User login
5-year-old boy • behavioral issues • elevated ALT and AST levels • Dx?
THE CASE
A 5-year-old boy was brought into his primary care clinic by his mother, who expressed concern about her son’s increasing impulsiveness, aggression, and difficulty staying on task at preschool and at home. The child’s medical history was unremarkable, and he was taking no medications. The family history was negative for hepatic or metabolic disease and positive for attention deficit-hyperactivity disorder (ADHD; father).
The child’s growth was normal. His physical exam was remarkable for a liver edge 1 cm below his costal margin. No Kayser-Fleischer rings were present.
Screening included a complete metabolic panel. Notable results included an alanine aminotransferase (ALT) level of 208 U/dL (normal range, < 30 U/dL), an aspartate transaminase (AST) level of 125 U/dL (normal range, 10-34 U/dL), and an alkaline phosphatase (ALP) of 470 U/dL (normal range, 93-309 U/dL). Subsequent repeat laboratory testing confirmed these elevations (ALT, 248 U/dL; AST, 137 U/dL; ALP, 462 U/dL). Ceruloplasmin levels were low (11 mg/dL; normal range, 18-35 mg/dL), and 24-hour urinary copper was not obtainable. Prothrombin/partial thromboplastin time, ammonia, lactate, total and direct bilirubin, and gamma-glutamyltransferase levels were normal.
Further evaluation included abdominal ultrasound and brain magnetic resonance imaging, both of which yielded normal results. Testing for Epstein-Barr virus; cytomegalovirus; hepatitis A, B, and C titers; and antinuclear, anti-smooth muscle, and anti–liver-kidney microsomal antibodies was negative.
THE DIAGNOSIS
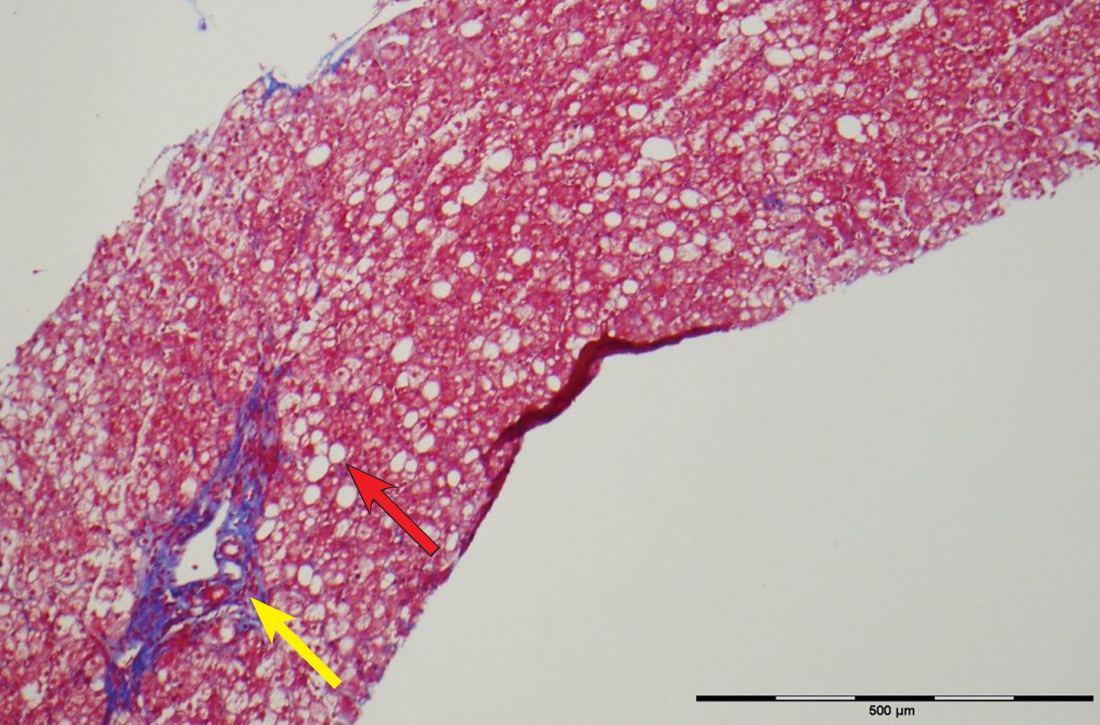
The patient’s low ceruloplasmin prompted referral to Pediatric Gastroenterology for consultation and liver biopsy due to concern for Wilson disease. Biopsy results were consistent with, and quantitative liver copper confirmatory for, this diagnosis (FIGURE).
