User login
When Should an IVC Filter Be Used to Treat a DVT?
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
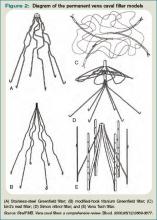
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
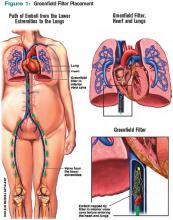
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
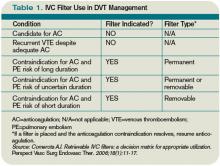
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
In the Literature: January 2010
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
The latest research you need to know
In This Edition
- Perioperative smoking cessation reduces postoperative complications.
- Quality of life is not diminished in heart failure patients receiving defibrillator therapy.
- Simplification of the revised Geneva score may be useful in assessing for pulmonary embolism.
- Non-cardiac surgery after drug eluting stents not associated with major cardiac risk.
- Major cardiac risk is lowest 90 days after bare metal stent percutaneous coronary intervention.
- Extensive cancer screening in unexplained VTE detects more malignancies, but does not affect cancer related mortality.
- Preadmission use of statins decreases after hospitalization mortality in pneumonia.
- Drug-eluting stents are better than bare-metal stents for PCI in acute MI, but additional randomized study is needed.
Does a short duration of perioperative smoking cessation lead to a reduction in postoperative complications?
Background: Prior studies have demonstrated a reduction in postoperative complications when patients stop smoking in the perioperative period. However, they have not clearly shown what effect a fairly short duration of cessation, such as a period of only four weeks, has on the frequency of complications.
Study design: Randomized controlled trial.
Setting: Four university-affiliated hospitals in Sweden.
Synopsis: Using 117 patients who were daily smokers for less than one year between the ages of 18-79 who were scheduled for elective general or orthopedic surgery, this study showed that a smoking-cessation intervention initiated as little as four weeks prior to surgery resulted in fewer postoperative complications. The complication rate was reduced from 41% in the control group to 21% in the intervention group, which received cessation counseling and nicotine-replacement therapy. The relative risk reduction was 49% (95% confidence interval, 3-40) with a number needed to treat of five.
Because this was a randomized controlled trial with a large observed benefit, it appears to be reasonable to endorse perioperative smoking cessation as late as four weeks before an elective surgery. The study was limited in its ability to detect a difference in wound infections by the small sample size and the possibility patients might have unblinded themselves to outcome assessors, causing an overestimation of the effect of the intervention on the primary outcome of all complications.
Bottom line: Perioperative smoking cessation reduces postoperative complications even when started just four weeks prior to surgery.
Citation: Lindstrom D, Azodi OS, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications. Ann Surg. 2008;248(5):739-745.
Does implantable-defibrillator therapy cause deterioration in quality of life for patients with heart failure?
Background: Patients with depressed left-ventricular function are known to have improved survival after receiving implantable cardioverter defibrillators (ICDs). However, there is concern ICD therapy can prolong survival at the expense of a diminished quality of life.
Study design: Randomized placebo-controlled trial.
Setting: Multiple centers in the U.S., Canada, and New Zealand.
Synopsis: Using 2,479 patients from the Sudden Cardiac Death in Heart Failure trial who were 18 and older and had stable heart failure and depressed left-ventricular function, this study demonstrated no significant quality-of-life difference at 30 months when compared with patients who received ICD, amiodarone, and state-of-the-art medical therapy or an amiodarone placebo and state-of-the-art medical therapy. While functional status did not differ at any time between the three groups, psychological well-being was improved in the ICD group at three months (p=0.01) and 12 months (p=0.03) when compared with the placebo group, but at 30 months there was no difference between the groups.
While the trial was randomized and placebo-controlled, the investigators were unable to blind patients or outcome assessors. Nevertheless, the lack of deterioration of quality of life in ICD patients is reassuring.
