User login
Erik Greb joined the staff of Neurology Reviews in January 2012. Since then, he has attended scientific conferences, conducted video interviews, and written about clinical research in multiple sclerosis, epilepsy, Parkinson's disease, Alzheimer's disease, stroke, and other neurologic disorders. In addition to news articles, Erik has written investigative stories about multiple sclerosis, headache, and epilepsy. He previously wrote about pharmaceutical manufacturing, drug formulation and delivery, quality assurance, and regulation for Pharmaceutical Technology.
Experts call to revise the Uniform Determination of Death Act
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
FROM ANNALS OF INTERNAL MEDICINE
Employment is associated with high likelihood of declining epilepsy surgery
BALTIMORE – , according to an analysis presented at the annual meeting of the American Epilepsy Society. “Future work should confirm this finding prospectively, determine if it holds in other patient populations, and explore the decision to proceed with or decline epilepsy surgery from a patient-centered perspective,” said Vishal Mandge, MD, MPH, a clinical neurophysiology fellow at Duke University in Durham, N.C., and colleagues. “Identifying the role that factors such as the fear of losing employment due to complications from surgery and inability to take medical leave for an extended period of time play in the patient’s decision to proceed with epilepsy surgery may identify needs and suggest strategies to reduce barriers to this underutilized treatment.”
Although epilepsy surgery is known to be safe and effective, many surgical candidates with drug-resistant epilepsy decline to undergo the procedure. Prior investigations of the barriers to epilepsy surgery have focused on access to epilepsy centers that offer epilepsy surgery and patients’ reluctance to undergo presurgical evaluation. Dr. Mandge and colleagues instead set out to evaluate the association between various demographic, disease-specific, and epilepsy-evaluation variables and patients’ decision to decline surgery after they have been identified as candidates.
A retrospective case-control study
The investigators conducted a retrospective case-control study of patients who were discussed at the epilepsy surgery conference of a tertiary care hospital serving an urban New York community between Jan. 1, 2009, and June 30, 2017. They identified patients who were considered candidates for resective epilepsy surgery. Dr. Mandge and colleagues used the chi-squared test for nominal variables and analysis of variance for scale variables to evaluate these variables’ associations with a patient’s decision to decline epilepsy surgery. They also performed multivariate binary logistic regression to identify variables that predict a patient’s decision to decline surgery.
Dr. Mandge and colleagues identified 159 patients who were discussed during the study period. Of this group, 87 patients were eligible for resective epilepsy surgery after a thorough evaluation. Thirty-four (40%) of the eligible patients declined to undergo surgery. Approximately 20% of eligible patients were employed, and 70% of patients had a high school diploma or higher education.
Univariate analysis indicated that employment (odds ratio, 4.2), temporal lesion on MRI (OR, 0.35), temporal EEG localization (OR, 0.21), and temporal seizure onset zone (OR, 0.19) were independently and significantly associated with a patient’s decision to decline surgery. Multivariate logistic regression analysis indicated that current employment (OR, 7.5), the number of current antiepileptic drugs (AEDs; OR, 3.5), and concordance between seizure semiology, seizure onset on EEG, and imaging (OR, 0.08) were significantly associated with a patient’s decision to decline surgery.
Fear of unemployment may explain results
“With each additional AED, the patients were 3.5 times more likely to decline surgery, even after adjusting for other variables,” said Alexis D. Boro, MD, a neurologist at Montefiore Medical Center in New York and one of the investigators. “My suspicion is that some of this reflects the burden of taking a lot of seizure medication. While the medications are much, much safer than seizures, and looking for and dealing with side effects is a lot of what we do, people often don’t feel great when they are taking multiple seizure medications. We counsel our patients that they should generally expect to stay on some seizure medications after surgery. The reason for surgery is to stop the seizures, not to stop the medications. We are often able to reduce medications after a period of time after surgery, and for many patients, this is one of the benefits.”
The association between employment and increased likelihood of declining surgery was unexpected and may not hold everywhere, said Dr. Boro. “We had expected the opposite result because we assumed that employed patients would be concerned that a seizure at work might result in loss of work. But it may be that many of our patients who are employed are concerned about losing their jobs if they miss work for a medical procedure. Some of our patients may be concerned about sharing medical information with their employers. For some of our patients, being employed may imply limited insurance coverage.”
The study was not supported by external funding, and the investigators did not report any disclosures.
SOURCE: Mandge VA et al. AES 2019, Abstract 1.362.
BALTIMORE – , according to an analysis presented at the annual meeting of the American Epilepsy Society. “Future work should confirm this finding prospectively, determine if it holds in other patient populations, and explore the decision to proceed with or decline epilepsy surgery from a patient-centered perspective,” said Vishal Mandge, MD, MPH, a clinical neurophysiology fellow at Duke University in Durham, N.C., and colleagues. “Identifying the role that factors such as the fear of losing employment due to complications from surgery and inability to take medical leave for an extended period of time play in the patient’s decision to proceed with epilepsy surgery may identify needs and suggest strategies to reduce barriers to this underutilized treatment.”
Although epilepsy surgery is known to be safe and effective, many surgical candidates with drug-resistant epilepsy decline to undergo the procedure. Prior investigations of the barriers to epilepsy surgery have focused on access to epilepsy centers that offer epilepsy surgery and patients’ reluctance to undergo presurgical evaluation. Dr. Mandge and colleagues instead set out to evaluate the association between various demographic, disease-specific, and epilepsy-evaluation variables and patients’ decision to decline surgery after they have been identified as candidates.
A retrospective case-control study
The investigators conducted a retrospective case-control study of patients who were discussed at the epilepsy surgery conference of a tertiary care hospital serving an urban New York community between Jan. 1, 2009, and June 30, 2017. They identified patients who were considered candidates for resective epilepsy surgery. Dr. Mandge and colleagues used the chi-squared test for nominal variables and analysis of variance for scale variables to evaluate these variables’ associations with a patient’s decision to decline epilepsy surgery. They also performed multivariate binary logistic regression to identify variables that predict a patient’s decision to decline surgery.
Dr. Mandge and colleagues identified 159 patients who were discussed during the study period. Of this group, 87 patients were eligible for resective epilepsy surgery after a thorough evaluation. Thirty-four (40%) of the eligible patients declined to undergo surgery. Approximately 20% of eligible patients were employed, and 70% of patients had a high school diploma or higher education.
Univariate analysis indicated that employment (odds ratio, 4.2), temporal lesion on MRI (OR, 0.35), temporal EEG localization (OR, 0.21), and temporal seizure onset zone (OR, 0.19) were independently and significantly associated with a patient’s decision to decline surgery. Multivariate logistic regression analysis indicated that current employment (OR, 7.5), the number of current antiepileptic drugs (AEDs; OR, 3.5), and concordance between seizure semiology, seizure onset on EEG, and imaging (OR, 0.08) were significantly associated with a patient’s decision to decline surgery.
Fear of unemployment may explain results
“With each additional AED, the patients were 3.5 times more likely to decline surgery, even after adjusting for other variables,” said Alexis D. Boro, MD, a neurologist at Montefiore Medical Center in New York and one of the investigators. “My suspicion is that some of this reflects the burden of taking a lot of seizure medication. While the medications are much, much safer than seizures, and looking for and dealing with side effects is a lot of what we do, people often don’t feel great when they are taking multiple seizure medications. We counsel our patients that they should generally expect to stay on some seizure medications after surgery. The reason for surgery is to stop the seizures, not to stop the medications. We are often able to reduce medications after a period of time after surgery, and for many patients, this is one of the benefits.”