Genetic testing for mutations in the ATP7B gene was performed on the patient, his mother, and his siblings (his father was unavailable). The patient, his mother, and his sister were all positive for His1069Gln mutation; only the patient was positive for a 3990_3993 del mutation (his half-brother was negative for both mutations). The presence of 2 different mutant alleles for the ATP7B gene, one on each chromosome—the common substitution mutation, His1069Gln, in exon 14 and a 3990_3993 del TTAT mutation in exon 19—qualified the patient as a compound heterozygote.
The 3990_3993 del TTAT mutation—which to our knowledge has not been previously reported—produced a translational frame shift and premature stop codon. As others have pointed out, frame shift and missense mutations produce a more severe phenotype.1
Continue to: Further testing was prompted...
Further testing was prompted by a report suggesting that codon 129 mutations of the human prion gene (HPG) influence Wilson disease.2 Compared with patients who are heterozygous (M129V) or homozygous (V129V) for valine, those who are homozygous for methionine (M129M) have delayed symptom onset.2 Our patient was heterozygous (M129V). It is interesting to speculate that HPG heterozygosity, combined with a mutation causing a stop codon, predisposed our patient to more rapid accumulation of copper and earlier age of onset.
DISCUSSION
Wilson disease is an inherited disorder of copper metabolism.3 An inherent difficulty in its recognition, diagnosis, and management is its rarity: global prevalence is estimated as 1/30,000, although this varies by geographic location.1 In contrast, ADHD has a prevalence of 7.2%,4 making it 2400 times more prevalent than Wilson disease. Furthermore, abnormal liver function tests are common in children; the differential diagnosis includes etiologies such as infection (both viral and nonviral), immune-mediated inflammatory disease, drug toxicity (iatrogenic or medication-induced), anatomic abnormalities, and nonalcoholic fatty liver disease.5
Wilson disease is remarkable, however, for being easily treatable if detected and devastating if not. Although liver abnormalities often improve with treatment, delayed diagnosis and management significantly impact neurologic recovery: An 18-month delay results in 38% of patients continuing to have major neurologic disabilities.6 Untreated, Wilson disease may be fatal within 5 years of development of neurologic symptoms.7 Thus, it has been suggested that evaluation for Wilson disease be considered in any child older than 1 year who presents with unexplained liver disease, including asymptomatic elevations of serum transaminases.8
Mutations in ATP7B on chromosome 13 are responsible for the pathology of Wilson disease9; more than 250 mutations have been identified, including substitutions, deletions, and missense mutations.10 Affected patients may be compound heterozygotes11 and/or may possess new mutations, as seen in our patient.
Although copper absorption is normal, impaired excretion causes toxic accumulation in affected organs. ATP7B’s product, ATPase 2, regulates copper excretion, as well as copper binding to apoceruloplasmin to form the carrier protein ceruloplasmin. An ATP7B abnormality would prevent the latter—making ceruloplasmin a useful screening biomarker and a reliable marker for Wilson disease by age 1 year.8
Continue to: Hepatic and neurocognitive effects
Hepatic and neurocognitive effects. Excess copper in hepatocytes causes oxidative damage and release of copper into the circulation, with accumulation in susceptible organs (eg, brain, kidneys). Hepatocyte apoptosis is accelerated by copper’s negative effect on inhibitor of apoptosis protein.12,13 Renal tubular damage leads to Fanconi syndrome,14 in which substances such as glucose, phosphates, and potassium are excreted in urine rather than absorbed into the bloodstream by the kidneys. Excess copper deposition in the Descemet membrane may lead to Kayser-Fleisher ring formation.15 In the brain, copper deposition may occur in the lenticular nuclei,3 as well as in the thalamus, subthalamus, brainstem, and frontal cortex—resulting in extrapyramidal, cerebral, and mild cerebellar symptoms.6
Cognitive impairment, which may be subtle, includes increased impulsivity, impaired judgment, apathy, poor decision making, decreased attention, increased lability, slowed thinking, and memory loss.6 Behavioral manifestations include changes in school or work performance and outbursts mimicking ADHD12,16,17 as well as paranoia, depression, and bizarre behaviors.16,18 Neuropsychiatric abnormalities include personality changes, pseudoparkinsonism, dyskinesia/dysarthria, and ataxia/tremor. Younger patients with psychiatric symptoms may be labelled with depression, anxiety, obsessive-compulsive disorder, bipolar disorder, or antisocial disorder.6,16,18
Hepatic disease manifestations range from asymptomatic elevations in AST/ALT to acute hepatitis, mimicking infectious processes. Cirrhosis is the end result of untreated Wilson disease, with liver transplantation required if end-stage liver disease results. Rarely, patients present in fulminant hepatic failure, with death occurring if emergent liver transplantation is not performed.6,8,10
Of note, before age 10, > 80% of patients with Wilson disease present with hepatic symptoms; those ages 10 to 18 often manifest psychiatric changes.17 Kayser-Fleisher rings are common in patients with neurologic manifestations but less so in those who have hepatic presentations or are presymptomatic.6,15
Effective disease-mitigating treatment is indicated and available for both symptomatic and asymptomatic individuals and includes the copper chelators D-penicillamine (starting dose, 150-300 mg/d with a gradual weekly increase to 20 mg/kg/d) and trientine hydrochloride (a heavy metal chelating compound; starting dose, 20 mg/kg/d to a maximum of 1000 mg/d in young adults). Adverse effects of D-penicillamine include cutaneous eruptions, neutropenia, thrombocytopenia, proteinuria, and a lupus-like syndrome; therefore, trientine is increasingly being used as first-line therapy.8
Continue to: For asymptomatic...
