vertebral fracture | 39 (3.8%) | 21 (1.7%) | 0.54 (0.32–0.92) | .02 |
| Death | 141 (13.3%) | 101 (9.6%) | 0.72 (0.56-0.93) | .01 |
| Rates of fracture were calculated by Kaplan-Meier methods at 24 months and are not simple percentages | ||||
What’s new: The adherence advantage
The obvious advantage of zoledronic acid over other bisphosphonates is the high level of adherence that is possible under the controlled environment of a once yearly infusion administered under medical supervision. Considering the low rates of adherence to oral bisphosphonates, this is a significant medical advance.
This study shows that a yearly infusion of zoledronic acid is highly effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture. It is the first randomized-controlled trial of a bisphosphonate for secondary prevention in patients with recent hip fracture, regardless of their bone mineral density status.
In a previous 3-year randomized controlled trial of 5mg zoledronic acid once yearly vs placebo for postmenopausal women with osteoporosis, the risk of vertebral fractures was reduced by 70% (3.3% vs 10.9% placebo) and the risk of hip fracture was reduced by 41% (1.4% vs 2.5% placebo).9
The NNT of 19 for 2 years to prevent one clinical fracture and NNT of 27 for 2 years to prevent one death strikes us as a good bargain compared to many modern medical interventions. Are these results too good to be true? We don’t think so.
No serious design flaws
This was a well done trial with no serious flaws in design. The number of deaths, however, was relatively small, so the NNT may be as high as 50 by our rough calculations. As a “worst case” for the benefit, however, this still seems worthwhile.
Caveats
Patients with uncorrected hypocalcemia or creatinine clearance <35 mL/min should not take bisphosphonates, including zoledronic acid. Although this study did not show any evidence of an increased risk of atrial fibrillation or report any cases of osteonecrosis of the jaw, providers should monitor patients for these potential adverse events.
Challenges to implementation: $1200 per dose
The FDA approved Reclast (zoledronic acid 5 mg) as a once-yearly treatment for postmenopausal osteoporosis and Paget’s disease in August 2007. (Zometa is the brand name for zoledronic acid 4 mg that is indicated for multiple myeloma, bone metastasis, and hypercalcemia of malignancy.) Medicare and most insurance plans will reimburse Reclast infusion for these FDA-approved indications when billed by a provider using a CPT code. It is administered intravenously over 15-minutes and there are no risks beyond those associated with local infiltration.
The greatest barrier to implementing this practice for solo physicians or small group practices will likely be the up front expense of buying the drug; one dose is approximately $1200. Patients can be referred to larger practices or hospitals with the capital to have zoledronic acid on hand and the capability of providing the infusion.
The cost is comparable to the annual cost of oral bisphosphonates and less than the cost of the other IV bisphosphonate (ibandronate), which is administered every 3 months ( TABLE 2 ).
TABLE 2
Bisphosphonates for osteoporosis: Routes, dosage, and cost
| GENERIC NAME | BRAND NAME | ROUTE OF ADMINISTRATION | DOSE AND FREQUENCY | APPROXIMATE ANNUAL COST |
|---|---|---|---|---|
| Alendronate | Fosamax | Oral | 10 mg/d or 70 mg/week | $960–$1120 |
| Ibandronate | Boniva | Oral or IV | 2.5 mg/d, 150 mg monthly (PO) or 3 mg IV every 3 months | $1140–$1200 PO, $1980 IV |
| Risedronate | Actonel | Oral | 5 mg/d or 35 mg/week | $1000–$1100 |
| Zoledronic acid | Reclast | IV | 5 mg once a year | $1200 |
PURLS methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at here.
For patients with a recent hip fracture, intravenous zoledronic acid annually is an option for reducing the risk of new fractures and death.1
Strength of recommendation (SOR)
B: based on one well-designed randomized controlled trial
Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357: 1799–1809. Epub Sep 17.
Illustrative Case
A 75-year-old woman comes to see you 1 month after she had surgery to repair a hip fracture. She was diagnosed with osteoporosis 3 years prior to the hip fracture and is currently taking calcium and vitamin D. She tried taking an oral bisphosphonate but couldn’t tolerate the gastrointestinal side effects. What treatment can you recommend to reduce her risk of sustaining another fracture?
Background: First fracture heightens risk
Patients with a prior hip fracture have 2.5 times the risk of a new fracture compared to age-matched persons without a previous hip fracture.2 Women who have hip fractures are 3 times more likely to die in the first 6 months after the fracture than women of the same age and health status without fractures.3 Ten million people in the US have osteoporosis and 300,000 per year suffer hip fracture.4
Guidelines from the National Osteoporosis Foundation (NOF) and the Institute for Clinical Systems Improvement (ICSI) include these recommendations for hip fracture patients: discuss adequacy of total calcium and vitamin D intake; address home safety and falls prevention; and encourage specific exercises for muscle strength. They also recommend treating all patients with a prior hip or vertebral fracture with an antiresorptive agent. Options include oral bisphosphonates (alendronate, ibandronate, or risedronate), calcitonin intranasal spray or subcutaneous calcitonin, hormone therapy, parathyroid hormone, and raloxifene.5,6
Clinical context: Are we doing our best?
Most patients with hip fracture are not properly evaluated or treated for osteoporosis. A 2002 study of 500 hip fracture patients treated at 4 Midwestern health systems found that only 12% to 24% of patients had a DXA (dual-energy x-ray absorptiometry) scan either before or after hip fracture, 5% to 27% of the patients received documented advice to take adequate calcium and vitamin D, and 5% to 37% received a prescription for any antiresorptive medication (bisphosphonate [2% to 10%], estrogen, calcitonin, or raloxifene).7
Bisphosphonates are effective but compliance is poor
Bisphosphonates are effective in preventing recurrence of hip fracture. One cohort study that included over 35,000 women over age 45 who had received a bisphosphonate prescription showed that patients who are adherent to treatment have a 44.5% relative risk reduction over 2 years and an absolute risk reduction of 0.8%, for an NNT of 125.
However, compliance with oral bisphosphonate therapy is poor; only 20% of the women in this study persisted with the therapy for 24 months.8 Patients must take these medications first thing in the morning with 8 ounces of water and then remain upright for 30 to 60 minutes before eating or drinking. Gastrointestinal side effects, including dyspepsia, nausea, and reflux disease, occur in about 25% of patients, and there is a small risk of developing gastric or duodenal ulcers.