Bottom line: Placement of ICDs in heart failure patients with a high risk of sudden cardiac death does not appear to decrease quality of life.
Citation: Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999-1008.
Can a simplified, revised Geneva score retain diagnostic accuracy and clinical utility?
Background: The revised Geneva score is a validated and objective clinical decision rule, but has multiple variables with different weights. This can make the tool cumbersome and difficult to remember, and could lead to inaccurate calculations and misjudgments in patient care.
Study design: Retrospective cohort study.
Setting: Four university-affiliated European hospitals.
Synopsis: Using data from two prior prospective trials involving patients with suspected pulmonary embolism (PE), this study showed re-analysis of these patients with a simplified, revised Geneva score, which gives only one point to each clinical factor, resulted in the same level of diagnostic accuracy. Specifically, data from 1,049 patients was used to construct a receiver-operating characteristic curve analysis comparing the standardized and simplified Geneva score, which showed areas under the curve of 0.75 (95% confidence interval 0.71-0.78) and 0.74 (0.70-0.77), respectively. Additionally, the safety of using this clinical tool to rule out PE was demonstrated when using both a three-level (low-intermediate probability) and a dichotomized scheme (PE unlikely) in combination with a negative D-dimer test.
The retrospective nature of the study was its major limitation. The authors suggest a prospective study to complete validation of the simplified, revised Geneva score.
Bottom line: With prospective analysis, it might be possible to further validate a simplified, revised Geneva score.
Citation: Klok FA, Mos ICM, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131-2136.
Is the rate of postoperative major adverse cardiac events (MACEs) inversely related to time after percutaneous coronary intervention (PCI) with a drug-eluting stent (DES)?
Background: The American College of Cardiology and the American Heart Association recently released an advisory that included a recommendation to delay elective noncardiac surgery (NCS) for one year after DES placement. However, no large study addresses the timing of NCS after PCI with DES.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 520 patients who had NCS after DES at the Mayo Clinic, 5.4% experienced MACEs, but the rate of MACEs was not significantly associated with the time after stent placement to surgery (p=0.337). However, observed rates of MACEs were lower after one year. Elderly patients and those going for emergent surgery are at the highest risk for MACE. Bleeding complications were not associated with antiplatelet use.
Although this study does not provide a clear cutoff time for when it is safe to proceed to NCS after DES, it is somewhat reassuring to see the relatively small number of MACEs and the lack of bleeding complications associated with antiplatelet use. However, careful coordination between hospitalists, cardiologists, anesthesiologists, and surgeons is still needed when coordinating NCS after DES, especially in the elderly or during emergent situations.
Bottom line: While time to noncardiac surgery after drug-eluting stent placement is not associated with major adverse cardiac events, observed rates of events are lower after one year.
Citation: Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of non-cardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology.2008;109: 596-604.
Is the risk of MACEs and bleeding events for patients undergoing NCS related to the time interval between PCI with bare-metal stent?
Background: In order to prevent thrombosis of bare-metal stents (BMS) placed during percutaneous coronary intervention (PCI), antiplatelet therapy is used. This poses a risk of bleeding, if surgery is needed during the antiplatelet therapy. The American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 899 patients who had NCS within one year of PCI with BMS at the Mayo Clinic between Jan. 1, 1990, and Jan. 1, 2005, this study found that when NCS was done 30 days or less after PCI with BMS, the MACEs rate was 10.5%, compared with 2.8% when NCS was done 91 or more days after PCI with BMS. After a multivariable analysis, it also was shown bleeding events were not associated with time between PCI with BMS and NCS.
While the American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS, waiting at least 90 days would permit completion of antiplatelet therapy and re-endothelialization of the stent.
Bottom line: The risk of MACEs with noncardiac surgery is lowest when performed at least 90 days after PCI with bare-metal stent.
Citation: Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent, percutaneous coronary intervention. Anesthesiology. 2008;109: 588-595.