For asymptomatic patients who have had effective chelation therapy and proven de-coppering, zinc salts are a useful follow-on therapy. Zinc’s proposed mechanism of action is induction of metallothionein in enterocytes, which promotes copper trapping and eventual excretion into the lumen. Importantly, treatment for Wilson disease is lifelong and monitoring of compliance is essential.8
Our 5-year-old patient was started on oral trientine at 20 mg/kg/d and a low copper diet. In response to this initial treatment, the patient’s liver function tests (LFTs) normalized, and he was switched to 25 mg tid of a zinc chelate, with continuation of the low copper diet. His LFTs have remained normal, although his urine copper levels are still elevated. He continues to be monitored periodically with LFTs and measurement of urine copper levels. He is also being treated for ADHD, as his presenting behavioral abnormalities suggestive of ADHD have not resolved.
THE TAKEAWAY
Although children presenting with symptoms consistent with ADHD often have ADHD, as was true in this case, it is important to consider other diagnoses. Unexplained elevations of liver function test values in children older than 1 year should prompt screening for Wilson disease.5,8 Additionally, other family members should be evaluated; if they have the disease, treatment should be started by age 2 years, even if the patient is asymptomatic.
In our patient’s case, routine screening saved the day. The complete metabolic panel revealed elevated ALT and AST levels, prompting further evaluation. Without this testing, his diagnosis likely would have been delayed, leading to progressive liver and central nervous system disease. With early identification and treatment, it is possible to stop the progression of Wilson disease.
CORRESPONDENCE
Jeffrey Taylor, MD, MS, Evangelical Community Hospital, Department of Pediatrics, 1 Hospital Drive, Lewisburg PA, 17837; [email protected].
1. Wu F, Wang J, Pu C, et al. Wilson’s disease: a comprehensive review of the molecular mechanisms. Int J Mol Sci. 2015;16:6419-6431.
2. Merle U, Stremmel W, Gessner R. Influence of homozygosity for methionine at codon 129 of the human prion gene on the onset of neurological and hepatic symptoms in Wilson disease. Arch Neurol. 2006;63:982-985.
3. Compston A. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, by S. A. Kinnier Wilson, (From the National Hospital, and the Laboratory of the National Hospital, Queen Square, London) Brain 1912: 34; 295-509. Brain. 2009;132(pt 8):1997-2001.
4. Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994-e1001.
5. Kang K. Abnormality on liver function test. Pediatr Gastroenterol Hepatol Nutr. 2013;16:225-232.
6. Lorincz M. Neurologic Wilson’s disease. Ann NY Acad Sci. 2010;1184:173-187.
7. Dening TR, Berrios GE, Walshe JM. Wilson’s disease and epilepsy. Brain. 1988;111(pt 5):1139-1155.
8. Socha P, Janczyk W, Dhawan A, et al. Wilson’s disease in children: a position paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutrit. 2018;66:334-344.
9. Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337.
10. Ala A, Schilsky ML. Wilson disease: pathophysiology, diagnosis, treatment and screening. Clin Liver Dis. 2004;8:787-805, viii.
11. Thomas GR, Forbes JR, Roberts EA, et al. The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet. 1995;9:210-217.
12. Pfeiffer RF. Wilson’s disease. Semin Neurol. 2007;27:123-132.
13. Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol. 2006;2:482-493.
14. Morgan HG, Stewart WK, Lowe KG, et al. Wilson’s disease and the Fanconi syndrome. Q J Med. 1962;31:361-384.
15. Wiebers DO, Hollenhorst RW, Goldstein NP. The ophthalmologic manifestations of Wilson’s disease. Mayo Clin Proc. 1977;52:409-416.
16. Jackson GH, Meyer A, Lippmann S. Wilson’s disease: psychiatric manifestations may be the clinical presentation. Postgrad Med. 1994;95:135-138.