The association between employment and increased likelihood of declining surgery was unexpected and may not hold everywhere, said Dr. Boro. “We had expected the opposite result because we assumed that employed patients would be concerned that a seizure at work might result in loss of work. But it may be that many of our patients who are employed are concerned about losing their jobs if they miss work for a medical procedure. Some of our patients may be concerned about sharing medical information with their employers. For some of our patients, being employed may imply limited insurance coverage.”
The study was not supported by external funding, and the investigators did not report any disclosures.
SOURCE: Mandge VA et al. AES 2019, Abstract 1.362.
BALTIMORE – , according to an analysis presented at the annual meeting of the American Epilepsy Society. “Future work should confirm this finding prospectively, determine if it holds in other patient populations, and explore the decision to proceed with or decline epilepsy surgery from a patient-centered perspective,” said Vishal Mandge, MD, MPH, a clinical neurophysiology fellow at Duke University in Durham, N.C., and colleagues. “Identifying the role that factors such as the fear of losing employment due to complications from surgery and inability to take medical leave for an extended period of time play in the patient’s decision to proceed with epilepsy surgery may identify needs and suggest strategies to reduce barriers to this underutilized treatment.”
Although epilepsy surgery is known to be safe and effective, many surgical candidates with drug-resistant epilepsy decline to undergo the procedure. Prior investigations of the barriers to epilepsy surgery have focused on access to epilepsy centers that offer epilepsy surgery and patients’ reluctance to undergo presurgical evaluation. Dr. Mandge and colleagues instead set out to evaluate the association between various demographic, disease-specific, and epilepsy-evaluation variables and patients’ decision to decline surgery after they have been identified as candidates.
A retrospective case-control study
The investigators conducted a retrospective case-control study of patients who were discussed at the epilepsy surgery conference of a tertiary care hospital serving an urban New York community between Jan. 1, 2009, and June 30, 2017. They identified patients who were considered candidates for resective epilepsy surgery. Dr. Mandge and colleagues used the chi-squared test for nominal variables and analysis of variance for scale variables to evaluate these variables’ associations with a patient’s decision to decline epilepsy surgery. They also performed multivariate binary logistic regression to identify variables that predict a patient’s decision to decline surgery.
Dr. Mandge and colleagues identified 159 patients who were discussed during the study period. Of this group, 87 patients were eligible for resective epilepsy surgery after a thorough evaluation. Thirty-four (40%) of the eligible patients declined to undergo surgery. Approximately 20% of eligible patients were employed, and 70% of patients had a high school diploma or higher education.
Univariate analysis indicated that employment (odds ratio, 4.2), temporal lesion on MRI (OR, 0.35), temporal EEG localization (OR, 0.21), and temporal seizure onset zone (OR, 0.19) were independently and significantly associated with a patient’s decision to decline surgery. Multivariate logistic regression analysis indicated that current employment (OR, 7.5), the number of current antiepileptic drugs (AEDs; OR, 3.5), and concordance between seizure semiology, seizure onset on EEG, and imaging (OR, 0.08) were significantly associated with a patient’s decision to decline surgery.
Fear of unemployment may explain results
“With each additional AED, the patients were 3.5 times more likely to decline surgery, even after adjusting for other variables,” said Alexis D. Boro, MD, a neurologist at Montefiore Medical Center in New York and one of the investigators. “My suspicion is that some of this reflects the burden of taking a lot of seizure medication. While the medications are much, much safer than seizures, and looking for and dealing with side effects is a lot of what we do, people often don’t feel great when they are taking multiple seizure medications. We counsel our patients that they should generally expect to stay on some seizure medications after surgery. The reason for surgery is to stop the seizures, not to stop the medications. We are often able to reduce medications after a period of time after surgery, and for many patients, this is one of the benefits.”
The association between employment and increased likelihood of declining surgery was unexpected and may not hold everywhere, said Dr. Boro. “We had expected the opposite result because we assumed that employed patients would be concerned that a seizure at work might result in loss of work. But it may be that many of our patients who are employed are concerned about losing their jobs if they miss work for a medical procedure. Some of our patients may be concerned about sharing medical information with their employers. For some of our patients, being employed may imply limited insurance coverage.”
The study was not supported by external funding, and the investigators did not report any disclosures.
SOURCE: Mandge VA et al. AES 2019, Abstract 1.362.
REPORTING FROM AES 2019
Reduction in convulsive seizure frequency is associated with improved executive function in Dravet syndrome
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
REPORTING FROM AES 2019
Outcomes of epilepsy surgery at 1 year may be better among older patients
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
REPORTING FROM AES 2019
Women with epilepsy less likely than controls to breastfeed
BALTIMORE – , according to data presented at the annual meeting of the American Epilepsy Society. Seizure control, education by the treating neurologist, and postpartum lactation consultative support are associated with adherence to breastfeeding, said the researchers.
“We need to understand and address the challenges that women with epilepsy face beyond seizure control and medication management when they are being seen by various health care providers to ensure the best quality of life for them and their babies,” Abrar Al-Faraj, MD, instructor of neurology at Boston University, said in a press release. “The strong efforts to advocate for breastfeeding in the general population should include women with chronic diseases such as epilepsy.”
A retrospective study of women who underwent pregnancy
Data have established the benefits of breastfeeding in the general population. Recent studies have confirmed that for women with epilepsy and their children, breastfeeding is safe and may provide neurodevelopmental benefits. Data also indicate, however, that rates of breastfeeding are significantly lower in women with epilepsy than in the general population. Dr. Al-Faraj and colleagues sought to compare the rates of initiation of and adherence to breastfeeding in women with epilepsy with those in healthy controls. They also intended to identify the factors that affect breastfeeding in women with epilepsy and assess the influence of support systems (e.g., lactation consult services) on breastfeeding.
The investigators retrospectively studied 102 women with epilepsy who were treated at the Beth Israel Deaconess Medical Center (BIDMC) Epilepsy Clinic and underwent pregnancies between 2009 and 2018. They compared these women to 113 healthy controls without epilepsy who were treated at the obstetrical service at BIDMC during the same period. Dr. Al-Faraj and colleagues reviewed patients’ medical records for demographic information, epilepsy type, degree of seizure control during pregnancy and post partum, number of antiepileptic medications (AEDs), breastfeeding education by providers (i.e., neurologists and epilepsy nurses), lactation consult, and rate of initiation of and adherence to breastfeeding at 6 weeks and 3 and 6 months. The investigators excluded from their analysis patients with other chronic medical conditions, those taking medications other than AEDs that may affect breastfeeding, and those with limited follow-up during pregnancy and post partum.
Education and support were correlated with breastfeeding
Participants’ ages ranged from 20 years to 40 years. The rate of breastfeeding initiation was significantly lower in women with epilepsy (51%) than in controls (87%). The rate declined significantly to 38.2% at 6 weeks in women with epilepsy, compared to 76% in controls. The rate of adherence at 3 months was 36.2% in women with epilepsy, and adherence at 6 months was 18.6%.