Study summary
The HORIZON Recurrent Fracture Trial was an international, randomized, double blind, placebo-controlled trial of 2127 patients with a recent hip fracture.
- The primary endpoint was a new clinical fracture.
- Secondary endpoints included the change in bone mineral density in the non-fractured hip, new vertebral and hip fractures, and pre-specified safety endpoints, including death.
Patients. Women and men age 50 or older who had undergone a surgical repair of a minimal trauma hip fracture in the previous 90 days were eligible for the study. Ninety-one percent of the patients were white, 76% were female, and the mean age was 74.5 years. Forty-one percent of patients had a T score at the femoral neck of –2.5 or less at baseline (meeting diagnostic criteria for osteoporosis).
Method. Patients were randomized to receive either intravenous zoledronic acid 5 mg or placebo within 90 days of surgical repair of a hip fracture and yearly thereafter for the duration of the study. Patients with serum 25-hydroxyvitamin D levels <15 ng/mL received a loading dose of vitamin D (50,000–125,000 IU) 14 days before the first dose of the study drug. All patients were given daily calcium (1000–1500 mg) and vitamin D (800–1200 IU) supplements. Simultaneous treatment with nasal calcitonin, selective estrogen-receptor modulators, hormone replacement, tobolone, and external hip protectors was permitted “at the discretion of the investigators,” and 10.5% of the study patients did receive one of these other osteoporosis therapies.
Patients were followed for up to 5 years. Bone mineral density was tested by DXA at the hip and femoral neck at baseline and then annually. The median follow-up time was 1.9 years; 71.3% of patients completing the trial, and 3% of patients were lost to follow-up.
Results. The patients assigned to zoledronic acid had a 5.3% absolute risk reduction for new clinical fractures, yielding an NNT of 19 over 2 years to prevent one new clinical fracture ( TABLE 1 ). Bone mineral density of the contralateral hip increased in the zoledronic acid group by 2.6% after 1 year, 4.7% after 2 years, and 5.5% after 3 years and declined in the placebo group by 1.0%, 0.7%, and 0.9% respectively.
There was a 3.7% absolute risk reduction of death, with an NNT of 27 for 2 years to prevent one death.
Adverse events that were more common in the zoledronic acid group included fever (8.7% vs 3.1%) and musculoskeletal pain (3.1% vs. 1.2%). There were no reported cases of jaw osteonecrosis in either group and no statistically significant delay in the healing of fractures with zoledronic acid. Both groups had similar rates of renal and cardiovascular events, including atrial fibrillation and stroke.
Novartis provided funding for the study. An independent data and safety monitoring board oversaw the conduct and safety of the study and recommended that it be stopped early after having surpassed the pre-specified efficacy boundaries. Independent statisticians confirmed the data analysis that was performed by the sponsor.1
TABLE 1
The HORIZON study: Rates of fracture and death were lower in the zoledronic group compared to placebo1
| OUTCOME | PLACEBO (N=1062) | ZOLEDRONIC ACID (N=1065) | HAZARD RATIO (95% CI) | P VALUE |
|---|---|---|---|---|
| Any fracture | 139 (13.9%) | 92 (8.6%) | 0.65 (0.50-0.84) | .001 |
| Hip fracture | 33 (3.5%) | 23 (2.0%) | 0.70 (0.41-1.19) | .18 |
| Vertebral fracture | 39 (3.8%) | 21 (1.7%) | 0.54 (0.32–0.92) | .02 |
| Death | 141 (13.3%) | 101 (9.6%) | 0.72 (0.56-0.93) | .01 |
| Rates of fracture were calculated by Kaplan-Meier methods at 24 months and are not simple percentages | ||||
What’s new: The adherence advantage
The obvious advantage of zoledronic acid over other bisphosphonates is the high level of adherence that is possible under the controlled environment of a once yearly infusion administered under medical supervision. Considering the low rates of adherence to oral bisphosphonates, this is a significant medical advance.
This study shows that a yearly infusion of zoledronic acid is highly effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture. It is the first randomized-controlled trial of a bisphosphonate for secondary prevention in patients with recent hip fracture, regardless of their bone mineral density status.
In a previous 3-year randomized controlled trial of 5mg zoledronic acid once yearly vs placebo for postmenopausal women with osteoporosis, the risk of vertebral fractures was reduced by 70% (3.3% vs 10.9% placebo) and the risk of hip fracture was reduced by 41% (1.4% vs 2.5% placebo).9
The NNT of 19 for 2 years to prevent one clinical fracture and NNT of 27 for 2 years to prevent one death strikes us as a good bargain compared to many modern medical interventions. Are these results too good to be true? We don’t think so.
No serious design flaws
This was a well done trial with no serious flaws in design. The number of deaths, however, was relatively small, so the NNT may be as high as 50 by our rough calculations. As a “worst case” for the benefit, however, this still seems worthwhile.
Caveats
Patients with uncorrected hypocalcemia or creatinine clearance <35 mL/min should not take bisphosphonates, including zoledronic acid. Although this study did not show any evidence of an increased risk of atrial fibrillation or report any cases of osteonecrosis of the jaw, providers should monitor patients for these potential adverse events.
Challenges to implementation: $1200 per dose
The FDA approved Reclast (zoledronic acid 5 mg) as a once-yearly treatment for postmenopausal osteoporosis and Paget’s disease in August 2007. (Zometa is the brand name for zoledronic acid 4 mg that is indicated for multiple myeloma, bone metastasis, and hypercalcemia of malignancy.) Medicare and most insurance plans will reimburse Reclast infusion for these FDA-approved indications when billed by a provider using a CPT code. It is administered intravenously over 15-minutes and there are no risks beyond those associated with local infiltration.
The greatest barrier to implementing this practice for solo physicians or small group practices will likely be the up front expense of buying the drug; one dose is approximately $1200. Patients can be referred to larger practices or hospitals with the capital to have zoledronic acid on hand and the capability of providing the infusion.
The cost is comparable to the annual cost of oral bisphosphonates and less than the cost of the other IV bisphosphonate (ibandronate), which is administered every 3 months ( TABLE 2 ).