Should we screen extensively for cancer in patients with newly diagnosed venous thromboembolism (VTE)?
Background: It is well known VTE can be the first manifestation of previously undiagnosed cancer. Retrospective studies have suggested “limited” cancer screening, including a history and physical examination, along with basic blood work, adequately identifies malignancy in patients with unexplained VTE. However, more recent prospective studies have suggested more extensive screening, which includes imaging studies or tumor-marker measurement, can increase the rate of cancer detection.
Study design: Systematic review.
Setting: Literature search using MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, and evidence-based medicine reviews.
Synopsis: Thirty-six studies of 9,516 patients with VTE reported the period prevalence of previously undiagnosed cancer from baseline to 12 months was 6.3% (95% confidence interval (CI) of 5.6% to 6.9%) in all patients with VTE, and was even higher in patients with unprovoked VTE, 10% (95% CI 8.6% to 11.3%). Of the 34 articles used for prevalence assessment, an extensive screening strategy using CT scans of the abdomen and pelvis increased the proportion of previously undiagnosed cancer detection from 49.4% (CI, 40.2% to 58.5%; limited screening) to 69.7% (CI, 61.1% to 77.8%) in patients with unprovoked VTE. Ultrasonography of the abdomen and pelvis and tumor-marker screening did not result in a clinically significant increase in the frequency of cancer detection.
Four studies compared the rate of detection of early-stage, previously undiagnosed cancer between the limited and extensive screening strategies. Extensive screening led to an absolute decrease in cancer-related mortality of 1.9%, but this difference was not statistically significant.
In this systematic review, there is a great deal of heterogeneity in the studies. Most of the studies did not look at whether an increase in detection of new malignant conditions resulted in a change in the detection rate of early-stage cancer, or a decrease in cancer-related morbidity, cancer-related mortality, or overall mortality. Furthermore, the studies did not assess the consequences of extensive screening, such as patient anxiety and discomfort, testing complications, burden of additional tests for false-positive results, or cost-effectiveness. However, it is important for hospitalists to recognize undiagnosed cancer is common in unexplained VTE and warrants at least a limited-screening approach with more extensive screening.
Bottom line: Although the prevalence of undiagnosed cancer is common in VTE, extensive screening did not offer a cancer-related mortality benefit. CT of the abdomen and pelvis did, however, lead to a greater number of cancer diagnoses in patients with unexplained VTE.
Citation: Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149: 323-333.
Does the use of preadmission statins decrease the risk of death, bacteremia, and pulmonary complications in patients admitted with pneumonia?
Background: Both experimental and clinical studies have suggested statins improve outcomes in severe infections, such as sepsis. This is thought to be due to the antithrombotic, anti-inflammatory, and immunomodulatory effects of statins. However, previous studies on the effect of statins on pneumonia have conflicting outcomes.
Study design: Population-based cohort study of 29,900 patients.
Setting: Danish Health Registry.
Synopsis: Researchers studied patients ages 15 years and older hospitalized with pneumonia for the first time between January 1997 and December 2004. While statin users had more co-morbidities than nonusers, the 30-day mortality was 10.3% in users, compared with 15.7% in nonusers, corresponding to an adjusted 30-day mortality rate ratio of 0.69 (95% CI of 0.58-0.82). The 90-day mortality ratio was 16.8% in users, compared with 22.4% in nonusers, corresponding to an adjusted 90-day mortality ratio of 0.75 (95% CI of 0.65-0.86). Former use of statins was not associated with a decreased risk of death. The adjusted risk for bacteremia and pulmonary complications was not significantly different between nonusers and users.
Because this was an observational study, a causal relationship cannot be determined. Hospitalists should be alerted to the possibility statins might, in the future, prove to be a standard treatment modality in pneumonia. A randomized, double-blind trial might help further determine the effect of the acute use of statins on pneumonia outcomes.
Bottom line: Preadmission statin use is associated with a decrease in 30- and 90-day mortality in pneumonia.