The reasons for not breastfeeding were known for 17.6% of women with epilepsy. These reasons included fear of AED exposure through breast milk, recommendations by providers (e.g., pediatricians and obstetricians) not to breastfeed, failed breastfeeding attempts because of technical difficulties (e.g., the baby’s inability to latch), and lack of milk supply. Treating neurologists discussed breastfeeding with 52.9% of women with epilepsy, and epilepsy nurses discussed it with 91% of women with epilepsy. Among the 66% of patients who received obstetrical care at BIDMC, 13% of women with epilepsy had lactation consultation post partum, compared with 58% of controls. Breastfeeding education by the treating neurologist was significantly and positively correlated with decision to breastfeed and initiation of breastfeeding. Postpartum lactation consult support was also associated with a significantly higher rate of breastfeeding initiation, adherence at 6 weeks, adherence at 3 months, and adherence at 6 months. Women with well-controlled seizures were more likely to continue breastfeeding at 6 weeks, compared with women with uncontrolled seizures. The researchers found no statistically significant difference in the breastfeeding initiation rate, however, between women with controlled seizures and those with uncontrolled seizures.
“Women with poor seizure control are a particularly vulnerable group and have the greatest need for intervention to improve breastfeeding rates,” said Dr. Al-Faraj and colleagues. Focused physician education and support measures such as lactation consultation may be potential interventions to improve the treatment of women with epilepsy, they added. “Further prospective investigations are needed to identify other factors that prevent the decision to initiate or adhere to breastfeeding in women with epilepsy and evaluate interventions that may be implemented as a public health measure to support this vulnerable population.”
The study did not have external funding, and the investigators reported no disclosures.
SOURCE: Al-Faraj AO et al. AES 2019, Abstract 1.246.
BALTIMORE – , according to data presented at the annual meeting of the American Epilepsy Society. Seizure control, education by the treating neurologist, and postpartum lactation consultative support are associated with adherence to breastfeeding, said the researchers.
“We need to understand and address the challenges that women with epilepsy face beyond seizure control and medication management when they are being seen by various health care providers to ensure the best quality of life for them and their babies,” Abrar Al-Faraj, MD, instructor of neurology at Boston University, said in a press release. “The strong efforts to advocate for breastfeeding in the general population should include women with chronic diseases such as epilepsy.”
A retrospective study of women who underwent pregnancy
Data have established the benefits of breastfeeding in the general population. Recent studies have confirmed that for women with epilepsy and their children, breastfeeding is safe and may provide neurodevelopmental benefits. Data also indicate, however, that rates of breastfeeding are significantly lower in women with epilepsy than in the general population. Dr. Al-Faraj and colleagues sought to compare the rates of initiation of and adherence to breastfeeding in women with epilepsy with those in healthy controls. They also intended to identify the factors that affect breastfeeding in women with epilepsy and assess the influence of support systems (e.g., lactation consult services) on breastfeeding.
The investigators retrospectively studied 102 women with epilepsy who were treated at the Beth Israel Deaconess Medical Center (BIDMC) Epilepsy Clinic and underwent pregnancies between 2009 and 2018. They compared these women to 113 healthy controls without epilepsy who were treated at the obstetrical service at BIDMC during the same period. Dr. Al-Faraj and colleagues reviewed patients’ medical records for demographic information, epilepsy type, degree of seizure control during pregnancy and post partum, number of antiepileptic medications (AEDs), breastfeeding education by providers (i.e., neurologists and epilepsy nurses), lactation consult, and rate of initiation of and adherence to breastfeeding at 6 weeks and 3 and 6 months. The investigators excluded from their analysis patients with other chronic medical conditions, those taking medications other than AEDs that may affect breastfeeding, and those with limited follow-up during pregnancy and post partum.
Education and support were correlated with breastfeeding
Participants’ ages ranged from 20 years to 40 years. The rate of breastfeeding initiation was significantly lower in women with epilepsy (51%) than in controls (87%). The rate declined significantly to 38.2% at 6 weeks in women with epilepsy, compared to 76% in controls. The rate of adherence at 3 months was 36.2% in women with epilepsy, and adherence at 6 months was 18.6%.
The reasons for not breastfeeding were known for 17.6% of women with epilepsy. These reasons included fear of AED exposure through breast milk, recommendations by providers (e.g., pediatricians and obstetricians) not to breastfeed, failed breastfeeding attempts because of technical difficulties (e.g., the baby’s inability to latch), and lack of milk supply. Treating neurologists discussed breastfeeding with 52.9% of women with epilepsy, and epilepsy nurses discussed it with 91% of women with epilepsy. Among the 66% of patients who received obstetrical care at BIDMC, 13% of women with epilepsy had lactation consultation post partum, compared with 58% of controls. Breastfeeding education by the treating neurologist was significantly and positively correlated with decision to breastfeed and initiation of breastfeeding. Postpartum lactation consult support was also associated with a significantly higher rate of breastfeeding initiation, adherence at 6 weeks, adherence at 3 months, and adherence at 6 months. Women with well-controlled seizures were more likely to continue breastfeeding at 6 weeks, compared with women with uncontrolled seizures. The researchers found no statistically significant difference in the breastfeeding initiation rate, however, between women with controlled seizures and those with uncontrolled seizures.
“Women with poor seizure control are a particularly vulnerable group and have the greatest need for intervention to improve breastfeeding rates,” said Dr. Al-Faraj and colleagues. Focused physician education and support measures such as lactation consultation may be potential interventions to improve the treatment of women with epilepsy, they added. “Further prospective investigations are needed to identify other factors that prevent the decision to initiate or adhere to breastfeeding in women with epilepsy and evaluate interventions that may be implemented as a public health measure to support this vulnerable population.”
The study did not have external funding, and the investigators reported no disclosures.
SOURCE: Al-Faraj AO et al. AES 2019, Abstract 1.246.
BALTIMORE – , according to data presented at the annual meeting of the American Epilepsy Society. Seizure control, education by the treating neurologist, and postpartum lactation consultative support are associated with adherence to breastfeeding, said the researchers.
“We need to understand and address the challenges that women with epilepsy face beyond seizure control and medication management when they are being seen by various health care providers to ensure the best quality of life for them and their babies,” Abrar Al-Faraj, MD, instructor of neurology at Boston University, said in a press release. “The strong efforts to advocate for breastfeeding in the general population should include women with chronic diseases such as epilepsy.”
A retrospective study of women who underwent pregnancy
Data have established the benefits of breastfeeding in the general population. Recent studies have confirmed that for women with epilepsy and their children, breastfeeding is safe and may provide neurodevelopmental benefits. Data also indicate, however, that rates of breastfeeding are significantly lower in women with epilepsy than in the general population. Dr. Al-Faraj and colleagues sought to compare the rates of initiation of and adherence to breastfeeding in women with epilepsy with those in healthy controls. They also intended to identify the factors that affect breastfeeding in women with epilepsy and assess the influence of support systems (e.g., lactation consult services) on breastfeeding.
The investigators retrospectively studied 102 women with epilepsy who were treated at the Beth Israel Deaconess Medical Center (BIDMC) Epilepsy Clinic and underwent pregnancies between 2009 and 2018. They compared these women to 113 healthy controls without epilepsy who were treated at the obstetrical service at BIDMC during the same period. Dr. Al-Faraj and colleagues reviewed patients’ medical records for demographic information, epilepsy type, degree of seizure control during pregnancy and post partum, number of antiepileptic medications (AEDs), breastfeeding education by providers (i.e., neurologists and epilepsy nurses), lactation consult, and rate of initiation of and adherence to breastfeeding at 6 weeks and 3 and 6 months. The investigators excluded from their analysis patients with other chronic medical conditions, those taking medications other than AEDs that may affect breastfeeding, and those with limited follow-up during pregnancy and post partum.
Education and support were correlated with breastfeeding
Participants’ ages ranged from 20 years to 40 years. The rate of breastfeeding initiation was significantly lower in women with epilepsy (51%) than in controls (87%). The rate declined significantly to 38.2% at 6 weeks in women with epilepsy, compared to 76% in controls. The rate of adherence at 3 months was 36.2% in women with epilepsy, and adherence at 6 months was 18.6%.