17. O’Conner JA, Sokol RJ. Copper metabolism and copper storage disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 3rd ed. New York, NY: Cambridge University Press; 2007:626-660.
18. Dening TR, Berrios GE. Wilson’s disease: a longitudinal study of psychiatric symptoms. Biol Psychiatry. 1990;28:255-265.
THE CASE
A 5-year-old boy was brought into his primary care clinic by his mother, who expressed concern about her son’s increasing impulsiveness, aggression, and difficulty staying on task at preschool and at home. The child’s medical history was unremarkable, and he was taking no medications. The family history was negative for hepatic or metabolic disease and positive for attention deficit-hyperactivity disorder (ADHD; father).
The child’s growth was normal. His physical exam was remarkable for a liver edge 1 cm below his costal margin. No Kayser-Fleischer rings were present.
Screening included a complete metabolic panel. Notable results included an alanine aminotransferase (ALT) level of 208 U/dL (normal range, < 30 U/dL), an aspartate transaminase (AST) level of 125 U/dL (normal range, 10-34 U/dL), and an alkaline phosphatase (ALP) of 470 U/dL (normal range, 93-309 U/dL). Subsequent repeat laboratory testing confirmed these elevations (ALT, 248 U/dL; AST, 137 U/dL; ALP, 462 U/dL). Ceruloplasmin levels were low (11 mg/dL; normal range, 18-35 mg/dL), and 24-hour urinary copper was not obtainable. Prothrombin/partial thromboplastin time, ammonia, lactate, total and direct bilirubin, and gamma-glutamyltransferase levels were normal.
Further evaluation included abdominal ultrasound and brain magnetic resonance imaging, both of which yielded normal results. Testing for Epstein-Barr virus; cytomegalovirus; hepatitis A, B, and C titers; and antinuclear, anti-smooth muscle, and anti–liver-kidney microsomal antibodies was negative.
THE DIAGNOSIS
The patient’s low ceruloplasmin prompted referral to Pediatric Gastroenterology for consultation and liver biopsy due to concern for Wilson disease. Biopsy results were consistent with, and quantitative liver copper confirmatory for, this diagnosis (FIGURE).

Genetic testing for mutations in the ATP7B gene was performed on the patient, his mother, and his siblings (his father was unavailable). The patient, his mother, and his sister were all positive for His1069Gln mutation; only the patient was positive for a 3990_3993 del mutation (his half-brother was negative for both mutations). The presence of 2 different mutant alleles for the ATP7B gene, one on each chromosome—the common substitution mutation, His1069Gln, in exon 14 and a 3990_3993 del TTAT mutation in exon 19—qualified the patient as a compound heterozygote.
The 3990_3993 del TTAT mutation—which to our knowledge has not been previously reported—produced a translational frame shift and premature stop codon. As others have pointed out, frame shift and missense mutations produce a more severe phenotype.1
Continue to: Further testing was prompted...
Further testing was prompted by a report suggesting that codon 129 mutations of the human prion gene (HPG) influence Wilson disease.2 Compared with patients who are heterozygous (M129V) or homozygous (V129V) for valine, those who are homozygous for methionine (M129M) have delayed symptom onset.2 Our patient was heterozygous (M129V). It is interesting to speculate that HPG heterozygosity, combined with a mutation causing a stop codon, predisposed our patient to more rapid accumulation of copper and earlier age of onset.
DISCUSSION
Wilson disease is an inherited disorder of copper metabolism.3 An inherent difficulty in its recognition, diagnosis, and management is its rarity: global prevalence is estimated as 1/30,000, although this varies by geographic location.1 In contrast, ADHD has a prevalence of 7.2%,4 making it 2400 times more prevalent than Wilson disease. Furthermore, abnormal liver function tests are common in children; the differential diagnosis includes etiologies such as infection (both viral and nonviral), immune-mediated inflammatory disease, drug toxicity (iatrogenic or medication-induced), anatomic abnormalities, and nonalcoholic fatty liver disease.5
Wilson disease is remarkable, however, for being easily treatable if detected and devastating if not. Although liver abnormalities often improve with treatment, delayed diagnosis and management significantly impact neurologic recovery: An 18-month delay results in 38% of patients continuing to have major neurologic disabilities.6 Untreated, Wilson disease may be fatal within 5 years of development of neurologic symptoms.7 Thus, it has been suggested that evaluation for Wilson disease be considered in any child older than 1 year who presents with unexplained liver disease, including asymptomatic elevations of serum transaminases.8
Mutations in ATP7B on chromosome 13 are responsible for the pathology of Wilson disease9; more than 250 mutations have been identified, including substitutions, deletions, and missense mutations.10 Affected patients may be compound heterozygotes11 and/or may possess new mutations, as seen in our patient.