TABLE 2
Bisphosphonates for osteoporosis: Routes, dosage, and cost
| GENERIC NAME | BRAND NAME | ROUTE OF ADMINISTRATION | DOSE AND FREQUENCY | APPROXIMATE ANNUAL COST |
|---|---|---|---|---|
| Alendronate | Fosamax | Oral | 10 mg/d or 70 mg/week | $960–$1120 |
| Ibandronate | Boniva | Oral or IV | 2.5 mg/d, 150 mg monthly (PO) or 3 mg IV every 3 months | $1140–$1200 PO, $1980 IV |
| Risedronate | Actonel | Oral | 5 mg/d or 35 mg/week | $1000–$1100 |
| Zoledronic acid | Reclast | IV | 5 mg once a year | $1200 |
PURLS methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at here.
1. Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799-1809.Epub Sept 17.
2. Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporosis Int 2003;14:879-883.
3. Empana JP, Dargent-Molina P, Breart G, et al. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc 2004;52:685-690.
4. National Osteoporosis Foundation. About osteoporosis: fast facts. Available at: www.nof.org/osteoporosis/diseasefacts.htm. Accessed on October 4, 2007.
5. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2005.
6. Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of osteoporosis. Bloomington (MN): Institute for Clinical Systems Improvement; 2006.
7. Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum 2002;47:651-654.
8. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 2006;81:1013-1022.
9. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-1822.
1. Lyles KW, Colon-Emeric CS, Magaziner JF, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799-1809.Epub Sept 17.
2. Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporosis Int 2003;14:879-883.
3. Empana JP, Dargent-Molina P, Breart G, et al. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc 2004;52:685-690.
4. National Osteoporosis Foundation. About osteoporosis: fast facts. Available at: www.nof.org/osteoporosis/diseasefacts.htm. Accessed on October 4, 2007.
5. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2005.
6. Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of osteoporosis. Bloomington (MN): Institute for Clinical Systems Improvement; 2006.
7. Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum 2002;47:651-654.
8. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 2006;81:1013-1022.
9. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-1822.
Copyright © 2007 The Family Physicians Inquiries Network.
All rights reserved.
“Can you call in a Z-Pak for me?”
Frank is a high school history teacher who reads The Wall Street Journal and The New York Times every morning. So I was a bit taken aback when, just as we stepped onto the court for Sunday morning doubles last month, he asked, “Say John, can you call in a Z-Pak to Walgreens for me today?” Being a typical nice-guy family doc, I said with only slight hesitation, “Why sure, Frank. Which Walgreens?”
Because I’d recently written an editorial about the overuse of antibiotics for respiratory infections,1 I felt obliged to lay on a few facts about the dubious value of antibiotics for acute bronchitis, the illness he clearly had, judging from the hacking cough.
“You know, Frank,” I said, “you’ll probably get better just as fast without the Z-Pak.”
To which he replied, “The Walgreens on the corner of Lake Park and 55th.”
Okay, I didn’t win that round. But I have been keeping score for the last 2 weeks. I am batting .800 at the office; 4 of the last 5 patients with a variety of upper respiratory infections have walked out of the office without an antibiotic prescription. In the spirit of full disclosure, however, I should tell you that one patient was pregnant and another gentleman said that his symptoms were already clearing up since he made the appointment for a cough lasting 2 weeks. I could not resist pulling out the pad, however, for a woman with mild depression, hypertension, and chronic back pain (which was flaring), who complained that she’d “been blowing green stuff out of [her] nose for the past three days” and wanted an antibiotic.
Just say no
The science, of course, does not back up this practice and is the reason for the “just say no to antibiotics for acute respiratory infections” campaign being waged by the Centers for Disease Control and Prevention. For years we’ve known that viral upper respiratory infections do not respond to antibiotics. Research during the past 5 years has demonstrated repeatedly and convincingly that acute sinusitis, acute bronchitis, and most cases of pharyngitis and otitis media resolve as rapidly or nearly as rapidly without antibiotics, as well.
Why is this so? These syndromes, too, are predominately viral in origin, and even those caused by bacterial infection resolve nearly as rapidly without an antibiotic. For example, the duration of strep pharyngitis is shortened by only 1 to 2 days with antibiotic treatment, and complications of strep pharyngitis are now rare in the US. It’s reasonable to treat mildly symptomatic cases with supportive care; in fact, that’s the official, evidence-based recommendation in the Netherlands. Nonetheless, despite strong evidence of the limited effectiveness of antibiotics for acute respiratory infections, a recent study shows that 65% of upper respiratory infections, 78% of acute bronchitis, 65% of acute pharyngitis, and 81% of acute sinusitis episodes in primary care are treated with an antibiotic.2
Techniques for turning the tide
How are we to improve our prescribing for acute respiratory infections? First, it’s important to put 1 simple fact on the table: Most acute respiratory infections do not resolve in 1 week. The sad truth is that most acute respiratory infections for which patients consult a physician run their course in 10 to 21 days. Knowing this fact allows me to tell patients who present during the first week of illness that I am not at all surprised they are not yet well.
Next, I tell patients they do not need an antibiotic and that they will recover as rapidly without one. Sometimes this does the trick. For patients who insist that they need an antibiotic, the “wait and see” prescription reduces antibiotic use by about 50%.3 In this approach, one gives the patient a prescription but tells him to fill it only if symptoms do not improve in the next few days.
Two studies published recently in the Annals of Family Medicine provide new evidence for additional approaches. A study of patients’ expectations for treatment of their sore throat revealed that patients’ main purpose in consulting a physician was not to obtain a prescription but to find out the cause of the sore throat and to get pain relief.4 This suggests that aggressive treatment of throat pain might satisfy many patients more than a worthless antibiotic that might cause diarrhea and other adverse effects.
In the second study, researchers performed a secondary analysis of data from an earlier randomized controlled trial and attempted to identify clinical features of acute sinusitis that might predict a good response to antibiotic treatment.5 In the original randomized clinical trial, patients treated with amoxicillin did not improve more rapidly than those treated with placebo.6 In the recent analysis, the investigators found that even the classic symptoms of purulent nasal discharge and facial pain did not predict which patient would improve more rapidly with an antibiotic. This study prompted me to reflect on the futility of giving any patient with sinusitis symptoms an antibiotic, save for those who appear significantly ill or who are at risk for complications. But change comes slowly.
Yesterday I was driving home from Sunday tennis doubles with Frank, my friend who’d requested the Z-Pak, and a fellow player Jerry. We were shooting the breeze when the topic of the high cost of health care came up. After my short treatise on the waste we create with over-testing and over-treatment, Frank piped up, “You know, John, I don’t think that Z-Pak did me any good.”
