User login
CDI: The Scope of the Problem
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
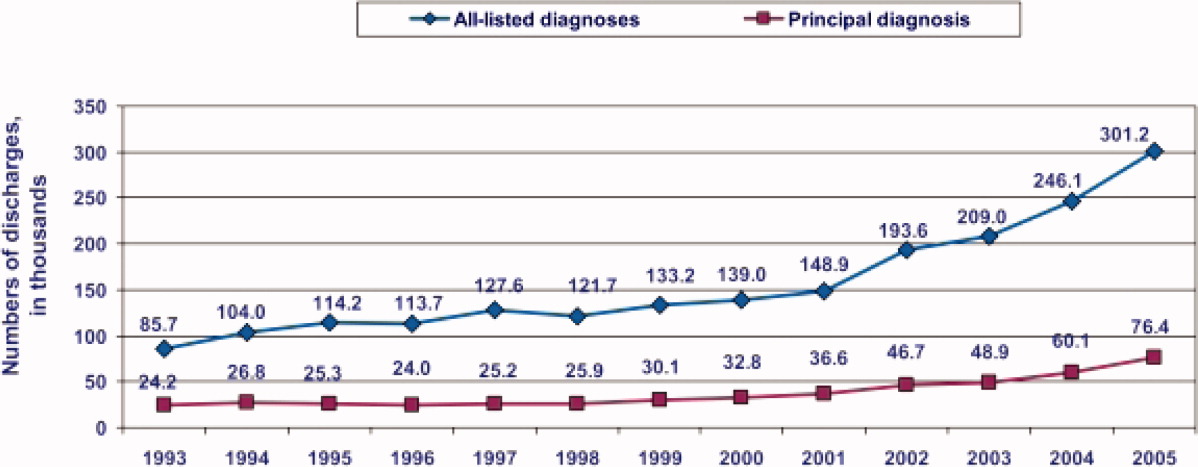
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.
8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.

8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
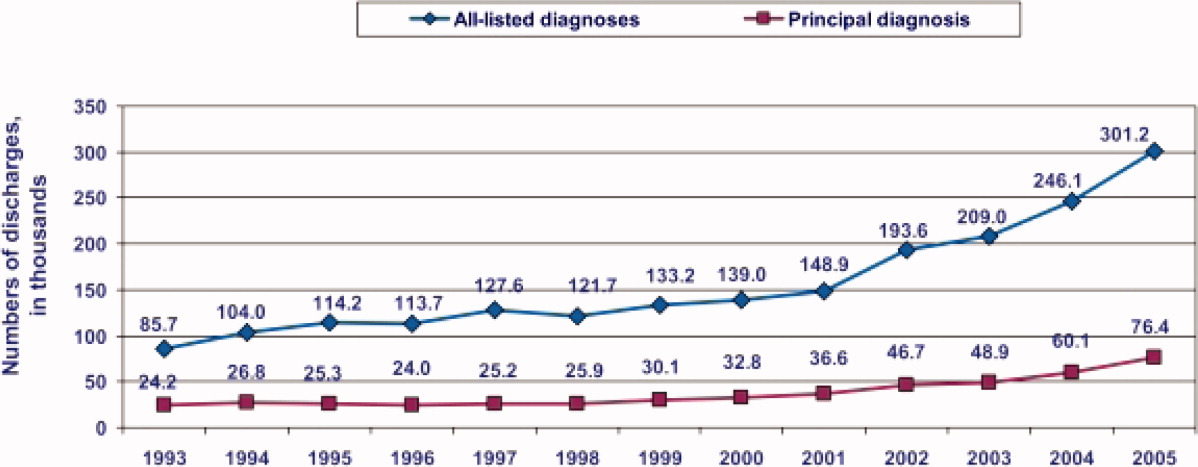
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.

8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
Strategies for Prevention of CDI
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.