User login
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a
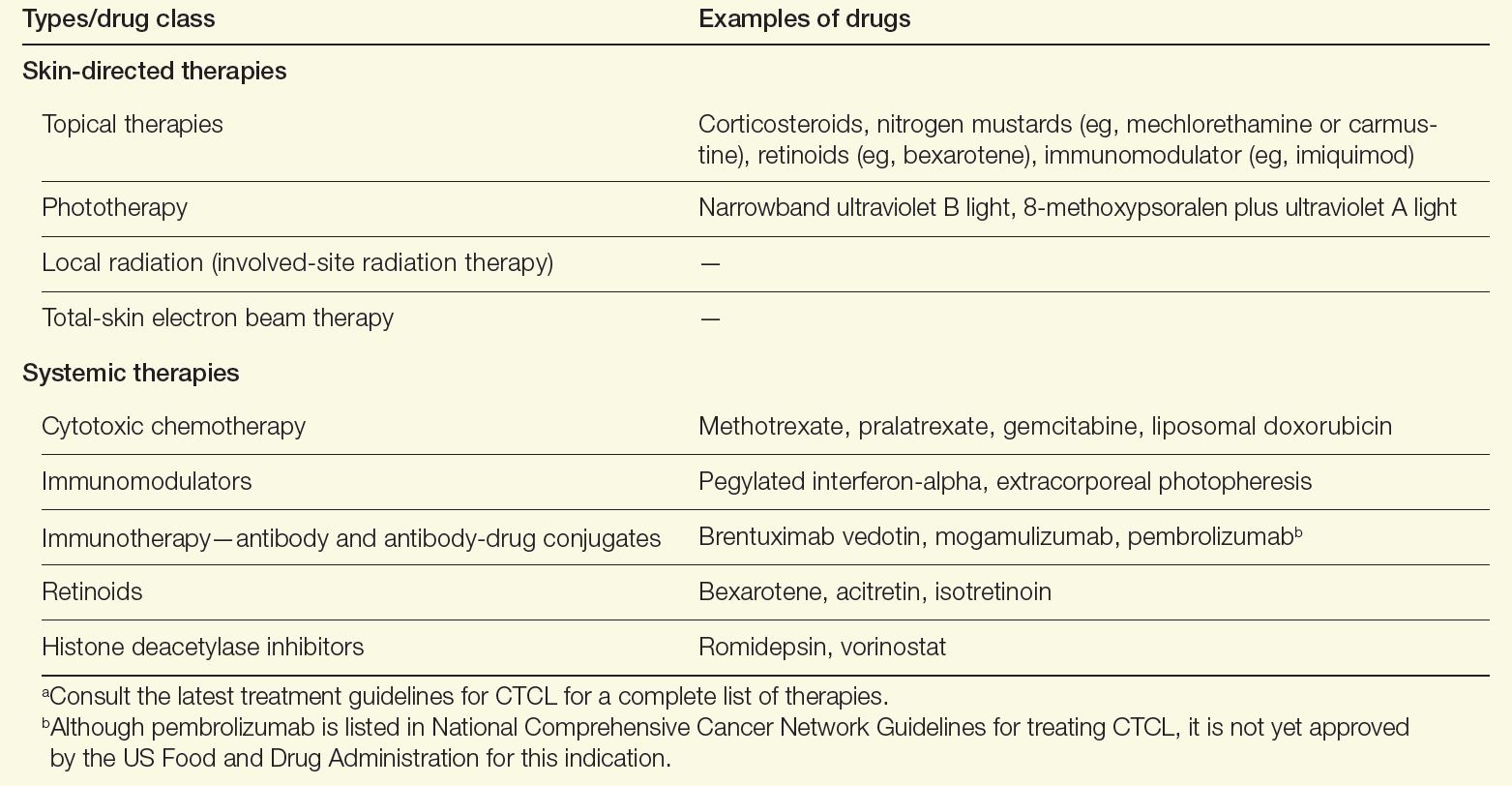
Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a

Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a

Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Magnitude of Potentially Inappropriate Thrombophilia Testing in the Inpatient Hospital Setting
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
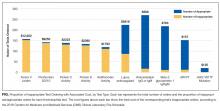
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
Venous thromboembolism (VTE) affects more than 1 million patients and costs the US healthcare system more than $1.5 billion annually.1 Inherited and acquired thrombophilias have been perceived as important risk factors in assessing the risk of VTE recurrence and guiding the duration of anticoagulation.