I had not won the first round with Frank, but I had sowed the seeds of doubt.
1. Hickner J. A new look at an old problem: inappropriate antibiotics for acute respiratory infections. Ann Fam Med 2006;4:484-485.
2. Gill JM, Fleischut P, Haas S, Pellini B, Crawford A, Nash DB. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med 2006;38:349-354.
3. Couchman GR, Rascoe TG, Forjuoh SN. Back-up antibiotic prescriptions for common respiratory symptoms. Patient satisfaction and fill rates. J Fam Pract. 2000;49:907-913.
4. van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med 2006;4:494-499.
5. De Sutter A, Lemiengre M, Van Maele G, et al. Predicting prognosis and effect of antibiotic treatment in rhinosinusitis. Ann Fam Med 2006;4:486-493.
6. De Sutter AI, De Meyere MJ, Christiaens TC, Van Driel ML, Peersman W, DeMaeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323.
Frank is a high school history teacher who reads The Wall Street Journal and The New York Times every morning. So I was a bit taken aback when, just as we stepped onto the court for Sunday morning doubles last month, he asked, “Say John, can you call in a Z-Pak to Walgreens for me today?” Being a typical nice-guy family doc, I said with only slight hesitation, “Why sure, Frank. Which Walgreens?”
Because I’d recently written an editorial about the overuse of antibiotics for respiratory infections,1 I felt obliged to lay on a few facts about the dubious value of antibiotics for acute bronchitis, the illness he clearly had, judging from the hacking cough.
“You know, Frank,” I said, “you’ll probably get better just as fast without the Z-Pak.”
To which he replied, “The Walgreens on the corner of Lake Park and 55th.”
Okay, I didn’t win that round. But I have been keeping score for the last 2 weeks. I am batting .800 at the office; 4 of the last 5 patients with a variety of upper respiratory infections have walked out of the office without an antibiotic prescription. In the spirit of full disclosure, however, I should tell you that one patient was pregnant and another gentleman said that his symptoms were already clearing up since he made the appointment for a cough lasting 2 weeks. I could not resist pulling out the pad, however, for a woman with mild depression, hypertension, and chronic back pain (which was flaring), who complained that she’d “been blowing green stuff out of [her] nose for the past three days” and wanted an antibiotic.
Just say no
The science, of course, does not back up this practice and is the reason for the “just say no to antibiotics for acute respiratory infections” campaign being waged by the Centers for Disease Control and Prevention. For years we’ve known that viral upper respiratory infections do not respond to antibiotics. Research during the past 5 years has demonstrated repeatedly and convincingly that acute sinusitis, acute bronchitis, and most cases of pharyngitis and otitis media resolve as rapidly or nearly as rapidly without antibiotics, as well.
Why is this so? These syndromes, too, are predominately viral in origin, and even those caused by bacterial infection resolve nearly as rapidly without an antibiotic. For example, the duration of strep pharyngitis is shortened by only 1 to 2 days with antibiotic treatment, and complications of strep pharyngitis are now rare in the US. It’s reasonable to treat mildly symptomatic cases with supportive care; in fact, that’s the official, evidence-based recommendation in the Netherlands. Nonetheless, despite strong evidence of the limited effectiveness of antibiotics for acute respiratory infections, a recent study shows that 65% of upper respiratory infections, 78% of acute bronchitis, 65% of acute pharyngitis, and 81% of acute sinusitis episodes in primary care are treated with an antibiotic.2
Techniques for turning the tide
How are we to improve our prescribing for acute respiratory infections? First, it’s important to put 1 simple fact on the table: Most acute respiratory infections do not resolve in 1 week. The sad truth is that most acute respiratory infections for which patients consult a physician run their course in 10 to 21 days. Knowing this fact allows me to tell patients who present during the first week of illness that I am not at all surprised they are not yet well.
Next, I tell patients they do not need an antibiotic and that they will recover as rapidly without one. Sometimes this does the trick. For patients who insist that they need an antibiotic, the “wait and see” prescription reduces antibiotic use by about 50%.3 In this approach, one gives the patient a prescription but tells him to fill it only if symptoms do not improve in the next few days.
Two studies published recently in the Annals of Family Medicine provide new evidence for additional approaches. A study of patients’ expectations for treatment of their sore throat revealed that patients’ main purpose in consulting a physician was not to obtain a prescription but to find out the cause of the sore throat and to get pain relief.4 This suggests that aggressive treatment of throat pain might satisfy many patients more than a worthless antibiotic that might cause diarrhea and other adverse effects.
In the second study, researchers performed a secondary analysis of data from an earlier randomized controlled trial and attempted to identify clinical features of acute sinusitis that might predict a good response to antibiotic treatment.5 In the original randomized clinical trial, patients treated with amoxicillin did not improve more rapidly than those treated with placebo.6 In the recent analysis, the investigators found that even the classic symptoms of purulent nasal discharge and facial pain did not predict which patient would improve more rapidly with an antibiotic. This study prompted me to reflect on the futility of giving any patient with sinusitis symptoms an antibiotic, save for those who appear significantly ill or who are at risk for complications. But change comes slowly.
Yesterday I was driving home from Sunday tennis doubles with Frank, my friend who’d requested the Z-Pak, and a fellow player Jerry. We were shooting the breeze when the topic of the high cost of health care came up. After my short treatise on the waste we create with over-testing and over-treatment, Frank piped up, “You know, John, I don’t think that Z-Pak did me any good.”
I had not won the first round with Frank, but I had sowed the seeds of doubt.
Frank is a high school history teacher who reads The Wall Street Journal and The New York Times every morning. So I was a bit taken aback when, just as we stepped onto the court for Sunday morning doubles last month, he asked, “Say John, can you call in a Z-Pak to Walgreens for me today?” Being a typical nice-guy family doc, I said with only slight hesitation, “Why sure, Frank. Which Walgreens?”
Because I’d recently written an editorial about the overuse of antibiotics for respiratory infections,1 I felt obliged to lay on a few facts about the dubious value of antibiotics for acute bronchitis, the illness he clearly had, judging from the hacking cough.
“You know, Frank,” I said, “you’ll probably get better just as fast without the Z-Pak.”
To which he replied, “The Walgreens on the corner of Lake Park and 55th.”