The reasons for not breastfeeding were known for 17.6% of women with epilepsy. These reasons included fear of AED exposure through breast milk, recommendations by providers (e.g., pediatricians and obstetricians) not to breastfeed, failed breastfeeding attempts because of technical difficulties (e.g., the baby’s inability to latch), and lack of milk supply. Treating neurologists discussed breastfeeding with 52.9% of women with epilepsy, and epilepsy nurses discussed it with 91% of women with epilepsy. Among the 66% of patients who received obstetrical care at BIDMC, 13% of women with epilepsy had lactation consultation post partum, compared with 58% of controls. Breastfeeding education by the treating neurologist was significantly and positively correlated with decision to breastfeed and initiation of breastfeeding. Postpartum lactation consult support was also associated with a significantly higher rate of breastfeeding initiation, adherence at 6 weeks, adherence at 3 months, and adherence at 6 months. Women with well-controlled seizures were more likely to continue breastfeeding at 6 weeks, compared with women with uncontrolled seizures. The researchers found no statistically significant difference in the breastfeeding initiation rate, however, between women with controlled seizures and those with uncontrolled seizures.
“Women with poor seizure control are a particularly vulnerable group and have the greatest need for intervention to improve breastfeeding rates,” said Dr. Al-Faraj and colleagues. Focused physician education and support measures such as lactation consultation may be potential interventions to improve the treatment of women with epilepsy, they added. “Further prospective investigations are needed to identify other factors that prevent the decision to initiate or adhere to breastfeeding in women with epilepsy and evaluate interventions that may be implemented as a public health measure to support this vulnerable population.”
The study did not have external funding, and the investigators reported no disclosures.
SOURCE: Al-Faraj AO et al. AES 2019, Abstract 1.246.
REPORTING FROM AES 2019
CDC finds that efforts to reduce new HIV infections have stalled
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.
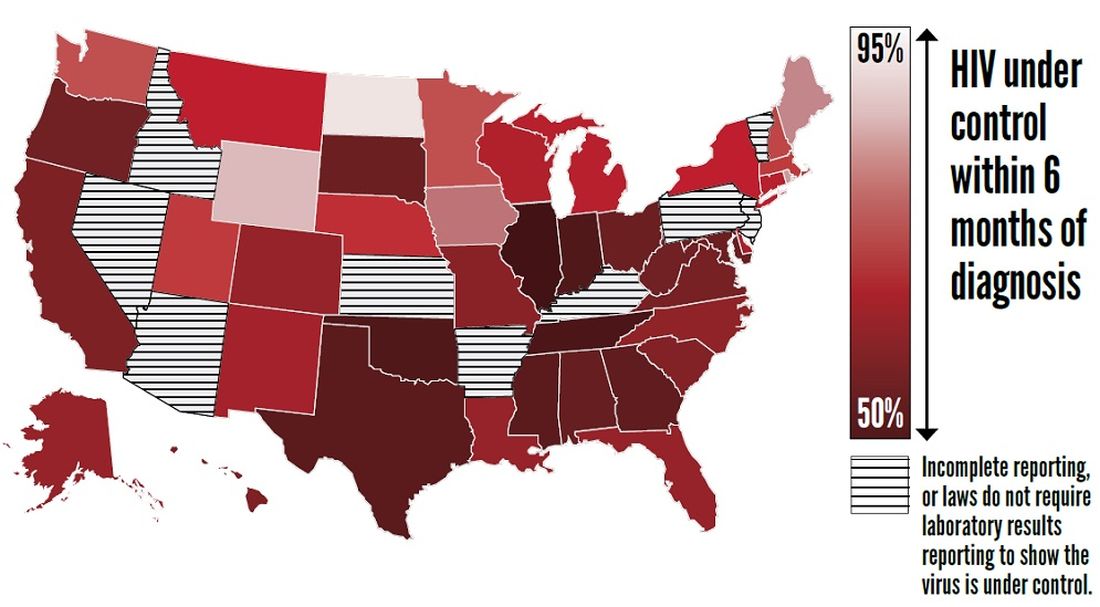
The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.

The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.

The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
FROM THE CDC
Headache may be a significant outcome of pediatric hemispherectomy
CHARLOTTE, N.C. – , according to a study presented at the annual meeting of the Child Neurology Society. Patients with headache before hemispherectomy may be at risk of increased headache frequency after the procedure. The most common type of headache reported is nonmigrainous headache.
“We recommend that hemispherectomy patients be evaluated and followed closely postoperatively and questioned regarding the presence of headache,” said William Bingaman, MD, vice-chair of the neurological institute and head of the section of epilepsy surgery at the Cleveland Clinic, and colleagues. “Patients should be assessed and treated quickly to decrease prolonged pain and suffering and increase function.”
The two main types of hemispherectomy are anatomical and functional. Anatomical hemispherectomy entails the removal of a large amount of brain mass. Functional hemispherectomy entails the removal of a smaller amount of brain mass, as well as the disconnection of the brain’s hemispheres. Most patients are seizure free or have reduced seizure frequency after hemispherectomy. Other common postoperative outcomes include changes in behavior, cognition, motor function, and speech. Many studies have explored these outcomes, but few have examined the frequency of postoperative headache in children who undergo hemispherectomy.
Dr. Bingaman and colleagues retrospectively reviewed the charts of 74 children who underwent hemispherectomy at the Cleveland Clinic during the previous 5 years. They excluded 14 patients who were too young to respond for the analysis. The investigators sent the remaining 60 patients an informative letter and followed up with a 10-minute phone questionnaire about patients’ postoperative headache symptoms.
Twenty-two (36.7%) eligible patients completed the questionnaire. Thirty-eight patients could not be reached or declined to participate. Half of the 22 respondents were male. Participants’ median age at surgery was 6.5 years. The most common types of hemispherectomy were left functional (50%) and right functional (31.8%).
Nine (39.1%) of the 22 respondents had headache before surgery, and seven (31.8%) reported a family history of headache. In all, 19 (86.4%) patients had headache after surgery, and 10 (45.5%) had headaches that began after the surgery. Of the nine patients with preoperative headache, six (66.6%) had an increase in headache frequency after surgery, two (22.2%) reported no change, and one (11.1%) had decreased headache frequency. The most common type of headache reported was tension-type headache, and migraine was the second most common.
“The cause of any posthemispherectomy headache has thus far only been attributed to hydrocephalus,” wrote Dr. Bingaman and colleagues. “The tendency of headache to worsen following stresses such as illness, stroke, trauma, etc. has been studied extensively. The pathophysiology of posthemispherectomy headache can be investigated further by classifying hemispherectomy as a type of trauma or injury, which would explain the development of postoperative headache. Previous studies on posttraumatic headache have ascribed these headaches to neuroinflammation following the injury. Additionally, posthemispherectomy headache could also be due to the buildup of debris and fluid following the operation. Future hemispherectomy patients should be treated prophylactically for headache.”
The authors did not report funding for their study or declare any disclosures.
SOURCE: Pandit I et al. CNS 2019. Abstract 99.
CHARLOTTE, N.C. – , according to a study presented at the annual meeting of the Child Neurology Society. Patients with headache before hemispherectomy may be at risk of increased headache frequency after the procedure. The most common type of headache reported is nonmigrainous headache.
