User login
Erik Greb joined the staff of Neurology Reviews in January 2012. Since then, he has attended scientific conferences, conducted video interviews, and written about clinical research in multiple sclerosis, epilepsy, Parkinson's disease, Alzheimer's disease, stroke, and other neurologic disorders. In addition to news articles, Erik has written investigative stories about multiple sclerosis, headache, and epilepsy. He previously wrote about pharmaceutical manufacturing, drug formulation and delivery, quality assurance, and regulation for Pharmaceutical Technology.
Galcanezumab benefits patients with migraine and medication overuse
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
REPORTING FROM AHS 2019
Can dietary therapies treat GERD effectively?
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
REPORTING FROM FRESTON CONFERENCE 2019
Two genetic variants modify risk of Alzheimer’s disease
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Two variants of MS4A are associated with the risk of Alzheimer’s disease.
Major finding: The rs1582763 SNP is associated with reduced risk for Alzheimer’s disease, and rs6591561 is associated with increased risk of Alzheimer’s disease.
Study details: A genome-wide association study of 813 participants in the Alzheimer’s Disease Neuroimaging Initiative.
Disclosures: Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
Source: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
Serum neurofilament light chain level may indicate MS disease activity
according to an investigation published online August 12 in JAMA Neurology. Furthermore, changes in sNfL levels are associated with disability worsening, and sNfL levels may be influenced by treatment. These data support the potential of sNfL as an objective surrogate of ongoing MS disease activity, according to the researchers.
Neuronal and axonal loss increase levels of NfL in cerebrospinal fluid (CSF) in patients with MS. Previous research indicated that sNfL levels are correlated with CSF levels of NfL and are associated with clinical and imaging measures of disease activity. For the purpose of repeated sampling, collecting blood from patients would be more practical than performing lumbar punctures, said the investigators. No long-term studies of sNfL concentrations and their associations with MS disease outcomes had been performed, however.
Ester Cantó, PhD, of the University of California, San Francisco (UCSF), and colleagues examined data from the prospective Expression, Proteomics, Imaging, Clinical (EPIC) study to assess sNfL as a biomarker of MS disease activity and progression. The ongoing EPIC study is being conducted at UCSF. Dr. Cantó and colleagues analyzed data collected from July 1, 2004, through August 31, 2017, for 607 patients with MS. Participants underwent clinical examinations and serum sample collections annually for 5 years, then at various time points for as long as 12 years. The median follow-up duration was 10 years. The researchers measured sNfL levels with a sensitive single-molecule array platform and compared them with clinical and MRI variables using univariable and multivariable analyses. Dr. Cantó and colleagues chose disability progression, defined as clinically significant worsening on the Expanded Disability Status Scale (EDSS) score, and brain fraction atrophy as their primary outcomes.
The population’s mean age was 42.5 years. About 70% of participants were women, and all were of non-Hispanic European descent. At baseline, sNfL levels were significantly associated with EDSS score, MS subtype, and treatment status.
Dr. Cantó and colleagues found a significant interaction between EDSS worsening and change in levels of sNfL over time. Baseline sNfL levels were associated with approximately 11.6% of the variance in participants’ brain fraction atrophy at year 10. When the investigators controlled for sex, age, and disease duration, they found that baseline sNfL levels were associated with 18% of the variance in brain fraction atrophy at year 10. After 5 years’ follow-up, active treatment was associated with lower levels of sNfL. High-efficacy treatments were associated with greater decreases in sNfL levels, compared with platform therapies.
More frequent sample acquisition could provide greater detail about changes in sNfL levels, wrote Dr. Cantó and colleagues. They acknowledged that their study had insufficient power for the researchers to assess the outcomes of individual MS therapies. Other limitations included the lack of data on NfL stability and the lack of a group of healthy controls.
“For an individual patient, the biomarker prognostic power of sNfL level for clinical and MRI outcomes was limited,” said the investigators. “Further prospective studies are necessary to assess the assay’s utility for decision making in individual patients.”
The National Institutes of Health and the U.S. National MS Society supported the study. Several of the investigators received compensation from Novartis, which provided funds for the reagents needed for the single-molecule array assay.
SOURCE: Cantó E et al. JAMA Neurol. 2019 Aug. 12. doi: 10.1001/jamaneurol.2019.2137.
according to an investigation published online August 12 in JAMA Neurology. Furthermore, changes in sNfL levels are associated with disability worsening, and sNfL levels may be influenced by treatment. These data support the potential of sNfL as an objective surrogate of ongoing MS disease activity, according to the researchers.