Okay, I didn’t win that round. But I have been keeping score for the last 2 weeks. I am batting .800 at the office; 4 of the last 5 patients with a variety of upper respiratory infections have walked out of the office without an antibiotic prescription. In the spirit of full disclosure, however, I should tell you that one patient was pregnant and another gentleman said that his symptoms were already clearing up since he made the appointment for a cough lasting 2 weeks. I could not resist pulling out the pad, however, for a woman with mild depression, hypertension, and chronic back pain (which was flaring), who complained that she’d “been blowing green stuff out of [her] nose for the past three days” and wanted an antibiotic.
Just say no
The science, of course, does not back up this practice and is the reason for the “just say no to antibiotics for acute respiratory infections” campaign being waged by the Centers for Disease Control and Prevention. For years we’ve known that viral upper respiratory infections do not respond to antibiotics. Research during the past 5 years has demonstrated repeatedly and convincingly that acute sinusitis, acute bronchitis, and most cases of pharyngitis and otitis media resolve as rapidly or nearly as rapidly without antibiotics, as well.
Why is this so? These syndromes, too, are predominately viral in origin, and even those caused by bacterial infection resolve nearly as rapidly without an antibiotic. For example, the duration of strep pharyngitis is shortened by only 1 to 2 days with antibiotic treatment, and complications of strep pharyngitis are now rare in the US. It’s reasonable to treat mildly symptomatic cases with supportive care; in fact, that’s the official, evidence-based recommendation in the Netherlands. Nonetheless, despite strong evidence of the limited effectiveness of antibiotics for acute respiratory infections, a recent study shows that 65% of upper respiratory infections, 78% of acute bronchitis, 65% of acute pharyngitis, and 81% of acute sinusitis episodes in primary care are treated with an antibiotic.2
Techniques for turning the tide
How are we to improve our prescribing for acute respiratory infections? First, it’s important to put 1 simple fact on the table: Most acute respiratory infections do not resolve in 1 week. The sad truth is that most acute respiratory infections for which patients consult a physician run their course in 10 to 21 days. Knowing this fact allows me to tell patients who present during the first week of illness that I am not at all surprised they are not yet well.
Next, I tell patients they do not need an antibiotic and that they will recover as rapidly without one. Sometimes this does the trick. For patients who insist that they need an antibiotic, the “wait and see” prescription reduces antibiotic use by about 50%.3 In this approach, one gives the patient a prescription but tells him to fill it only if symptoms do not improve in the next few days.
Two studies published recently in the Annals of Family Medicine provide new evidence for additional approaches. A study of patients’ expectations for treatment of their sore throat revealed that patients’ main purpose in consulting a physician was not to obtain a prescription but to find out the cause of the sore throat and to get pain relief.4 This suggests that aggressive treatment of throat pain might satisfy many patients more than a worthless antibiotic that might cause diarrhea and other adverse effects.
In the second study, researchers performed a secondary analysis of data from an earlier randomized controlled trial and attempted to identify clinical features of acute sinusitis that might predict a good response to antibiotic treatment.5 In the original randomized clinical trial, patients treated with amoxicillin did not improve more rapidly than those treated with placebo.6 In the recent analysis, the investigators found that even the classic symptoms of purulent nasal discharge and facial pain did not predict which patient would improve more rapidly with an antibiotic. This study prompted me to reflect on the futility of giving any patient with sinusitis symptoms an antibiotic, save for those who appear significantly ill or who are at risk for complications. But change comes slowly.
Yesterday I was driving home from Sunday tennis doubles with Frank, my friend who’d requested the Z-Pak, and a fellow player Jerry. We were shooting the breeze when the topic of the high cost of health care came up. After my short treatise on the waste we create with over-testing and over-treatment, Frank piped up, “You know, John, I don’t think that Z-Pak did me any good.”
I had not won the first round with Frank, but I had sowed the seeds of doubt.
1. Hickner J. A new look at an old problem: inappropriate antibiotics for acute respiratory infections. Ann Fam Med 2006;4:484-485.
2. Gill JM, Fleischut P, Haas S, Pellini B, Crawford A, Nash DB. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med 2006;38:349-354.
3. Couchman GR, Rascoe TG, Forjuoh SN. Back-up antibiotic prescriptions for common respiratory symptoms. Patient satisfaction and fill rates. J Fam Pract. 2000;49:907-913.
4. van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med 2006;4:494-499.
5. De Sutter A, Lemiengre M, Van Maele G, et al. Predicting prognosis and effect of antibiotic treatment in rhinosinusitis. Ann Fam Med 2006;4:486-493.
6. De Sutter AI, De Meyere MJ, Christiaens TC, Van Driel ML, Peersman W, DeMaeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323.
1. Hickner J. A new look at an old problem: inappropriate antibiotics for acute respiratory infections. Ann Fam Med 2006;4:484-485.
2. Gill JM, Fleischut P, Haas S, Pellini B, Crawford A, Nash DB. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med 2006;38:349-354.
3. Couchman GR, Rascoe TG, Forjuoh SN. Back-up antibiotic prescriptions for common respiratory symptoms. Patient satisfaction and fill rates. J Fam Pract. 2000;49:907-913.
4. van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med 2006;4:494-499.
5. De Sutter A, Lemiengre M, Van Maele G, et al. Predicting prognosis and effect of antibiotic treatment in rhinosinusitis. Ann Fam Med 2006;4:486-493.
6. De Sutter AI, De Meyere MJ, Christiaens TC, Van Driel ML, Peersman W, DeMaeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323.
Acute sinusitis, antibiotics, and the Holy Grail
In 1998 the British Journal of General Practice published a study by W. Stalman1 entitled “The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice?” In this Dutch randomized trial, doxycycline was no more effective than placebo for clinically diagnosed acute sinusitis. Stallman’s trial was unique, because the diagnosis of acute sinusitis was based on clinical findings rather than xrays, computed tomography (CT) scans, or sinus puncture and culture—the gold standard for diagnosing bacterial sinusitis. He based the diagnosis on clinical findings because that is how we practice in family medicine. We rarely use x-rays and CT scans to diagnose sinusitis. X-ray findings are not all that accurate,2 and CT scans are expensive, not immediately available, and should be reserved for difficult cases.