“We recommend that hemispherectomy patients be evaluated and followed closely postoperatively and questioned regarding the presence of headache,” said William Bingaman, MD, vice-chair of the neurological institute and head of the section of epilepsy surgery at the Cleveland Clinic, and colleagues. “Patients should be assessed and treated quickly to decrease prolonged pain and suffering and increase function.”
The two main types of hemispherectomy are anatomical and functional. Anatomical hemispherectomy entails the removal of a large amount of brain mass. Functional hemispherectomy entails the removal of a smaller amount of brain mass, as well as the disconnection of the brain’s hemispheres. Most patients are seizure free or have reduced seizure frequency after hemispherectomy. Other common postoperative outcomes include changes in behavior, cognition, motor function, and speech. Many studies have explored these outcomes, but few have examined the frequency of postoperative headache in children who undergo hemispherectomy.
Dr. Bingaman and colleagues retrospectively reviewed the charts of 74 children who underwent hemispherectomy at the Cleveland Clinic during the previous 5 years. They excluded 14 patients who were too young to respond for the analysis. The investigators sent the remaining 60 patients an informative letter and followed up with a 10-minute phone questionnaire about patients’ postoperative headache symptoms.
Twenty-two (36.7%) eligible patients completed the questionnaire. Thirty-eight patients could not be reached or declined to participate. Half of the 22 respondents were male. Participants’ median age at surgery was 6.5 years. The most common types of hemispherectomy were left functional (50%) and right functional (31.8%).
Nine (39.1%) of the 22 respondents had headache before surgery, and seven (31.8%) reported a family history of headache. In all, 19 (86.4%) patients had headache after surgery, and 10 (45.5%) had headaches that began after the surgery. Of the nine patients with preoperative headache, six (66.6%) had an increase in headache frequency after surgery, two (22.2%) reported no change, and one (11.1%) had decreased headache frequency. The most common type of headache reported was tension-type headache, and migraine was the second most common.
“The cause of any posthemispherectomy headache has thus far only been attributed to hydrocephalus,” wrote Dr. Bingaman and colleagues. “The tendency of headache to worsen following stresses such as illness, stroke, trauma, etc. has been studied extensively. The pathophysiology of posthemispherectomy headache can be investigated further by classifying hemispherectomy as a type of trauma or injury, which would explain the development of postoperative headache. Previous studies on posttraumatic headache have ascribed these headaches to neuroinflammation following the injury. Additionally, posthemispherectomy headache could also be due to the buildup of debris and fluid following the operation. Future hemispherectomy patients should be treated prophylactically for headache.”
The authors did not report funding for their study or declare any disclosures.
SOURCE: Pandit I et al. CNS 2019. Abstract 99.
CHARLOTTE, N.C. – , according to a study presented at the annual meeting of the Child Neurology Society. Patients with headache before hemispherectomy may be at risk of increased headache frequency after the procedure. The most common type of headache reported is nonmigrainous headache.
“We recommend that hemispherectomy patients be evaluated and followed closely postoperatively and questioned regarding the presence of headache,” said William Bingaman, MD, vice-chair of the neurological institute and head of the section of epilepsy surgery at the Cleveland Clinic, and colleagues. “Patients should be assessed and treated quickly to decrease prolonged pain and suffering and increase function.”
The two main types of hemispherectomy are anatomical and functional. Anatomical hemispherectomy entails the removal of a large amount of brain mass. Functional hemispherectomy entails the removal of a smaller amount of brain mass, as well as the disconnection of the brain’s hemispheres. Most patients are seizure free or have reduced seizure frequency after hemispherectomy. Other common postoperative outcomes include changes in behavior, cognition, motor function, and speech. Many studies have explored these outcomes, but few have examined the frequency of postoperative headache in children who undergo hemispherectomy.
Dr. Bingaman and colleagues retrospectively reviewed the charts of 74 children who underwent hemispherectomy at the Cleveland Clinic during the previous 5 years. They excluded 14 patients who were too young to respond for the analysis. The investigators sent the remaining 60 patients an informative letter and followed up with a 10-minute phone questionnaire about patients’ postoperative headache symptoms.
Twenty-two (36.7%) eligible patients completed the questionnaire. Thirty-eight patients could not be reached or declined to participate. Half of the 22 respondents were male. Participants’ median age at surgery was 6.5 years. The most common types of hemispherectomy were left functional (50%) and right functional (31.8%).
Nine (39.1%) of the 22 respondents had headache before surgery, and seven (31.8%) reported a family history of headache. In all, 19 (86.4%) patients had headache after surgery, and 10 (45.5%) had headaches that began after the surgery. Of the nine patients with preoperative headache, six (66.6%) had an increase in headache frequency after surgery, two (22.2%) reported no change, and one (11.1%) had decreased headache frequency. The most common type of headache reported was tension-type headache, and migraine was the second most common.
“The cause of any posthemispherectomy headache has thus far only been attributed to hydrocephalus,” wrote Dr. Bingaman and colleagues. “The tendency of headache to worsen following stresses such as illness, stroke, trauma, etc. has been studied extensively. The pathophysiology of posthemispherectomy headache can be investigated further by classifying hemispherectomy as a type of trauma or injury, which would explain the development of postoperative headache. Previous studies on posttraumatic headache have ascribed these headaches to neuroinflammation following the injury. Additionally, posthemispherectomy headache could also be due to the buildup of debris and fluid following the operation. Future hemispherectomy patients should be treated prophylactically for headache.”
The authors did not report funding for their study or declare any disclosures.
SOURCE: Pandit I et al. CNS 2019. Abstract 99.
REPORTING FROM CNS 2019
Use of PEP prophylaxis techniques may diverge from the evidence
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic pancreatic duct stent placement and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used pancreatic duct stent placement to prevent PEP. Most responders (72%) used pancreatic duct stent placement only in patients at high risk of PEP, and 64% reported using pancreatic duct stent placement in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used pancreatic duct stent placement for PEP in more than half of ERCPs. Among endoscopists who rarely use pancreatic duct stent placement for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that pancreatic duct stent placement was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
AGA offers resources to help your patients understand ECRP and scope safety. Learn more at https://www.gastro.org/
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic pancreatic duct stent placement and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used pancreatic duct stent placement to prevent PEP. Most responders (72%) used pancreatic duct stent placement only in patients at high risk of PEP, and 64% reported using pancreatic duct stent placement in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used pancreatic duct stent placement for PEP in more than half of ERCPs. Among endoscopists who rarely use pancreatic duct stent placement for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that pancreatic duct stent placement was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
AGA offers resources to help your patients understand ECRP and scope safety. Learn more at https://www.gastro.org/
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic pancreatic duct stent placement and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used pancreatic duct stent placement to prevent PEP. Most responders (72%) used pancreatic duct stent placement only in patients at high risk of PEP, and 64% reported using pancreatic duct stent placement in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used pancreatic duct stent placement for PEP in more than half of ERCPs. Among endoscopists who rarely use pancreatic duct stent placement for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that pancreatic duct stent placement was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
AGA offers resources to help your patients understand ECRP and scope safety. Learn more at https://www.gastro.org/
FROM GASTROINTESTINAL ENDOSCOPY
Use of PEP prophylaxis techniques may diverge from the evidence
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used to prevent PEP. Most responders (72%) used only in patients at high risk of PEP, and 64% reported using in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used for PEP in more than half of ERCPs. Among endoscopists who rarely use for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
* This story was updated on 12/2/2019.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used to prevent PEP. Most responders (72%) used only in patients at high risk of PEP, and 64% reported using in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used for PEP in more than half of ERCPs. Among endoscopists who rarely use for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
* This story was updated on 12/2/2019.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
(PEP), according to research published online in Gastrointestinal Endoscopy. In addition, methods for PEP prophylaxis in clinical practice are not implemented to the extent that the current evidence warrants.