Although copper absorption is normal, impaired excretion causes toxic accumulation in affected organs. ATP7B’s product, ATPase 2, regulates copper excretion, as well as copper binding to apoceruloplasmin to form the carrier protein ceruloplasmin. An ATP7B abnormality would prevent the latter—making ceruloplasmin a useful screening biomarker and a reliable marker for Wilson disease by age 1 year.8
Continue to: Hepatic and neurocognitive effects
Hepatic and neurocognitive effects. Excess copper in hepatocytes causes oxidative damage and release of copper into the circulation, with accumulation in susceptible organs (eg, brain, kidneys). Hepatocyte apoptosis is accelerated by copper’s negative effect on inhibitor of apoptosis protein.12,13 Renal tubular damage leads to Fanconi syndrome,14 in which substances such as glucose, phosphates, and potassium are excreted in urine rather than absorbed into the bloodstream by the kidneys. Excess copper deposition in the Descemet membrane may lead to Kayser-Fleisher ring formation.15 In the brain, copper deposition may occur in the lenticular nuclei,3 as well as in the thalamus, subthalamus, brainstem, and frontal cortex—resulting in extrapyramidal, cerebral, and mild cerebellar symptoms.6
Cognitive impairment, which may be subtle, includes increased impulsivity, impaired judgment, apathy, poor decision making, decreased attention, increased lability, slowed thinking, and memory loss.6 Behavioral manifestations include changes in school or work performance and outbursts mimicking ADHD12,16,17 as well as paranoia, depression, and bizarre behaviors.16,18 Neuropsychiatric abnormalities include personality changes, pseudoparkinsonism, dyskinesia/dysarthria, and ataxia/tremor. Younger patients with psychiatric symptoms may be labelled with depression, anxiety, obsessive-compulsive disorder, bipolar disorder, or antisocial disorder.6,16,18
Hepatic disease manifestations range from asymptomatic elevations in AST/ALT to acute hepatitis, mimicking infectious processes. Cirrhosis is the end result of untreated Wilson disease, with liver transplantation required if end-stage liver disease results. Rarely, patients present in fulminant hepatic failure, with death occurring if emergent liver transplantation is not performed.6,8,10
Of note, before age 10, > 80% of patients with Wilson disease present with hepatic symptoms; those ages 10 to 18 often manifest psychiatric changes.17 Kayser-Fleisher rings are common in patients with neurologic manifestations but less so in those who have hepatic presentations or are presymptomatic.6,15
Effective disease-mitigating treatment is indicated and available for both symptomatic and asymptomatic individuals and includes the copper chelators D-penicillamine (starting dose, 150-300 mg/d with a gradual weekly increase to 20 mg/kg/d) and trientine hydrochloride (a heavy metal chelating compound; starting dose, 20 mg/kg/d to a maximum of 1000 mg/d in young adults). Adverse effects of D-penicillamine include cutaneous eruptions, neutropenia, thrombocytopenia, proteinuria, and a lupus-like syndrome; therefore, trientine is increasingly being used as first-line therapy.8
Continue to: For asymptomatic...
For asymptomatic patients who have had effective chelation therapy and proven de-coppering, zinc salts are a useful follow-on therapy. Zinc’s proposed mechanism of action is induction of metallothionein in enterocytes, which promotes copper trapping and eventual excretion into the lumen. Importantly, treatment for Wilson disease is lifelong and monitoring of compliance is essential.8
Our 5-year-old patient was started on oral trientine at 20 mg/kg/d and a low copper diet. In response to this initial treatment, the patient’s liver function tests (LFTs) normalized, and he was switched to 25 mg tid of a zinc chelate, with continuation of the low copper diet. His LFTs have remained normal, although his urine copper levels are still elevated. He continues to be monitored periodically with LFTs and measurement of urine copper levels. He is also being treated for ADHD, as his presenting behavioral abnormalities suggestive of ADHD have not resolved.
THE TAKEAWAY
Although children presenting with symptoms consistent with ADHD often have ADHD, as was true in this case, it is important to consider other diagnoses. Unexplained elevations of liver function test values in children older than 1 year should prompt screening for Wilson disease.5,8 Additionally, other family members should be evaluated; if they have the disease, treatment should be started by age 2 years, even if the patient is asymptomatic.
In our patient’s case, routine screening saved the day. The complete metabolic panel revealed elevated ALT and AST levels, prompting further evaluation. Without this testing, his diagnosis likely would have been delayed, leading to progressive liver and central nervous system disease. With early identification and treatment, it is possible to stop the progression of Wilson disease.
CORRESPONDENCE
Jeffrey Taylor, MD, MS, Evangelical Community Hospital, Department of Pediatrics, 1 Hospital Drive, Lewisburg PA, 17837; [email protected].