Since Stallman’s study, 2 more randomized placebo-controlled trials of antibiotic treatment for sinusitis-like symptoms in general practice— one of amoxicillin and the other of amoxicillin-clavulanate—found no benefit.3,4 Even for patients with “x-ray confirmed” acute sinusitis in general practice, a randomized trial showed no benefit for amoxicillin.5
In this issue of The journal of family practice, Merenstein et al6 report the results of a similar randomized trial of clinically diagnosed sinusitis in primary care, showing once again that antibiotics are not very effective for treating sinusitislike symptoms, even for patients who have had symptoms for at least 7 days. At 14 days follow-up, 32 (48%) of the amoxicillin group vs 25 (37%) of the placebo group were cured, a nonsignificant difference. Even assuming that this 11% absolute difference was statistically significant—which might have occurred with a larger study—the number needed to treat would be 9. That is a lot of unnecessary antibiotic prescribing. Most readers of JFP will be familiar with the reason for the disappointing outcome of Merenstein’s and others sinusitis antibiotic treatment trials in primary care. That is, most cases of clinically diagnosed acute sinusitis are caused by viruses and do not respond to antibiotics. Furthermore, there is no reliable way clinically to distinguish viral from bacterial sinus infection; there is too much overlap in symptoms.
We now have 5 good placebo-controlled randomized trials of antibiotic treatment of clinically diagnosed acute sinusitis, all with negative results. This approaches “A” level of evidence. Is it time we “just say no” to treating sinusitis-like complaints with antibiotics? Was Stalman right? Is this the end of the story? Not quite, in my opinion.
For the most part, I agree that being stingy with antibiotics for patients with sinusitis-like complaints is a good thing, especially patients with mild symptoms. Most patients will have viral infections and will recover as quickly with symptomatic treatment. But we know that some patients we see do have acute bacterial sinusitis and will benefit from an antibiotic. We just don’t know who they are. On average, patients with acute sinusitis and air-fluid levels on CT scan improve more quickly with antibiotics.7 This point brings me back to the Merenstein study. The investigators noted that, despite similar cure rates at 14 days, the amoxicillin group improved more rapidly, and the time to complete improvement for those who fully recovered by 14 days was 8.1 days for the amoxicillin group and 10.7 days for the placebo group. This suggests to me there are “antibiotic responsive” cases buried in the group of patients in this study. I can’t get too excited about this, however, because patients’ average symptom severity ratings at 3, 7, and 14 days were not significantly different in the amoxicillin and placebo groups (as noted in Merenstein’s Table 2) Nonetheless, it is not yet time to give up the search for identifying “antibiotic responsive” acute sinusitis.
Here is another piece of evidence to support this assertion. Hansen found that patients with a clinical diagnosis of acute sinusitis who had significant facial pain did benefit from penicillin.8 As soon as 3 days after treatment started, pain was significantly diminished in the penicillin treated group compared with placebo.
What we really need is a reliable, easy, and inexpensive point-of-care test to distinguish viral from bacterial sinusitis—the “strep screen” for acute sinusitis. That test is the Holy Grail of acute sinusitis treatment. CT scans are too expensive, and plain x-rays and sinus transillumination are not accurate enough.2 The Europeans have had limited success with C-reactive protein, but the predictive value is not terrific. I expect that a clever microbiologist will eventually discover the right test. Then we can target antibiotic treatment to those who will really benefit.
In the meantime, I recommend following the CDC principles of appropriate antibiotic use for acute rhinosinusitis.9 Temporize at least a week for those who are not that ill, treat pain, and do not hesitate to use an antibiotic for those with more severe, classical symptoms of acute bacterial sinusitis—maxillary pain and purulent (not green or yellow) nasal discharge. Despite concerns about antibiotic resistance, amoxicillin is still a good initial choice, reserving broad-spectrum antibiotics for resistant cases. JFP has published 3 “Clinical Inquires” that nicely summarize the current scientific evidence for diagnosis and treatment of acute sinusitis.10,11,12
1. Stalman W, van Essen GA, van der Graaf Y, de Melker RA. The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebocontrolled double-blind randomized doxycycline trial [see comments]. British J Gen Pract 1997;47:794-799
2. Zucher D, Balk E, Engels E, et al. Diagnosis and Treatment of Acute Bacterial Sinusitis. Publication no 99-E016: Evidence report/technology assessment number 9. Rockville, Md: Agency for Health Care Policy and Research, 1999. Available at www.ncbi.nlm.nih.gov/ books/bv.fcgi?rid=hstat1.chapter.13219.
3. DeSutter Al, DeMeyere MJ, Christiaens TC, Van Drie ML, Peersman W, DE, Maeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323
4. Bucher HC, Tschudi P, Young J, et al. Effect of amoxicillinclavulanate in clinically diagnosed acute rhinosinusitis: a placebo-controlled, double-blind, randomized trial in general practice. Arch Intern Med 2003;163:1793-1798
5. van Buchem FL, Knottnerus JA, Schrijnemaekers VJ, Peeters MF. Primary-care-based randomised placebo-controlled trial of antibiotic treatment in acute maxillary sinusitis [see comments]. Lancet 1997;349:683-687
6. Merenstein D, Whittaker C, Chadwell T, Wegner B, D’Amico F. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract 2005;54:144-152
7. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults [see comments]. BMJ 1996;313:325-329
8. Hansen JG, Schmidt H, Grinsted P. Randomized, double blind, placebo controlled trial of penicillin Vi in the treatment of actue maxillary sinusitis in adults in general practice. Scan J Prim Health Care 2000;18:44-47
9. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505
10. Reider JM, Nashelsky J, Neher J. Do imaging studies aid diagnosis of acute sinusitis? J Fam Pract 2003;52:565-567
11. DeAlleaume L, Parker S, Reider JM. What findings distinguish acute bacterial sinusitis? J Fam Pract 2003;52:563-565
12. Theis J, Oubichon T, Barnette DJ. Are antibiotics helpful for acute maxillary sinusitis? J Fam Pract 2003;52:490-492
In 1998 the British Journal of General Practice published a study by W. Stalman1 entitled “The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice?” In this Dutch randomized trial, doxycycline was no more effective than placebo for clinically diagnosed acute sinusitis. Stallman’s trial was unique, because the diagnosis of acute sinusitis was based on clinical findings rather than xrays, computed tomography (CT) scans, or sinus puncture and culture—the gold standard for diagnosing bacterial sinusitis. He based the diagnosis on clinical findings because that is how we practice in family medicine. We rarely use x-rays and CT scans to diagnose sinusitis. X-ray findings are not all that accurate,2 and CT scans are expensive, not immediately available, and should be reserved for difficult cases.