Thrombophilias increase the risk of a first thrombotic event, but existing data have failed to demonstrate the usefulness of routine thrombophilia screening on subsequent management.2,3 Moreover, thrombophilia testing ordered in the context of an inpatient hospitalization is limited by confounding factors, especially during an acute thrombotic event or in the setting of concurrent anticoagulation.4
Recognizing the costliness of routine thrombophilia testing, The American Society of Hematology introduced its Choosing Wisely campaign in 2013 in an effort to reduce test ordering in the setting of provoked VTEs with a major transient risk factor.5 In order to define current practice behavior at our institution, we conducted a retrospective study to determine the magnitude and financial impact of potentially inappropriate thrombophilia testing in the inpatient setting.
METHODS
We performed a retrospective analysis of thrombophilia testing across all inpatient services at a large, quaternary-care academic institution over a 2-year period. Electronic medical record data containing all thrombophilia tests ordered on inpatients from June 2013 to June 2015 were obtained. This study was exempt from institutional review board approval.
Inclusion criteria included any inpatient for which thrombophilia testing occurred. Patients were excluded if testing was ordered in the absence of VTE or arterial thrombosis or if it was ordered as part of a work-up for another medical condition (see Supplementary Material).
Thrombophilia testing was defined as any of the following: inherited thrombophilias (Factor V Leiden or prothrombin 20210 gene mutations, antithrombin, or protein C or S activity levels) or acquired thrombophilias (lupus anticoagulant [Testing refers to the activated partial thromboplastin time lupus assay.], beta-2 glycoprotein 1 immunoglobulins M and G, anticardiolipin immunoglobulins M and G, dilute Russell’s viper venom time, or JAK2 V617F mutations).
Extracted data included patient age, sex, type of thrombophilia test ordered, ordering primary service, admission diagnosis, and objective confirmation of thrombotic events. The indication for test ordering was determined via medical record review of the patient’s corresponding hospitalization. Each test was evaluated in the context of the patient’s presenting history, hospital course, active medications, accompanying laboratory and radiographic studies, and consultant recommendations to arrive at a conclusion regarding both the test’s reason for ordering and whether its indication was “inappropriate,” “appropriate,” or “equivocal.” Cost data were obtained through the Centers for Medicare & Medicaid Services (CMS) Clinical Laboratory Fee Schedule for 2016 (see Supplementary Material).6
The criteria for defining test appropriateness were formulated by utilizing a combination of major society guidelines and literature review.5,7-10 The criteria placed emphasis upon the ordered tests’ clinical relevance and reliability and were subsequently reviewed by a senior hematologist with specific expertise in thrombosis (see Supplementary Material).
Two internal medicine resident physician data reviewers independently evaluated the ordered tests. To ensure consistency between reviewers, a sample of identical test orders was compared for concordance, and a Cohen’s kappa coefficient was calculated. For purposes of analysis, equivocal orders were included under the appropriate category, as this study focused on the quantification of potentially inappropriate ordering practices. Pearson chi-square testing was performed in order to compare ordering practices between services using Stata.11
RESULTS
In total, we reviewed 2179 individual tests, of which 362 (16.6%) were excluded. The remaining 1817 tests involved 299 patients across 26 primary specialties. Fifty-two (2.9% of orders) were ultimately deemed equivocal. The Table illustrates the overall proportion and cost of inappropriate test ordering as well as testing characteristics of the most commonly encountered thrombotic diagnoses. The Figure illustrates the proportion of potentially inappropriate test ordering with its associated cost by test type.
Orders for Factor V Leiden, prothrombin 20210, and protein C and S activity levels were most commonly deemed inappropriate due to the test results’ failure to alter clinical management (97.3%, 99.2%, 99.4% of their inappropriate orders, respectively). Antithrombin testing (59.4%) was deemed inappropriate most commonly in the setting of acute thrombosis. The lupus anticoagulant (82.8%) was inappropriately ordered most frequently in the setting of concurrent anticoagulation.
Ordering practices were then compared between nonteaching and teaching inpatient general medicine services. We observed a higher proportion of inappropriate tests ordered by the nonteaching services as compared to the teaching services (120 of 173 orders [69.4%] versus 125 of 320 [39.1%], respectively; P < 0.001).
The interreviewer kappa coefficient was 0.82 (P < 0.0001).
DISCUSSION
This retrospective analysis represents one of the largest examinations of inpatient thrombophilia testing practices to date. Our results illustrate the high prevalence and significant financial impact of potentially inappropriate thrombophilia testing conducted in the inpatient setting. The data confirm that, per our defined criteria, more than 90% of inherited thrombophilia testing was potentially inappropriate while the majority of acquired thrombophilia testing was appropriate, with the exception of the lupus anticoagulant.