THE CASE
A 5-year-old boy was brought into his primary care clinic by his mother, who expressed concern about her son’s increasing impulsiveness, aggression, and difficulty staying on task at preschool and at home. The child’s medical history was unremarkable, and he was taking no medications. The family history was negative for hepatic or metabolic disease and positive for attention deficit-hyperactivity disorder (ADHD; father).
The child’s growth was normal. His physical exam was remarkable for a liver edge 1 cm below his costal margin. No Kayser-Fleischer rings were present.
Screening included a complete metabolic panel. Notable results included an alanine aminotransferase (ALT) level of 208 U/dL (normal range, < 30 U/dL), an aspartate transaminase (AST) level of 125 U/dL (normal range, 10-34 U/dL), and an alkaline phosphatase (ALP) of 470 U/dL (normal range, 93-309 U/dL). Subsequent repeat laboratory testing confirmed these elevations (ALT, 248 U/dL; AST, 137 U/dL; ALP, 462 U/dL). Ceruloplasmin levels were low (11 mg/dL; normal range, 18-35 mg/dL), and 24-hour urinary copper was not obtainable. Prothrombin/partial thromboplastin time, ammonia, lactate, total and direct bilirubin, and gamma-glutamyltransferase levels were normal.
Further evaluation included abdominal ultrasound and brain magnetic resonance imaging, both of which yielded normal results. Testing for Epstein-Barr virus; cytomegalovirus; hepatitis A, B, and C titers; and antinuclear, anti-smooth muscle, and anti–liver-kidney microsomal antibodies was negative.
THE DIAGNOSIS
The patient’s low ceruloplasmin prompted referral to Pediatric Gastroenterology for consultation and liver biopsy due to concern for Wilson disease. Biopsy results were consistent with, and quantitative liver copper confirmatory for, this diagnosis (FIGURE).

Genetic testing for mutations in the ATP7B gene was performed on the patient, his mother, and his siblings (his father was unavailable). The patient, his mother, and his sister were all positive for His1069Gln mutation; only the patient was positive for a 3990_3993 del mutation (his half-brother was negative for both mutations). The presence of 2 different mutant alleles for the ATP7B gene, one on each chromosome—the common substitution mutation, His1069Gln, in exon 14 and a 3990_3993 del TTAT mutation in exon 19—qualified the patient as a compound heterozygote.
The 3990_3993 del TTAT mutation—which to our knowledge has not been previously reported—produced a translational frame shift and premature stop codon. As others have pointed out, frame shift and missense mutations produce a more severe phenotype.1
Continue to: Further testing was prompted...
Further testing was prompted by a report suggesting that codon 129 mutations of the human prion gene (HPG) influence Wilson disease.2 Compared with patients who are heterozygous (M129V) or homozygous (V129V) for valine, those who are homozygous for methionine (M129M) have delayed symptom onset.2 Our patient was heterozygous (M129V). It is interesting to speculate that HPG heterozygosity, combined with a mutation causing a stop codon, predisposed our patient to more rapid accumulation of copper and earlier age of onset.
DISCUSSION
Wilson disease is an inherited disorder of copper metabolism.3 An inherent difficulty in its recognition, diagnosis, and management is its rarity: global prevalence is estimated as 1/30,000, although this varies by geographic location.1 In contrast, ADHD has a prevalence of 7.2%,4 making it 2400 times more prevalent than Wilson disease. Furthermore, abnormal liver function tests are common in children; the differential diagnosis includes etiologies such as infection (both viral and nonviral), immune-mediated inflammatory disease, drug toxicity (iatrogenic or medication-induced), anatomic abnormalities, and nonalcoholic fatty liver disease.5
Wilson disease is remarkable, however, for being easily treatable if detected and devastating if not. Although liver abnormalities often improve with treatment, delayed diagnosis and management significantly impact neurologic recovery: An 18-month delay results in 38% of patients continuing to have major neurologic disabilities.6 Untreated, Wilson disease may be fatal within 5 years of development of neurologic symptoms.7 Thus, it has been suggested that evaluation for Wilson disease be considered in any child older than 1 year who presents with unexplained liver disease, including asymptomatic elevations of serum transaminases.8
Mutations in ATP7B on chromosome 13 are responsible for the pathology of Wilson disease9; more than 250 mutations have been identified, including substitutions, deletions, and missense mutations.10 Affected patients may be compound heterozygotes11 and/or may possess new mutations, as seen in our patient.