Neuronal and axonal loss increase levels of NfL in cerebrospinal fluid (CSF) in patients with MS. Previous research indicated that sNfL levels are correlated with CSF levels of NfL and are associated with clinical and imaging measures of disease activity. For the purpose of repeated sampling, collecting blood from patients would be more practical than performing lumbar punctures, said the investigators. No long-term studies of sNfL concentrations and their associations with MS disease outcomes had been performed, however.
Ester Cantó, PhD, of the University of California, San Francisco (UCSF), and colleagues examined data from the prospective Expression, Proteomics, Imaging, Clinical (EPIC) study to assess sNfL as a biomarker of MS disease activity and progression. The ongoing EPIC study is being conducted at UCSF. Dr. Cantó and colleagues analyzed data collected from July 1, 2004, through August 31, 2017, for 607 patients with MS. Participants underwent clinical examinations and serum sample collections annually for 5 years, then at various time points for as long as 12 years. The median follow-up duration was 10 years. The researchers measured sNfL levels with a sensitive single-molecule array platform and compared them with clinical and MRI variables using univariable and multivariable analyses. Dr. Cantó and colleagues chose disability progression, defined as clinically significant worsening on the Expanded Disability Status Scale (EDSS) score, and brain fraction atrophy as their primary outcomes.
The population’s mean age was 42.5 years. About 70% of participants were women, and all were of non-Hispanic European descent. At baseline, sNfL levels were significantly associated with EDSS score, MS subtype, and treatment status.
Dr. Cantó and colleagues found a significant interaction between EDSS worsening and change in levels of sNfL over time. Baseline sNfL levels were associated with approximately 11.6% of the variance in participants’ brain fraction atrophy at year 10. When the investigators controlled for sex, age, and disease duration, they found that baseline sNfL levels were associated with 18% of the variance in brain fraction atrophy at year 10. After 5 years’ follow-up, active treatment was associated with lower levels of sNfL. High-efficacy treatments were associated with greater decreases in sNfL levels, compared with platform therapies.
More frequent sample acquisition could provide greater detail about changes in sNfL levels, wrote Dr. Cantó and colleagues. They acknowledged that their study had insufficient power for the researchers to assess the outcomes of individual MS therapies. Other limitations included the lack of data on NfL stability and the lack of a group of healthy controls.
“For an individual patient, the biomarker prognostic power of sNfL level for clinical and MRI outcomes was limited,” said the investigators. “Further prospective studies are necessary to assess the assay’s utility for decision making in individual patients.”
The National Institutes of Health and the U.S. National MS Society supported the study. Several of the investigators received compensation from Novartis, which provided funds for the reagents needed for the single-molecule array assay.
SOURCE: Cantó E et al. JAMA Neurol. 2019 Aug. 12. doi: 10.1001/jamaneurol.2019.2137.
according to an investigation published online August 12 in JAMA Neurology. Furthermore, changes in sNfL levels are associated with disability worsening, and sNfL levels may be influenced by treatment. These data support the potential of sNfL as an objective surrogate of ongoing MS disease activity, according to the researchers.
Neuronal and axonal loss increase levels of NfL in cerebrospinal fluid (CSF) in patients with MS. Previous research indicated that sNfL levels are correlated with CSF levels of NfL and are associated with clinical and imaging measures of disease activity. For the purpose of repeated sampling, collecting blood from patients would be more practical than performing lumbar punctures, said the investigators. No long-term studies of sNfL concentrations and their associations with MS disease outcomes had been performed, however.
Ester Cantó, PhD, of the University of California, San Francisco (UCSF), and colleagues examined data from the prospective Expression, Proteomics, Imaging, Clinical (EPIC) study to assess sNfL as a biomarker of MS disease activity and progression. The ongoing EPIC study is being conducted at UCSF. Dr. Cantó and colleagues analyzed data collected from July 1, 2004, through August 31, 2017, for 607 patients with MS. Participants underwent clinical examinations and serum sample collections annually for 5 years, then at various time points for as long as 12 years. The median follow-up duration was 10 years. The researchers measured sNfL levels with a sensitive single-molecule array platform and compared them with clinical and MRI variables using univariable and multivariable analyses. Dr. Cantó and colleagues chose disability progression, defined as clinically significant worsening on the Expanded Disability Status Scale (EDSS) score, and brain fraction atrophy as their primary outcomes.