Even when appropriately ordered, studies suggest that positive thrombophilia screening results fail to impact outcomes in most patients with VTE. In an effort to evaluate positive results’ potential to provide a basis from which to extend the duration of anticoagulation, and therefore reduce the risk of a recurrent VTE, a case-control analysis was performed on a series of patients with a first-VTE event (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis [MEGA] study).3 In examining the odds ratio (OR) for recurrence between patients who did or did not undergo testing for Factor V Leiden, antithrombin, or protein C or S activity, the data failed to show an impact of testing on the risk of VTE recurrence (OR 1.2; confidence interval, 0.8-1.8). In fact, decision making has increasingly relied on patients’ clinical characteristics rather than thrombophilia test results to guide anticoagulation duration after incident VTEs. A 2017 study illustrated that when using a clinical decision rule (Clinical Decision Rule Validation Study to Predict Low Recurrent Risk in Patients With Unprovoked Venous Thromboembolism [REVERSE criteria]) in patients with a first, unprovoked VTE, routine thrombophilia screening added little to determining the need for prolonged anticoagulation.12 These findings support the limited clinical utility of current test ordering practices for the prediction and management of recurrent venous thrombosis.
Regarding the acquired thrombophilias, antiphospholipid antibody testing was predominantly ordered in a justified manner, which is consistent with the notion that test results could affect clinical management, such as anticoagulation duration or choice of anticoagulant.13 However, the validity of lupus anticoagulant testing was limited by the frequency of patients on concurrent anticoagulation.
Financially, the cumulative cost associated with inappropriate ordering was substantial, regardless of the thrombotic event in question. Moreover, our calculated costs are derived from CMS reimbursement rates and likely underestimate the true financial impact of errant testing given that commercial laboratories frequently charge at rates several-fold higher. On a national scale, prior analyses have suggested that the annual cost of thrombophilia testing, based on typical commercial rates, ranges from $300 million to $672 million.14
Researchers in prior studies have similarly examined the frequency of inappropriate thrombophilia testing and methods to reduce it. Researchers in a 2014 study demonstrated initially high rates of inappropriate inherited thrombophilia testing, and then showed marked reductions in testing and cost savings across multiple specialties following the introduction of a flowchart on a preprinted order form.15 Our findings provide motivation to perform similar endeavors.
The proportional difference of inappropriate ordering observed between nonteaching- and teaching-medicine services indicates a potential role for educational interventions. We recently completed a series of lectures on high-value thrombophilia ordering for residents and are actively analyzing its impact on subsequent ordering practices. We are also piloting an electronic best practice advisory for thrombophilia test ordering. Though the advisory may be overridden, providers are asked to provide justification for doing so on a voluntary basis. We plan to evaluate its effect on our findings reported in this study.
We acknowledge that our exclusion criteria resulted in the omission of testing across a spectrum of nonthrombotic clinical conditions, raising the question of selection bias. Because there are no established guidelines to determine the appropriateness of testing in these scenarios, we chose to limit the analysis of errant ordering to the context of thrombotic events. Other limitations of this study include the analysis of equivocal orders as appropriate. However, because equivocal ordering represented less than 3% of all analyzed orders, including these as inappropriate would not have significantly altered our findings.
CONCLUSIONS
A review of thrombophilia testing practices at our institution demonstrated that inappropriate testing in the inpatient setting is a frequent phenomenon associated with a significant financial impact. This effect was more pronounced in inherited versus acquired thrombophilia testing. Testing was frequently confounded and often failed to impact patients’ short- or long-term clinical management, regardless of the result.
These findings serve as a strong impetus to reduce the burden of routine thrombophilia testing during hospital admissions. Our data demonstrate a need for institution-wide changes such as implementing best practice advisories, introducing ordering restrictions, and conducting educational interventions in order to reduce unnecessary expenditures and improve patient care.
Disclosure
The authors have nothing to disclose.
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
1. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. PubMed
2. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:Cd007069. PubMed
3. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477. PubMed
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
5. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. PubMed
6. Centers for Medicare & Medicaid Services: Clinical Laboratory Fee Schedule Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed October 2016
7. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
8. Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367-378. PubMed
9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352. PubMed
10. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
11. Stata Statistical Software [computer program]. Version Release 14. College Station, TX: StataCorp LP; 2015.
12. Garcia-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner A. Impact of thrombophilia screening on venous thromboembolism management practices. Thromb Res.149:76-80. PubMed
13. Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104(4):332-338. PubMed
14. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
15. Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345-349. PubMed
© 2017 Society of Hospital Medicine