Although copper absorption is normal, impaired excretion causes toxic accumulation in affected organs. ATP7B’s product, ATPase 2, regulates copper excretion, as well as copper binding to apoceruloplasmin to form the carrier protein ceruloplasmin. An ATP7B abnormality would prevent the latter—making ceruloplasmin a useful screening biomarker and a reliable marker for Wilson disease by age 1 year.8
Continue to: Hepatic and neurocognitive effects
Hepatic and neurocognitive effects. Excess copper in hepatocytes causes oxidative damage and release of copper into the circulation, with accumulation in susceptible organs (eg, brain, kidneys). Hepatocyte apoptosis is accelerated by copper’s negative effect on inhibitor of apoptosis protein.12,13 Renal tubular damage leads to Fanconi syndrome,14 in which substances such as glucose, phosphates, and potassium are excreted in urine rather than absorbed into the bloodstream by the kidneys. Excess copper deposition in the Descemet membrane may lead to Kayser-Fleisher ring formation.15 In the brain, copper deposition may occur in the lenticular nuclei,3 as well as in the thalamus, subthalamus, brainstem, and frontal cortex—resulting in extrapyramidal, cerebral, and mild cerebellar symptoms.6
Cognitive impairment, which may be subtle, includes increased impulsivity, impaired judgment, apathy, poor decision making, decreased attention, increased lability, slowed thinking, and memory loss.6 Behavioral manifestations include changes in school or work performance and outbursts mimicking ADHD12,16,17 as well as paranoia, depression, and bizarre behaviors.16,18 Neuropsychiatric abnormalities include personality changes, pseudoparkinsonism, dyskinesia/dysarthria, and ataxia/tremor. Younger patients with psychiatric symptoms may be labelled with depression, anxiety, obsessive-compulsive disorder, bipolar disorder, or antisocial disorder.6,16,18
Hepatic disease manifestations range from asymptomatic elevations in AST/ALT to acute hepatitis, mimicking infectious processes. Cirrhosis is the end result of untreated Wilson disease, with liver transplantation required if end-stage liver disease results. Rarely, patients present in fulminant hepatic failure, with death occurring if emergent liver transplantation is not performed.6,8,10
Of note, before age 10, > 80% of patients with Wilson disease present with hepatic symptoms; those ages 10 to 18 often manifest psychiatric changes.17 Kayser-Fleisher rings are common in patients with neurologic manifestations but less so in those who have hepatic presentations or are presymptomatic.6,15
Effective disease-mitigating treatment is indicated and available for both symptomatic and asymptomatic individuals and includes the copper chelators D-penicillamine (starting dose, 150-300 mg/d with a gradual weekly increase to 20 mg/kg/d) and trientine hydrochloride (a heavy metal chelating compound; starting dose, 20 mg/kg/d to a maximum of 1000 mg/d in young adults). Adverse effects of D-penicillamine include cutaneous eruptions, neutropenia, thrombocytopenia, proteinuria, and a lupus-like syndrome; therefore, trientine is increasingly being used as first-line therapy.8
Continue to: For asymptomatic...
For asymptomatic patients who have had effective chelation therapy and proven de-coppering, zinc salts are a useful follow-on therapy. Zinc’s proposed mechanism of action is induction of metallothionein in enterocytes, which promotes copper trapping and eventual excretion into the lumen. Importantly, treatment for Wilson disease is lifelong and monitoring of compliance is essential.8
Our 5-year-old patient was started on oral trientine at 20 mg/kg/d and a low copper diet. In response to this initial treatment, the patient’s liver function tests (LFTs) normalized, and he was switched to 25 mg tid of a zinc chelate, with continuation of the low copper diet. His LFTs have remained normal, although his urine copper levels are still elevated. He continues to be monitored periodically with LFTs and measurement of urine copper levels. He is also being treated for ADHD, as his presenting behavioral abnormalities suggestive of ADHD have not resolved.
THE TAKEAWAY
Although children presenting with symptoms consistent with ADHD often have ADHD, as was true in this case, it is important to consider other diagnoses. Unexplained elevations of liver function test values in children older than 1 year should prompt screening for Wilson disease.5,8 Additionally, other family members should be evaluated; if they have the disease, treatment should be started by age 2 years, even if the patient is asymptomatic.
In our patient’s case, routine screening saved the day. The complete metabolic panel revealed elevated ALT and AST levels, prompting further evaluation. Without this testing, his diagnosis likely would have been delayed, leading to progressive liver and central nervous system disease. With early identification and treatment, it is possible to stop the progression of Wilson disease.
CORRESPONDENCE
Jeffrey Taylor, MD, MS, Evangelical Community Hospital, Department of Pediatrics, 1 Hospital Drive, Lewisburg PA, 17837; [email protected].
1. Wu F, Wang J, Pu C, et al. Wilson’s disease: a comprehensive review of the molecular mechanisms. Int J Mol Sci. 2015;16:6419-6431.