Citation: Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia. Arch Intern Med. 2008;168(19):2081-2087.
Do outcomes differ when patients with acute myocardial infarction (MI) undergo PCI with drug-eluting stents (DES) compared with bare-metal stents?
Background: Randomized trials comparing drug-eluting stents with bare-metal stents in acute MI have been limited in size and duration. Concern exists regarding higher mortality among patients with ST-elevation MI treated with DES.
Study design: Observational, cohort study.
Setting: Patients were identified from a state-mandated database, in which all PCI performed in Massachusetts are reported.
Synopsis: Between April 2003 and September 2004, 7,217 eligible patients underwent stenting for acute MI. They were assigned to either the DES group or the bare-metal stent (BMS) group using propensity score matching. Patients in the DES group had lower mortality at two years, compared to a matched cohort of patients in the BMS group with MI (10.7% vs. 12.8%; absolute risk difference, -2.1%, CI, -3.8% to -0.4%). A statistically significant difference was noted among patients with or without ST-elevation MI.
The rates of target vessel revascularization at two years with MI were significantly lower among patients receiving DES than among those receiving BMS (9.6% vs. 14.5%; risk difference, -4.9%; CI, -6.1% to -3.1%).
The study is limited by its observational nature and residual confounding bias after matching. Importantly, this study was performed to determine if DESs were harmful, and the finding of reduced mortality was unanticipated.
Bottom line: Although patients with acute MI treated with drug-eluting stents had lower mortality and repeat revascularization rates compared with bare-metal stents, this outcome merits confirmation in randomized trials.
Citation: Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008;359 (13):1330-1342.
In This Edition
- Perioperative smoking cessation reduces postoperative complications.
- Quality of life is not diminished in heart failure patients receiving defibrillator therapy.
- Simplification of the revised Geneva score may be useful in assessing for pulmonary embolism.
- Non-cardiac surgery after drug eluting stents not associated with major cardiac risk.
- Major cardiac risk is lowest 90 days after bare metal stent percutaneous coronary intervention.
- Extensive cancer screening in unexplained VTE detects more malignancies, but does not affect cancer related mortality.
- Preadmission use of statins decreases after hospitalization mortality in pneumonia.
- Drug-eluting stents are better than bare-metal stents for PCI in acute MI, but additional randomized study is needed.
Does a short duration of perioperative smoking cessation lead to a reduction in postoperative complications?
Background: Prior studies have demonstrated a reduction in postoperative complications when patients stop smoking in the perioperative period. However, they have not clearly shown what effect a fairly short duration of cessation, such as a period of only four weeks, has on the frequency of complications.
Study design: Randomized controlled trial.
Setting: Four university-affiliated hospitals in Sweden.
Synopsis: Using 117 patients who were daily smokers for less than one year between the ages of 18-79 who were scheduled for elective general or orthopedic surgery, this study showed that a smoking-cessation intervention initiated as little as four weeks prior to surgery resulted in fewer postoperative complications. The complication rate was reduced from 41% in the control group to 21% in the intervention group, which received cessation counseling and nicotine-replacement therapy. The relative risk reduction was 49% (95% confidence interval, 3-40) with a number needed to treat of five.
Because this was a randomized controlled trial with a large observed benefit, it appears to be reasonable to endorse perioperative smoking cessation as late as four weeks before an elective surgery. The study was limited in its ability to detect a difference in wound infections by the small sample size and the possibility patients might have unblinded themselves to outcome assessors, causing an overestimation of the effect of the intervention on the primary outcome of all complications.
Bottom line: Perioperative smoking cessation reduces postoperative complications even when started just four weeks prior to surgery.
Citation: Lindstrom D, Azodi OS, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications. Ann Surg. 2008;248(5):739-745.
Does implantable-defibrillator therapy cause deterioration in quality of life for patients with heart failure?