“Future studies should not only further clarify the optimal PEP prophylaxis strategy, but should also focus on strategies to improve the implementation of evidence-based PEP prophylaxis techniques,” wrote Patrick Avila, MD, MPH, gastroenterologist at the University of California, San Francisco, and colleagues.
A survey of American endoscopists
Approximately 4% of patients who undergo biliopancreatic endoscopy develop PEP, which has a mortality rate of 3%. The American Society for Gastrointestinal Endoscopy (ASGE) recommends prophylactic and rectal NSAIDs to reduce the incidence and severity of PEP in patients at high risk. The ASGE further suggests that rectal indomethacin may decrease the risk and severity of PEP in patients at average risk.
The European Society for Gastrointestinal Endoscopy (ESGE) recommends rectal indomethacin for all patients undergoing ERCP (endoscopic retrograde cholangiopancreatography). Several surveys of European endoscopists, however, indicate that relatively few respondents have adopted the recommended prophylactic techniques. The literature does not contain information about practice patterns among American endoscopists, and Dr. Avila and colleagues decided to investigate this question.
The researchers developed a 16-question online survey to assess current practice patterns with regard to PEP prophylaxis. They defined ERCPs that entailed a high risk for PEP as any that involved pancreatic or precut sphincterotomy, traumatic biliary sphincterotomy, balloon dilation of the biliary sphincter, injection of the pancreatic duct, extensive pancreatic duct instrumentation, difficult cannulation, suspected sphincter of Oddi dysfunction, previous PEP, or a female patient. Dr. Avila and colleagues distributed the survey to 233 advanced endoscopists involved in advanced endoscopy fellowship training programs.
Respondents had years of experience
Sixty-two endoscopists (26.7%) completed the survey. Respondents’ mean age was 47 years, and most respondents (74.6%) had been performing ERCP for more than 5 years. Almost all respondents (95%) worked at a tertiary referral center, and all worked with fellows.
All respondents reported having used to prevent PEP. Most responders (72%) used only in patients at high risk of PEP, and 64% reported using in 25% or less of the ERCPs that they had performed. Four respondents (6.8%) used for PEP in more than half of ERCPs. Among endoscopists who rarely use for PEP, the major reasons cited included concern about increased risk of PEP with failed pancreatic duct insertion, the belief that stents do not provide additional benefit beyond pharmacologic prophylaxis, and difficulties in following up patients to ensure stent passage.
About 98% of respondents reported using rectal NSAIDs for PEP. Thirty-four respondents (59.7%) used this treatment only for patients at high risk, and 23 respondents (40.1%) used it for patients at average risk. Among respondents who used rectal NSAIDs to prevent PEP, 67.8% used the treatment in half or more of ERCPs. The NSAID of choice was indomethacin for all respondents. One respondent reported never using rectal NSAIDs for PEP because of doubts about its efficacy, in addition to cost and availability.
In addition, 49 respondents (83.0%) reported using rapid intravenous fluids to prevent PEP. None reported using octreotide or antibiotics to prevent PEP.
Results may reflect recall bias
The survey reveals “a significant divergence from the scientific evidence in how PEP techniques are used in routine clinical practice,” wrote Dr. Avila and colleagues. Several studies, including a randomized controlled trial, support the use of rectal NSAIDs as prophylaxis as patients at average risk of PEP, but less than half of respondents reported using it. The ASGE guidelines state that this treatment is “reasonable,” but do not advocate for it. “If appropriate, adopting a stronger stance in our practice guidelines may lead to further widespread use of rectal NSAIDs in this group of patients,” wrote Dr. Avila and colleagues.
Pancreatic duct stent placement is a difficult procedure to perform. The success rate in one British study was 51%, and a study of expert pancreaticobiliary endoscopists found a failure rate of 7%. It therefore may not be surprising that was used less often than rectal NSAIDs among respondents, according to the authors.
Dr. Avila and colleagues acknowledged that the survey’s low response rate could have introduced nonresponse bias into the findings. They also stated that the study may have been affected by selection and recall biases. The results thus may not be generalizable to other practice settings, they concluded.
The authors did not report any study funding or disclosures.
* This story was updated on 12/2/2019.
SOURCE: Avila P et al. Gastrointest Endosc. 2019 Nov 16. doi: 10.1016/j.gie.2019.11.013.
FROM GASTROINTESTINAL ENDOSCOPY
Benefits of focused ultrasound thalamotomy for essential tremor persist for 3 years
, according to data published Nov. 20 in Neurology. Improvement from baseline remains significant at that time point, although the magnitude of effect may decrease. In addition, the treatment is not associated with progressive or delayed complications.
“For people who have disabling essential tremor that is not responding to medication, this treatment should be considered as a safe and effective option,” Casey H. Halpern, MD, assistant professor of neurosurgery at Stanford (Calif.) University, said in a press release.
Long-term follow-up of a prospective trial
Focused ultrasound thalamotomy is an emerging treatment for essential tremor. The procedure, which does not require an incision, is conducted with the guidance of magnetic resonance thermometry and patient feedback. A randomized controlled trial conducted by Elias and colleagues indicated that focused ultrasound ventral intermediate nucleus thalamotomy significantly suppressed tremor, reduced disability, and improved quality of life at 3 months, compared with sham treatment. This improvement was sustained at 12 months, and a follow-up study showed that improvements in tremor and functional disability were sustained at 24 months.
Dr. Halpern and colleagues sought to evaluate the continued safety and efficacy of focused ultrasound thalamotomy at 3 years’ follow-up in patients who participated in the original trial. Movement disorder specialists evaluated participants’ tremor severity and functional impairment using the Clinical Rating Scale for Tremor (CRST) at baseline and at 12, 24, and 36 months after treatment. Patients responded to the Quality of Life in Essential Tremor (QUEST) questionnaire, which assesses quality of life at baseline and at each follow-up visit. Neurologists evaluated and recorded all adverse events that occurred during the trial.
Postural tremor was eliminated
The original population included 75 patients who underwent focused ultrasound thalamotomy during the randomized, blinded phase or in an unblinded fashion during the crossover phase. The mean age of all treated patients was 71 years, and disease duration at treatment was 16.8 years. Fifty-two participants were observed at 36 months, and the 3-year attrition rate thus was 31%.
Dr. Halpern and colleagues found that the hand combined tremor–motor score, which was the trial’s primary endpoint, was significantly improved from baseline at 3 years. The median improvement from baseline was 56%. The median disability score decreased by 63% from baseline. Postural tremor was eliminated at 36 months, and QUEST score improved by 50%.
For patients who were missing at 3-year follow-up, data obtained at 3 months was used for comparison. These patients had less improvement in hand tremor–motor score, less reduction in disability, and less reduction in postural tremor, compared with patients who presented for 3-year follow-up. When the investigators reanalyzed their results to account for missing data, they found that the improvement from baseline remained significant.
Dr. Halpern and colleagues compared scores at 36 months and at 6 months to evaluate the durability of the treatment effect. Data were available for 49 patients at both time points, and their combined tremor–motor score had increased by a median of one point at 36 months. Disability score increased by a median of 2 points at 36 months. Posture and QUEST scores did not change significantly. About 58% of patients had at least 50% improvement in hand combined tremor–motor score at 36 months, compared with 64% at 24 months and 61% at 12 months.