The population’s mean age was 42.5 years. About 70% of participants were women, and all were of non-Hispanic European descent. At baseline, sNfL levels were significantly associated with EDSS score, MS subtype, and treatment status.
Dr. Cantó and colleagues found a significant interaction between EDSS worsening and change in levels of sNfL over time. Baseline sNfL levels were associated with approximately 11.6% of the variance in participants’ brain fraction atrophy at year 10. When the investigators controlled for sex, age, and disease duration, they found that baseline sNfL levels were associated with 18% of the variance in brain fraction atrophy at year 10. After 5 years’ follow-up, active treatment was associated with lower levels of sNfL. High-efficacy treatments were associated with greater decreases in sNfL levels, compared with platform therapies.
More frequent sample acquisition could provide greater detail about changes in sNfL levels, wrote Dr. Cantó and colleagues. They acknowledged that their study had insufficient power for the researchers to assess the outcomes of individual MS therapies. Other limitations included the lack of data on NfL stability and the lack of a group of healthy controls.
“For an individual patient, the biomarker prognostic power of sNfL level for clinical and MRI outcomes was limited,” said the investigators. “Further prospective studies are necessary to assess the assay’s utility for decision making in individual patients.”
The National Institutes of Health and the U.S. National MS Society supported the study. Several of the investigators received compensation from Novartis, which provided funds for the reagents needed for the single-molecule array assay.
SOURCE: Cantó E et al. JAMA Neurol. 2019 Aug. 12. doi: 10.1001/jamaneurol.2019.2137.
FROM JAMA NEUROLOGY
Key clinical point: Serum neurofilament light chain level has potential as a surrogate of ongoing MS disease activity.
Major finding: Serum neurofilament light chain level is associated with brain fraction atrophy.
Study details: An ongoing, prospective, observational study of 607 patients with MS.
Disclosures: The National Institutes of Health and the U.S. National MS Society supported the study. Several of the investigators received compensation from Novartis, which provided funds for the reagents needed for the single-molecule array assay.
Source: Cantó E et al. JAMA Neurol. 2019 Aug 12. doi: 10.1001/jamaneurol.2019.2137.
Lasmiditan is associated with driving impairment
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
REPORTING FROM AHS 2019
Treatment of episodic cluster headache deviates from recommendations
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
REPORTING FROM AHS 2019
What is the future of celiac disease management?
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
CHICAGO – according to a lecture delivered at the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease.
Home testing services and portable gluten-detection devices enable patients to diagnose and manage themselves without medical supervision, but these strategies raise concerns about accuracy and efficacy, said Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University in New York.
Potential treatments on the horizon
The gluten-free diet is the only treatment proven effective for celiac disease, but it can be expensive or unpalatable for some patients. The diet also entails risks of bowel irregularity and weight gain. “The gluten-free diet remains an inadequate treatment for many people with celiac disease,” said Dr. Lebwohl.
Tennyson et al. found that 66% of patients with biopsy-proven celiac disease are interested in nondietary therapy (Therap Adv Gastroenterol. 2013;6[5]:358-64.). Such patients are more likely to be male and older than 50 years.
Latiglutenase, a gluten enzyme derived from bacteria and cereal, is among the pharmacotherapies being investigated as a treatment for nonresponsive celiac disease. It reduces or eliminates the toxicity of gluten. In a recent phase 2b trial, however, the treatment did not achieve the primary outcome measure of histologic improvement (Gastroenterology. 2017;152[4]:787-98.). Compared with placebo, the drug was not associated with significant improvements in histologic and symptom scores.
Another drug in development is the tight-junction modulator larazotide acetate. Studies of zonula occludens toxin and its mammalian analogue zonulin led to the development of larazotide acetate. Leffler et al. found that a 0.5-mg dose of the drug reduced symptoms of nonresponsive celiac disease in patients who were following a gluten-free diet, compared with patients treated with the diet alone (Gastroenterology. 2015;148[7]:1311-9.). Innovate Pharmaceuticals plans to study the drug in phase 3 trials, said Dr. Lebwohl.
ImmunosanT has studied Nexvax2, which promotes gluten peptide desensitization. A phase 2 study examined the drug’s efficacy in reducing symptoms during a masked food challenge. The company discontinued this study when an interim analysis showed that the drug provided no more protection from gluten exposure than placebo. Nexvax2 was safe and well tolerated, and the study revealed no new safety signals.