Since Stallman’s study, 2 more randomized placebo-controlled trials of antibiotic treatment for sinusitis-like symptoms in general practice— one of amoxicillin and the other of amoxicillin-clavulanate—found no benefit.3,4 Even for patients with “x-ray confirmed” acute sinusitis in general practice, a randomized trial showed no benefit for amoxicillin.5
In this issue of The journal of family practice, Merenstein et al6 report the results of a similar randomized trial of clinically diagnosed sinusitis in primary care, showing once again that antibiotics are not very effective for treating sinusitislike symptoms, even for patients who have had symptoms for at least 7 days. At 14 days follow-up, 32 (48%) of the amoxicillin group vs 25 (37%) of the placebo group were cured, a nonsignificant difference. Even assuming that this 11% absolute difference was statistically significant—which might have occurred with a larger study—the number needed to treat would be 9. That is a lot of unnecessary antibiotic prescribing. Most readers of JFP will be familiar with the reason for the disappointing outcome of Merenstein’s and others sinusitis antibiotic treatment trials in primary care. That is, most cases of clinically diagnosed acute sinusitis are caused by viruses and do not respond to antibiotics. Furthermore, there is no reliable way clinically to distinguish viral from bacterial sinus infection; there is too much overlap in symptoms.
We now have 5 good placebo-controlled randomized trials of antibiotic treatment of clinically diagnosed acute sinusitis, all with negative results. This approaches “A” level of evidence. Is it time we “just say no” to treating sinusitis-like complaints with antibiotics? Was Stalman right? Is this the end of the story? Not quite, in my opinion.
For the most part, I agree that being stingy with antibiotics for patients with sinusitis-like complaints is a good thing, especially patients with mild symptoms. Most patients will have viral infections and will recover as quickly with symptomatic treatment. But we know that some patients we see do have acute bacterial sinusitis and will benefit from an antibiotic. We just don’t know who they are. On average, patients with acute sinusitis and air-fluid levels on CT scan improve more quickly with antibiotics.7 This point brings me back to the Merenstein study. The investigators noted that, despite similar cure rates at 14 days, the amoxicillin group improved more rapidly, and the time to complete improvement for those who fully recovered by 14 days was 8.1 days for the amoxicillin group and 10.7 days for the placebo group. This suggests to me there are “antibiotic responsive” cases buried in the group of patients in this study. I can’t get too excited about this, however, because patients’ average symptom severity ratings at 3, 7, and 14 days were not significantly different in the amoxicillin and placebo groups (as noted in Merenstein’s Table 2) Nonetheless, it is not yet time to give up the search for identifying “antibiotic responsive” acute sinusitis.
Here is another piece of evidence to support this assertion. Hansen found that patients with a clinical diagnosis of acute sinusitis who had significant facial pain did benefit from penicillin.8 As soon as 3 days after treatment started, pain was significantly diminished in the penicillin treated group compared with placebo.
What we really need is a reliable, easy, and inexpensive point-of-care test to distinguish viral from bacterial sinusitis—the “strep screen” for acute sinusitis. That test is the Holy Grail of acute sinusitis treatment. CT scans are too expensive, and plain x-rays and sinus transillumination are not accurate enough.2 The Europeans have had limited success with C-reactive protein, but the predictive value is not terrific. I expect that a clever microbiologist will eventually discover the right test. Then we can target antibiotic treatment to those who will really benefit.
In the meantime, I recommend following the CDC principles of appropriate antibiotic use for acute rhinosinusitis.9 Temporize at least a week for those who are not that ill, treat pain, and do not hesitate to use an antibiotic for those with more severe, classical symptoms of acute bacterial sinusitis—maxillary pain and purulent (not green or yellow) nasal discharge. Despite concerns about antibiotic resistance, amoxicillin is still a good initial choice, reserving broad-spectrum antibiotics for resistant cases. JFP has published 3 “Clinical Inquires” that nicely summarize the current scientific evidence for diagnosis and treatment of acute sinusitis.10,11,12
In 1998 the British Journal of General Practice published a study by W. Stalman1 entitled “The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice?” In this Dutch randomized trial, doxycycline was no more effective than placebo for clinically diagnosed acute sinusitis. Stallman’s trial was unique, because the diagnosis of acute sinusitis was based on clinical findings rather than xrays, computed tomography (CT) scans, or sinus puncture and culture—the gold standard for diagnosing bacterial sinusitis. He based the diagnosis on clinical findings because that is how we practice in family medicine. We rarely use x-rays and CT scans to diagnose sinusitis. X-ray findings are not all that accurate,2 and CT scans are expensive, not immediately available, and should be reserved for difficult cases.
Since Stallman’s study, 2 more randomized placebo-controlled trials of antibiotic treatment for sinusitis-like symptoms in general practice— one of amoxicillin and the other of amoxicillin-clavulanate—found no benefit.3,4 Even for patients with “x-ray confirmed” acute sinusitis in general practice, a randomized trial showed no benefit for amoxicillin.5
In this issue of The journal of family practice, Merenstein et al6 report the results of a similar randomized trial of clinically diagnosed sinusitis in primary care, showing once again that antibiotics are not very effective for treating sinusitislike symptoms, even for patients who have had symptoms for at least 7 days. At 14 days follow-up, 32 (48%) of the amoxicillin group vs 25 (37%) of the placebo group were cured, a nonsignificant difference. Even assuming that this 11% absolute difference was statistically significant—which might have occurred with a larger study—the number needed to treat would be 9. That is a lot of unnecessary antibiotic prescribing. Most readers of JFP will be familiar with the reason for the disappointing outcome of Merenstein’s and others sinusitis antibiotic treatment trials in primary care. That is, most cases of clinically diagnosed acute sinusitis are caused by viruses and do not respond to antibiotics. Furthermore, there is no reliable way clinically to distinguish viral from bacterial sinus infection; there is too much overlap in symptoms.
We now have 5 good placebo-controlled randomized trials of antibiotic treatment of clinically diagnosed acute sinusitis, all with negative results. This approaches “A” level of evidence. Is it time we “just say no” to treating sinusitis-like complaints with antibiotics? Was Stalman right? Is this the end of the story? Not quite, in my opinion.