2. Merle U, Stremmel W, Gessner R. Influence of homozygosity for methionine at codon 129 of the human prion gene on the onset of neurological and hepatic symptoms in Wilson disease. Arch Neurol. 2006;63:982-985.
3. Compston A. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, by S. A. Kinnier Wilson, (From the National Hospital, and the Laboratory of the National Hospital, Queen Square, London) Brain 1912: 34; 295-509. Brain. 2009;132(pt 8):1997-2001.
4. Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994-e1001.
5. Kang K. Abnormality on liver function test. Pediatr Gastroenterol Hepatol Nutr. 2013;16:225-232.
6. Lorincz M. Neurologic Wilson’s disease. Ann NY Acad Sci. 2010;1184:173-187.
7. Dening TR, Berrios GE, Walshe JM. Wilson’s disease and epilepsy. Brain. 1988;111(pt 5):1139-1155.
8. Socha P, Janczyk W, Dhawan A, et al. Wilson’s disease in children: a position paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutrit. 2018;66:334-344.
9. Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337.
10. Ala A, Schilsky ML. Wilson disease: pathophysiology, diagnosis, treatment and screening. Clin Liver Dis. 2004;8:787-805, viii.
11. Thomas GR, Forbes JR, Roberts EA, et al. The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet. 1995;9:210-217.
12. Pfeiffer RF. Wilson’s disease. Semin Neurol. 2007;27:123-132.
13. Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol. 2006;2:482-493.
14. Morgan HG, Stewart WK, Lowe KG, et al. Wilson’s disease and the Fanconi syndrome. Q J Med. 1962;31:361-384.
15. Wiebers DO, Hollenhorst RW, Goldstein NP. The ophthalmologic manifestations of Wilson’s disease. Mayo Clin Proc. 1977;52:409-416.
16. Jackson GH, Meyer A, Lippmann S. Wilson’s disease: psychiatric manifestations may be the clinical presentation. Postgrad Med. 1994;95:135-138.
17. O’Conner JA, Sokol RJ. Copper metabolism and copper storage disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 3rd ed. New York, NY: Cambridge University Press; 2007:626-660.
18. Dening TR, Berrios GE. Wilson’s disease: a longitudinal study of psychiatric symptoms. Biol Psychiatry. 1990;28:255-265.
1. Wu F, Wang J, Pu C, et al. Wilson’s disease: a comprehensive review of the molecular mechanisms. Int J Mol Sci. 2015;16:6419-6431.
2. Merle U, Stremmel W, Gessner R. Influence of homozygosity for methionine at codon 129 of the human prion gene on the onset of neurological and hepatic symptoms in Wilson disease. Arch Neurol. 2006;63:982-985.
3. Compston A. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, by S. A. Kinnier Wilson, (From the National Hospital, and the Laboratory of the National Hospital, Queen Square, London) Brain 1912: 34; 295-509. Brain. 2009;132(pt 8):1997-2001.
4. Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994-e1001.
5. Kang K. Abnormality on liver function test. Pediatr Gastroenterol Hepatol Nutr. 2013;16:225-232.
6. Lorincz M. Neurologic Wilson’s disease. Ann NY Acad Sci. 2010;1184:173-187.
7. Dening TR, Berrios GE, Walshe JM. Wilson’s disease and epilepsy. Brain. 1988;111(pt 5):1139-1155.
8. Socha P, Janczyk W, Dhawan A, et al. Wilson’s disease in children: a position paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutrit. 2018;66:334-344.
9. Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337.
10. Ala A, Schilsky ML. Wilson disease: pathophysiology, diagnosis, treatment and screening. Clin Liver Dis. 2004;8:787-805, viii.
11. Thomas GR, Forbes JR, Roberts EA, et al. The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet. 1995;9:210-217.
12. Pfeiffer RF. Wilson’s disease. Semin Neurol. 2007;27:123-132.
13. Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol. 2006;2:482-493.
14. Morgan HG, Stewart WK, Lowe KG, et al. Wilson’s disease and the Fanconi syndrome. Q J Med. 1962;31:361-384.
15. Wiebers DO, Hollenhorst RW, Goldstein NP. The ophthalmologic manifestations of Wilson’s disease. Mayo Clin Proc. 1977;52:409-416.
16. Jackson GH, Meyer A, Lippmann S. Wilson’s disease: psychiatric manifestations may be the clinical presentation. Postgrad Med. 1994;95:135-138.
17. O’Conner JA, Sokol RJ. Copper metabolism and copper storage disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 3rd ed. New York, NY: Cambridge University Press; 2007:626-660.
18. Dening TR, Berrios GE. Wilson’s disease: a longitudinal study of psychiatric symptoms. Biol Psychiatry. 1990;28:255-265.