The investigators described all adverse events as mild or moderate. No new procedure-related adverse events occurred between 24 and 36 months of follow-up, and none worsened during this period. Two adverse events, however, resolved between 24 and 36 months: one case of dysarthria and one of imbalance.
Reduction in improvement may have many causes
“A reduction in improvement is not unexpected, as essential tremor is a progressive disease,” wrote Dr. Halpern and colleagues. “In addition, diminishing performance of motor–functional tasks over time, particularly in this elderly population, may be multifactorial.” Decrease in tremor control has been reported after all surgical treatments for essential tremor (e.g., deep brain stimulation [DBS] and radiofrequency thalamotomy). Retreatment with invasive therapies or ionizing irradiation would be more problematic than retreatment with focused ultrasound thalamotomy, they added.
The researchers acknowledged that the main limitations of their study were the 31% dropout rate at 3 years and the fact that the cohort at 3-year follow-up differed from those at 2-year follow-up and in the original trial. The results nevertheless “demonstrate persistent, significant tremor reduction, as well as functional and quality of life improvement, with a positive safety profile,” they wrote.
Study funding was provided by the Focused Ultrasound Foundation, the Binational Industrial Research and Development Foundation of Israel, and InSightec, the maker of the focused ultrasound equipment that the researchers used. Dr. Halpern and other investigators received research funding from InSightec. One of the researchers is on the company’s medical advisory board, and another served as a consultant to the company.
Effect on axial tremor is unclear
The 50% improvement in hand tremor, disability, and quality of life that Halpern et al. report is similar to the improvement observed following DBS therapy, said Aparna Wagle Shukla, MD, director of the neurophysiology laboratory at the University of Florida in Gainesville, in an interview. Although the results are promising, neurologists should bear several points in mind, she added.
“DBS-induced side effects often are amenable to programming adjustments. However, similar to radiofrequency thalamotomy, focused ultrasound thalamotomy causes lesion effects. While the study discusses the nature of thalamotomy-induced adverse effects, the clinical practitioners also will benefit from learning about the severity of side effects and how they were individually addressed,” said Dr. Wagle Shukla. “The study acknowledges that there was a 30% dropout rate at 3 years’ follow-up. As the original plan included a 5-year follow-up, it would be beneficial to know why a large fraction of participants discontinued participation earlier than expected.”
Furthermore, the study by Halpern et al. leaves several questions unanswered. It does not indicate, for example, whether focused ultrasound thalamotomy can affect the control of axial tremor, including head and voice tremor, said Dr. Wagle Shukla. “Also, the potential of focused ultrasound thalamotomy to treat complex tremors with possible targeting of multiple brain regions such as ventralis oralis anterior and posterior and zona incerta stimulation is currently not known.
“There is no doubt that focused ultrasound thalamotomy is useful for the control of hand tremors in patients diagnosed with essential tremor, with long-term improvements in quality of life,” Dr. Wagle Shukla continued. “However, it is presently limited in its scope as a unilateral, single-target brain procedure.”
SOURCE: Halpern CH et al. Neurology. 2019 Nov 20 (Epub ahead of print).
, according to data published Nov. 20 in Neurology. Improvement from baseline remains significant at that time point, although the magnitude of effect may decrease. In addition, the treatment is not associated with progressive or delayed complications.
“For people who have disabling essential tremor that is not responding to medication, this treatment should be considered as a safe and effective option,” Casey H. Halpern, MD, assistant professor of neurosurgery at Stanford (Calif.) University, said in a press release.
Long-term follow-up of a prospective trial
Focused ultrasound thalamotomy is an emerging treatment for essential tremor. The procedure, which does not require an incision, is conducted with the guidance of magnetic resonance thermometry and patient feedback. A randomized controlled trial conducted by Elias and colleagues indicated that focused ultrasound ventral intermediate nucleus thalamotomy significantly suppressed tremor, reduced disability, and improved quality of life at 3 months, compared with sham treatment. This improvement was sustained at 12 months, and a follow-up study showed that improvements in tremor and functional disability were sustained at 24 months.
Dr. Halpern and colleagues sought to evaluate the continued safety and efficacy of focused ultrasound thalamotomy at 3 years’ follow-up in patients who participated in the original trial. Movement disorder specialists evaluated participants’ tremor severity and functional impairment using the Clinical Rating Scale for Tremor (CRST) at baseline and at 12, 24, and 36 months after treatment. Patients responded to the Quality of Life in Essential Tremor (QUEST) questionnaire, which assesses quality of life at baseline and at each follow-up visit. Neurologists evaluated and recorded all adverse events that occurred during the trial.
Postural tremor was eliminated
The original population included 75 patients who underwent focused ultrasound thalamotomy during the randomized, blinded phase or in an unblinded fashion during the crossover phase. The mean age of all treated patients was 71 years, and disease duration at treatment was 16.8 years. Fifty-two participants were observed at 36 months, and the 3-year attrition rate thus was 31%.
Dr. Halpern and colleagues found that the hand combined tremor–motor score, which was the trial’s primary endpoint, was significantly improved from baseline at 3 years. The median improvement from baseline was 56%. The median disability score decreased by 63% from baseline. Postural tremor was eliminated at 36 months, and QUEST score improved by 50%.
For patients who were missing at 3-year follow-up, data obtained at 3 months was used for comparison. These patients had less improvement in hand tremor–motor score, less reduction in disability, and less reduction in postural tremor, compared with patients who presented for 3-year follow-up. When the investigators reanalyzed their results to account for missing data, they found that the improvement from baseline remained significant.
Dr. Halpern and colleagues compared scores at 36 months and at 6 months to evaluate the durability of the treatment effect. Data were available for 49 patients at both time points, and their combined tremor–motor score had increased by a median of one point at 36 months. Disability score increased by a median of 2 points at 36 months. Posture and QUEST scores did not change significantly. About 58% of patients had at least 50% improvement in hand combined tremor–motor score at 36 months, compared with 64% at 24 months and 61% at 12 months.
The investigators described all adverse events as mild or moderate. No new procedure-related adverse events occurred between 24 and 36 months of follow-up, and none worsened during this period. Two adverse events, however, resolved between 24 and 36 months: one case of dysarthria and one of imbalance.
Reduction in improvement may have many causes
“A reduction in improvement is not unexpected, as essential tremor is a progressive disease,” wrote Dr. Halpern and colleagues. “In addition, diminishing performance of motor–functional tasks over time, particularly in this elderly population, may be multifactorial.” Decrease in tremor control has been reported after all surgical treatments for essential tremor (e.g., deep brain stimulation [DBS] and radiofrequency thalamotomy). Retreatment with invasive therapies or ionizing irradiation would be more problematic than retreatment with focused ultrasound thalamotomy, they added.
The researchers acknowledged that the main limitations of their study were the 31% dropout rate at 3 years and the fact that the cohort at 3-year follow-up differed from those at 2-year follow-up and in the original trial. The results nevertheless “demonstrate persistent, significant tremor reduction, as well as functional and quality of life improvement, with a positive safety profile,” they wrote.
Study funding was provided by the Focused Ultrasound Foundation, the Binational Industrial Research and Development Foundation of Israel, and InSightec, the maker of the focused ultrasound equipment that the researchers used. Dr. Halpern and other investigators received research funding from InSightec. One of the researchers is on the company’s medical advisory board, and another served as a consultant to the company.