For the most part, I agree that being stingy with antibiotics for patients with sinusitis-like complaints is a good thing, especially patients with mild symptoms. Most patients will have viral infections and will recover as quickly with symptomatic treatment. But we know that some patients we see do have acute bacterial sinusitis and will benefit from an antibiotic. We just don’t know who they are. On average, patients with acute sinusitis and air-fluid levels on CT scan improve more quickly with antibiotics.7 This point brings me back to the Merenstein study. The investigators noted that, despite similar cure rates at 14 days, the amoxicillin group improved more rapidly, and the time to complete improvement for those who fully recovered by 14 days was 8.1 days for the amoxicillin group and 10.7 days for the placebo group. This suggests to me there are “antibiotic responsive” cases buried in the group of patients in this study. I can’t get too excited about this, however, because patients’ average symptom severity ratings at 3, 7, and 14 days were not significantly different in the amoxicillin and placebo groups (as noted in Merenstein’s Table 2) Nonetheless, it is not yet time to give up the search for identifying “antibiotic responsive” acute sinusitis.
Here is another piece of evidence to support this assertion. Hansen found that patients with a clinical diagnosis of acute sinusitis who had significant facial pain did benefit from penicillin.8 As soon as 3 days after treatment started, pain was significantly diminished in the penicillin treated group compared with placebo.
What we really need is a reliable, easy, and inexpensive point-of-care test to distinguish viral from bacterial sinusitis—the “strep screen” for acute sinusitis. That test is the Holy Grail of acute sinusitis treatment. CT scans are too expensive, and plain x-rays and sinus transillumination are not accurate enough.2 The Europeans have had limited success with C-reactive protein, but the predictive value is not terrific. I expect that a clever microbiologist will eventually discover the right test. Then we can target antibiotic treatment to those who will really benefit.
In the meantime, I recommend following the CDC principles of appropriate antibiotic use for acute rhinosinusitis.9 Temporize at least a week for those who are not that ill, treat pain, and do not hesitate to use an antibiotic for those with more severe, classical symptoms of acute bacterial sinusitis—maxillary pain and purulent (not green or yellow) nasal discharge. Despite concerns about antibiotic resistance, amoxicillin is still a good initial choice, reserving broad-spectrum antibiotics for resistant cases. JFP has published 3 “Clinical Inquires” that nicely summarize the current scientific evidence for diagnosis and treatment of acute sinusitis.10,11,12
1. Stalman W, van Essen GA, van der Graaf Y, de Melker RA. The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebocontrolled double-blind randomized doxycycline trial [see comments]. British J Gen Pract 1997;47:794-799
2. Zucher D, Balk E, Engels E, et al. Diagnosis and Treatment of Acute Bacterial Sinusitis. Publication no 99-E016: Evidence report/technology assessment number 9. Rockville, Md: Agency for Health Care Policy and Research, 1999. Available at www.ncbi.nlm.nih.gov/ books/bv.fcgi?rid=hstat1.chapter.13219.
3. DeSutter Al, DeMeyere MJ, Christiaens TC, Van Drie ML, Peersman W, DE, Maeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323
4. Bucher HC, Tschudi P, Young J, et al. Effect of amoxicillinclavulanate in clinically diagnosed acute rhinosinusitis: a placebo-controlled, double-blind, randomized trial in general practice. Arch Intern Med 2003;163:1793-1798
5. van Buchem FL, Knottnerus JA, Schrijnemaekers VJ, Peeters MF. Primary-care-based randomised placebo-controlled trial of antibiotic treatment in acute maxillary sinusitis [see comments]. Lancet 1997;349:683-687
6. Merenstein D, Whittaker C, Chadwell T, Wegner B, D’Amico F. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract 2005;54:144-152
7. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults [see comments]. BMJ 1996;313:325-329
8. Hansen JG, Schmidt H, Grinsted P. Randomized, double blind, placebo controlled trial of penicillin Vi in the treatment of actue maxillary sinusitis in adults in general practice. Scan J Prim Health Care 2000;18:44-47
9. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505
10. Reider JM, Nashelsky J, Neher J. Do imaging studies aid diagnosis of acute sinusitis? J Fam Pract 2003;52:565-567
11. DeAlleaume L, Parker S, Reider JM. What findings distinguish acute bacterial sinusitis? J Fam Pract 2003;52:563-565
12. Theis J, Oubichon T, Barnette DJ. Are antibiotics helpful for acute maxillary sinusitis? J Fam Pract 2003;52:490-492
1. Stalman W, van Essen GA, van der Graaf Y, de Melker RA. The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebocontrolled double-blind randomized doxycycline trial [see comments]. British J Gen Pract 1997;47:794-799
2. Zucher D, Balk E, Engels E, et al. Diagnosis and Treatment of Acute Bacterial Sinusitis. Publication no 99-E016: Evidence report/technology assessment number 9. Rockville, Md: Agency for Health Care Policy and Research, 1999. Available at www.ncbi.nlm.nih.gov/ books/bv.fcgi?rid=hstat1.chapter.13219.
3. DeSutter Al, DeMeyere MJ, Christiaens TC, Van Drie ML, Peersman W, DE, Maeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract 2002;51:317-323
4. Bucher HC, Tschudi P, Young J, et al. Effect of amoxicillinclavulanate in clinically diagnosed acute rhinosinusitis: a placebo-controlled, double-blind, randomized trial in general practice. Arch Intern Med 2003;163:1793-1798
5. van Buchem FL, Knottnerus JA, Schrijnemaekers VJ, Peeters MF. Primary-care-based randomised placebo-controlled trial of antibiotic treatment in acute maxillary sinusitis [see comments]. Lancet 1997;349:683-687
6. Merenstein D, Whittaker C, Chadwell T, Wegner B, D’Amico F. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract 2005;54:144-152
7. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults [see comments]. BMJ 1996;313:325-329
8. Hansen JG, Schmidt H, Grinsted P. Randomized, double blind, placebo controlled trial of penicillin Vi in the treatment of actue maxillary sinusitis in adults in general practice. Scan J Prim Health Care 2000;18:44-47
9. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505
10. Reider JM, Nashelsky J, Neher J. Do imaging studies aid diagnosis of acute sinusitis? J Fam Pract 2003;52:565-567
11. DeAlleaume L, Parker S, Reider JM. What findings distinguish acute bacterial sinusitis? J Fam Pract 2003;52:563-565
12. Theis J, Oubichon T, Barnette DJ. Are antibiotics helpful for acute maxillary sinusitis? J Fam Pract 2003;52:490-492