In addition to newly developed therapies, researchers are studying whether drugs marketed for other indications could be effective treatments for celiac disease. For example, budesonide, a treatment for asthma and chronic obstructive pulmonary disease, is being investigated for nonresponsive celiac disease and refractory celiac disease. Other research is examining whether budesonide could provide effective protection after inadvertent gluten exposure. Systemic steroids, immunosuppressants such as azathioprine, chemotherapeutics such as cladribine, and mesalamine, which is a treatment for inflammatory bowel disease, also are under investigation.
But several questions related to drug development for celiac disease remain unanswered. For example, whether researchers should choose clinical or histologic endpoints for their trials is a subject of debate. “Probably, we’re going to be looking for two endpoints,” said Dr. Lebwohl. No consensus has been established about whether trials should include patients for whom diagnosis is based on a test other than a biopsy. Also, the effect of nondietary therapy on adherence to the gluten-free diet remains to be clarified.
Self-management of celiac disease
“We’re in a new era” of self-monitoring and direct-to-consumer advertising aimed at patients with celiac disease, said Dr. Lebwohl. Products and services that enable patients to diagnose and manage themselves independently are broadly available. For example, 23andMe provides at-home testing for HLA-DQ2.5 and HLA-DQ8, which could support a diagnosis of celiac disease. The service does not, however, test for HLA-DQ2.2, which is present in about 5% of patients with celiac disease. This testing consequently has high negative-predictive value, but poor positive-predictive value, said Dr. Lebwohl.
Similarly, ImAware provides blood tests that patients can take at home and send to the company for results. The tests look for antibodies such as tissue transglutaminase immunoglobulin A/immunoglobulin G and deamidated gliadin peptide IgA/IgG. The company advises patients to share their results with a health care professional.
Furthermore, portable devices such as Nima are marketed as gluten detectors. One study of the device included 804 users from all 50 states. The device found gluten in 32% of all restaurant food tested advertised as gluten-free. The interpretation of these results should take into account the fact that the device may detect gluten levels lower than 20 ppm, which generally are safe for patients with celiac disease. Furthermore, the data were uploaded voluntarily by users, and thus are not a random sample (Am J Gastroenterol. 2019;114[5]:792-7.). The device cannot detect certain forms of gluten such as barley malt. Because of limitations like these, the Nima device has “vocal critics,” said Dr. Lebwohl.
A profusion of books that offer dietary advice for patients with celiac disease also has become available. Data from Google Trends indicate that the popularity of the gluten-free diet spread from small pockets of the country in 2006 to most of the states in 2015.
Yet this “do-it-yourself” approach to celiac disease raises several concerns, said Dr. Lebwohl. Patients are at risk of interpreting their test results incorrectly, for example. Failing to consult a dietitian or physician, each of whom could have expertise in the field, entails risks as well. “Knowledgeable and empathetic care-giving is more important than ever,” Dr. Lebwohl concluded.
Dr. Lebwohl is on the medical advisory board of Innovate Biopharmaceuticals, a consultant for Takeda, and an unpaid advisor for the Nima Sensor.
EXPERT ANALYSIS FROM THE 2019 FRESTON CONFERENCE
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).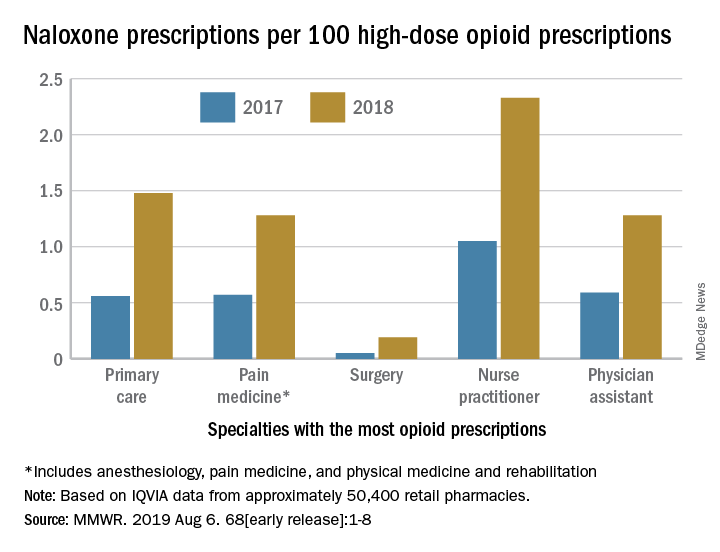
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Vaccination is not associated with increased risk of MS
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
FROM NEUROLOGY
Researchers examine potential causes of dementia in CTE
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
FROM JAMA NEUROLOGY