Background: Patients with depressed left-ventricular function are known to have improved survival after receiving implantable cardioverter defibrillators (ICDs). However, there is concern ICD therapy can prolong survival at the expense of a diminished quality of life.
Study design: Randomized placebo-controlled trial.
Setting: Multiple centers in the U.S., Canada, and New Zealand.
Synopsis: Using 2,479 patients from the Sudden Cardiac Death in Heart Failure trial who were 18 and older and had stable heart failure and depressed left-ventricular function, this study demonstrated no significant quality-of-life difference at 30 months when compared with patients who received ICD, amiodarone, and state-of-the-art medical therapy or an amiodarone placebo and state-of-the-art medical therapy. While functional status did not differ at any time between the three groups, psychological well-being was improved in the ICD group at three months (p=0.01) and 12 months (p=0.03) when compared with the placebo group, but at 30 months there was no difference between the groups.
While the trial was randomized and placebo-controlled, the investigators were unable to blind patients or outcome assessors. Nevertheless, the lack of deterioration of quality of life in ICD patients is reassuring.
Bottom line: Placement of ICDs in heart failure patients with a high risk of sudden cardiac death does not appear to decrease quality of life.
Citation: Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999-1008.
Can a simplified, revised Geneva score retain diagnostic accuracy and clinical utility?
Background: The revised Geneva score is a validated and objective clinical decision rule, but has multiple variables with different weights. This can make the tool cumbersome and difficult to remember, and could lead to inaccurate calculations and misjudgments in patient care.
Study design: Retrospective cohort study.
Setting: Four university-affiliated European hospitals.
Synopsis: Using data from two prior prospective trials involving patients with suspected pulmonary embolism (PE), this study showed re-analysis of these patients with a simplified, revised Geneva score, which gives only one point to each clinical factor, resulted in the same level of diagnostic accuracy. Specifically, data from 1,049 patients was used to construct a receiver-operating characteristic curve analysis comparing the standardized and simplified Geneva score, which showed areas under the curve of 0.75 (95% confidence interval 0.71-0.78) and 0.74 (0.70-0.77), respectively. Additionally, the safety of using this clinical tool to rule out PE was demonstrated when using both a three-level (low-intermediate probability) and a dichotomized scheme (PE unlikely) in combination with a negative D-dimer test.
The retrospective nature of the study was its major limitation. The authors suggest a prospective study to complete validation of the simplified, revised Geneva score.
Bottom line: With prospective analysis, it might be possible to further validate a simplified, revised Geneva score.
Citation: Klok FA, Mos ICM, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131-2136.
Is the rate of postoperative major adverse cardiac events (MACEs) inversely related to time after percutaneous coronary intervention (PCI) with a drug-eluting stent (DES)?
Background: The American College of Cardiology and the American Heart Association recently released an advisory that included a recommendation to delay elective noncardiac surgery (NCS) for one year after DES placement. However, no large study addresses the timing of NCS after PCI with DES.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 520 patients who had NCS after DES at the Mayo Clinic, 5.4% experienced MACEs, but the rate of MACEs was not significantly associated with the time after stent placement to surgery (p=0.337). However, observed rates of MACEs were lower after one year. Elderly patients and those going for emergent surgery are at the highest risk for MACE. Bleeding complications were not associated with antiplatelet use.
Although this study does not provide a clear cutoff time for when it is safe to proceed to NCS after DES, it is somewhat reassuring to see the relatively small number of MACEs and the lack of bleeding complications associated with antiplatelet use. However, careful coordination between hospitalists, cardiologists, anesthesiologists, and surgeons is still needed when coordinating NCS after DES, especially in the elderly or during emergent situations.
Bottom line: While time to noncardiac surgery after drug-eluting stent placement is not associated with major adverse cardiac events, observed rates of events are lower after one year.
Citation: Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of non-cardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology.2008;109: 596-604.