Effect on axial tremor is unclear
The 50% improvement in hand tremor, disability, and quality of life that Halpern et al. report is similar to the improvement observed following DBS therapy, said Aparna Wagle Shukla, MD, director of the neurophysiology laboratory at the University of Florida in Gainesville, in an interview. Although the results are promising, neurologists should bear several points in mind, she added.
“DBS-induced side effects often are amenable to programming adjustments. However, similar to radiofrequency thalamotomy, focused ultrasound thalamotomy causes lesion effects. While the study discusses the nature of thalamotomy-induced adverse effects, the clinical practitioners also will benefit from learning about the severity of side effects and how they were individually addressed,” said Dr. Wagle Shukla. “The study acknowledges that there was a 30% dropout rate at 3 years’ follow-up. As the original plan included a 5-year follow-up, it would be beneficial to know why a large fraction of participants discontinued participation earlier than expected.”
Furthermore, the study by Halpern et al. leaves several questions unanswered. It does not indicate, for example, whether focused ultrasound thalamotomy can affect the control of axial tremor, including head and voice tremor, said Dr. Wagle Shukla. “Also, the potential of focused ultrasound thalamotomy to treat complex tremors with possible targeting of multiple brain regions such as ventralis oralis anterior and posterior and zona incerta stimulation is currently not known.
“There is no doubt that focused ultrasound thalamotomy is useful for the control of hand tremors in patients diagnosed with essential tremor, with long-term improvements in quality of life,” Dr. Wagle Shukla continued. “However, it is presently limited in its scope as a unilateral, single-target brain procedure.”
SOURCE: Halpern CH et al. Neurology. 2019 Nov 20 (Epub ahead of print).
, according to data published Nov. 20 in Neurology. Improvement from baseline remains significant at that time point, although the magnitude of effect may decrease. In addition, the treatment is not associated with progressive or delayed complications.
“For people who have disabling essential tremor that is not responding to medication, this treatment should be considered as a safe and effective option,” Casey H. Halpern, MD, assistant professor of neurosurgery at Stanford (Calif.) University, said in a press release.
Long-term follow-up of a prospective trial
Focused ultrasound thalamotomy is an emerging treatment for essential tremor. The procedure, which does not require an incision, is conducted with the guidance of magnetic resonance thermometry and patient feedback. A randomized controlled trial conducted by Elias and colleagues indicated that focused ultrasound ventral intermediate nucleus thalamotomy significantly suppressed tremor, reduced disability, and improved quality of life at 3 months, compared with sham treatment. This improvement was sustained at 12 months, and a follow-up study showed that improvements in tremor and functional disability were sustained at 24 months.
Dr. Halpern and colleagues sought to evaluate the continued safety and efficacy of focused ultrasound thalamotomy at 3 years’ follow-up in patients who participated in the original trial. Movement disorder specialists evaluated participants’ tremor severity and functional impairment using the Clinical Rating Scale for Tremor (CRST) at baseline and at 12, 24, and 36 months after treatment. Patients responded to the Quality of Life in Essential Tremor (QUEST) questionnaire, which assesses quality of life at baseline and at each follow-up visit. Neurologists evaluated and recorded all adverse events that occurred during the trial.
Postural tremor was eliminated
The original population included 75 patients who underwent focused ultrasound thalamotomy during the randomized, blinded phase or in an unblinded fashion during the crossover phase. The mean age of all treated patients was 71 years, and disease duration at treatment was 16.8 years. Fifty-two participants were observed at 36 months, and the 3-year attrition rate thus was 31%.
Dr. Halpern and colleagues found that the hand combined tremor–motor score, which was the trial’s primary endpoint, was significantly improved from baseline at 3 years. The median improvement from baseline was 56%. The median disability score decreased by 63% from baseline. Postural tremor was eliminated at 36 months, and QUEST score improved by 50%.
For patients who were missing at 3-year follow-up, data obtained at 3 months was used for comparison. These patients had less improvement in hand tremor–motor score, less reduction in disability, and less reduction in postural tremor, compared with patients who presented for 3-year follow-up. When the investigators reanalyzed their results to account for missing data, they found that the improvement from baseline remained significant.
Dr. Halpern and colleagues compared scores at 36 months and at 6 months to evaluate the durability of the treatment effect. Data were available for 49 patients at both time points, and their combined tremor–motor score had increased by a median of one point at 36 months. Disability score increased by a median of 2 points at 36 months. Posture and QUEST scores did not change significantly. About 58% of patients had at least 50% improvement in hand combined tremor–motor score at 36 months, compared with 64% at 24 months and 61% at 12 months.
The investigators described all adverse events as mild or moderate. No new procedure-related adverse events occurred between 24 and 36 months of follow-up, and none worsened during this period. Two adverse events, however, resolved between 24 and 36 months: one case of dysarthria and one of imbalance.
Reduction in improvement may have many causes
“A reduction in improvement is not unexpected, as essential tremor is a progressive disease,” wrote Dr. Halpern and colleagues. “In addition, diminishing performance of motor–functional tasks over time, particularly in this elderly population, may be multifactorial.” Decrease in tremor control has been reported after all surgical treatments for essential tremor (e.g., deep brain stimulation [DBS] and radiofrequency thalamotomy). Retreatment with invasive therapies or ionizing irradiation would be more problematic than retreatment with focused ultrasound thalamotomy, they added.
The researchers acknowledged that the main limitations of their study were the 31% dropout rate at 3 years and the fact that the cohort at 3-year follow-up differed from those at 2-year follow-up and in the original trial. The results nevertheless “demonstrate persistent, significant tremor reduction, as well as functional and quality of life improvement, with a positive safety profile,” they wrote.
Study funding was provided by the Focused Ultrasound Foundation, the Binational Industrial Research and Development Foundation of Israel, and InSightec, the maker of the focused ultrasound equipment that the researchers used. Dr. Halpern and other investigators received research funding from InSightec. One of the researchers is on the company’s medical advisory board, and another served as a consultant to the company.
Effect on axial tremor is unclear
The 50% improvement in hand tremor, disability, and quality of life that Halpern et al. report is similar to the improvement observed following DBS therapy, said Aparna Wagle Shukla, MD, director of the neurophysiology laboratory at the University of Florida in Gainesville, in an interview. Although the results are promising, neurologists should bear several points in mind, she added.
“DBS-induced side effects often are amenable to programming adjustments. However, similar to radiofrequency thalamotomy, focused ultrasound thalamotomy causes lesion effects. While the study discusses the nature of thalamotomy-induced adverse effects, the clinical practitioners also will benefit from learning about the severity of side effects and how they were individually addressed,” said Dr. Wagle Shukla. “The study acknowledges that there was a 30% dropout rate at 3 years’ follow-up. As the original plan included a 5-year follow-up, it would be beneficial to know why a large fraction of participants discontinued participation earlier than expected.”
Furthermore, the study by Halpern et al. leaves several questions unanswered. It does not indicate, for example, whether focused ultrasound thalamotomy can affect the control of axial tremor, including head and voice tremor, said Dr. Wagle Shukla. “Also, the potential of focused ultrasound thalamotomy to treat complex tremors with possible targeting of multiple brain regions such as ventralis oralis anterior and posterior and zona incerta stimulation is currently not known.
“There is no doubt that focused ultrasound thalamotomy is useful for the control of hand tremors in patients diagnosed with essential tremor, with long-term improvements in quality of life,” Dr. Wagle Shukla continued. “However, it is presently limited in its scope as a unilateral, single-target brain procedure.”
SOURCE: Halpern CH et al. Neurology. 2019 Nov 20 (Epub ahead of print).
FROM NEUROLOGY