Is the risk of MACEs and bleeding events for patients undergoing NCS related to the time interval between PCI with bare-metal stent?
Background: In order to prevent thrombosis of bare-metal stents (BMS) placed during percutaneous coronary intervention (PCI), antiplatelet therapy is used. This poses a risk of bleeding, if surgery is needed during the antiplatelet therapy. The American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 899 patients who had NCS within one year of PCI with BMS at the Mayo Clinic between Jan. 1, 1990, and Jan. 1, 2005, this study found that when NCS was done 30 days or less after PCI with BMS, the MACEs rate was 10.5%, compared with 2.8% when NCS was done 91 or more days after PCI with BMS. After a multivariable analysis, it also was shown bleeding events were not associated with time between PCI with BMS and NCS.
While the American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS, waiting at least 90 days would permit completion of antiplatelet therapy and re-endothelialization of the stent.
Bottom line: The risk of MACEs with noncardiac surgery is lowest when performed at least 90 days after PCI with bare-metal stent.
Citation: Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent, percutaneous coronary intervention. Anesthesiology. 2008;109: 588-595.
Should we screen extensively for cancer in patients with newly diagnosed venous thromboembolism (VTE)?
Background: It is well known VTE can be the first manifestation of previously undiagnosed cancer. Retrospective studies have suggested “limited” cancer screening, including a history and physical examination, along with basic blood work, adequately identifies malignancy in patients with unexplained VTE. However, more recent prospective studies have suggested more extensive screening, which includes imaging studies or tumor-marker measurement, can increase the rate of cancer detection.
Study design: Systematic review.
Setting: Literature search using MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, and evidence-based medicine reviews.
Synopsis: Thirty-six studies of 9,516 patients with VTE reported the period prevalence of previously undiagnosed cancer from baseline to 12 months was 6.3% (95% confidence interval (CI) of 5.6% to 6.9%) in all patients with VTE, and was even higher in patients with unprovoked VTE, 10% (95% CI 8.6% to 11.3%). Of the 34 articles used for prevalence assessment, an extensive screening strategy using CT scans of the abdomen and pelvis increased the proportion of previously undiagnosed cancer detection from 49.4% (CI, 40.2% to 58.5%; limited screening) to 69.7% (CI, 61.1% to 77.8%) in patients with unprovoked VTE. Ultrasonography of the abdomen and pelvis and tumor-marker screening did not result in a clinically significant increase in the frequency of cancer detection.
Four studies compared the rate of detection of early-stage, previously undiagnosed cancer between the limited and extensive screening strategies. Extensive screening led to an absolute decrease in cancer-related mortality of 1.9%, but this difference was not statistically significant.
In this systematic review, there is a great deal of heterogeneity in the studies. Most of the studies did not look at whether an increase in detection of new malignant conditions resulted in a change in the detection rate of early-stage cancer, or a decrease in cancer-related morbidity, cancer-related mortality, or overall mortality. Furthermore, the studies did not assess the consequences of extensive screening, such as patient anxiety and discomfort, testing complications, burden of additional tests for false-positive results, or cost-effectiveness. However, it is important for hospitalists to recognize undiagnosed cancer is common in unexplained VTE and warrants at least a limited-screening approach with more extensive screening.
Bottom line: Although the prevalence of undiagnosed cancer is common in VTE, extensive screening did not offer a cancer-related mortality benefit. CT of the abdomen and pelvis did, however, lead to a greater number of cancer diagnoses in patients with unexplained VTE.
Citation: Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149: 323-333.
Does the use of preadmission statins decrease the risk of death, bacteremia, and pulmonary complications in patients admitted with pneumonia?
Background: Both experimental and clinical studies have suggested statins improve outcomes in severe infections, such as sepsis. This is thought to be due to the antithrombotic, anti-inflammatory, and immunomodulatory effects of statins. However, previous studies on the effect of statins on pneumonia have conflicting outcomes.
Study design: Population-based cohort study of 29,900 patients.
Setting: Danish Health Registry.
Synopsis: Researchers studied patients ages 15 years and older hospitalized with pneumonia for the first time between January 1997 and December 2004. While statin users had more co-morbidities than nonusers, the 30-day mortality was 10.3% in users, compared with 15.7% in nonusers, corresponding to an adjusted 30-day mortality rate ratio of 0.69 (95% CI of 0.58-0.82). The 90-day mortality ratio was 16.8% in users, compared with 22.4% in nonusers, corresponding to an adjusted 90-day mortality ratio of 0.75 (95% CI of 0.65-0.86). Former use of statins was not associated with a decreased risk of death. The adjusted risk for bacteremia and pulmonary complications was not significantly different between nonusers and users.
Because this was an observational study, a causal relationship cannot be determined. Hospitalists should be alerted to the possibility statins might, in the future, prove to be a standard treatment modality in pneumonia. A randomized, double-blind trial might help further determine the effect of the acute use of statins on pneumonia outcomes.
Bottom line: Preadmission statin use is associated with a decrease in 30- and 90-day mortality in pneumonia.
Citation: Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia. Arch Intern Med. 2008;168(19):2081-2087.
Do outcomes differ when patients with acute myocardial infarction (MI) undergo PCI with drug-eluting stents (DES) compared with bare-metal stents?
Background: Randomized trials comparing drug-eluting stents with bare-metal stents in acute MI have been limited in size and duration. Concern exists regarding higher mortality among patients with ST-elevation MI treated with DES.
Study design: Observational, cohort study.
Setting: Patients were identified from a state-mandated database, in which all PCI performed in Massachusetts are reported.
Synopsis: Between April 2003 and September 2004, 7,217 eligible patients underwent stenting for acute MI. They were assigned to either the DES group or the bare-metal stent (BMS) group using propensity score matching. Patients in the DES group had lower mortality at two years, compared to a matched cohort of patients in the BMS group with MI (10.7% vs. 12.8%; absolute risk difference, -2.1%, CI, -3.8% to -0.4%). A statistically significant difference was noted among patients with or without ST-elevation MI.
The rates of target vessel revascularization at two years with MI were significantly lower among patients receiving DES than among those receiving BMS (9.6% vs. 14.5%; risk difference, -4.9%; CI, -6.1% to -3.1%).
The study is limited by its observational nature and residual confounding bias after matching. Importantly, this study was performed to determine if DESs were harmful, and the finding of reduced mortality was unanticipated.
Bottom line: Although patients with acute MI treated with drug-eluting stents had lower mortality and repeat revascularization rates compared with bare-metal stents, this outcome merits confirmation in randomized trials.
Citation: Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008;359 (13):1330-1342.
In This Edition
- Perioperative smoking cessation reduces postoperative complications.
- Quality of life is not diminished in heart failure patients receiving defibrillator therapy.
- Simplification of the revised Geneva score may be useful in assessing for pulmonary embolism.
- Non-cardiac surgery after drug eluting stents not associated with major cardiac risk.
- Major cardiac risk is lowest 90 days after bare metal stent percutaneous coronary intervention.
- Extensive cancer screening in unexplained VTE detects more malignancies, but does not affect cancer related mortality.
- Preadmission use of statins decreases after hospitalization mortality in pneumonia.
- Drug-eluting stents are better than bare-metal stents for PCI in acute MI, but additional randomized study is needed.
Does a short duration of perioperative smoking cessation lead to a reduction in postoperative complications?
Background: Prior studies have demonstrated a reduction in postoperative complications when patients stop smoking in the perioperative period. However, they have not clearly shown what effect a fairly short duration of cessation, such as a period of only four weeks, has on the frequency of complications.
Study design: Randomized controlled trial.
Setting: Four university-affiliated hospitals in Sweden.
Synopsis: Using 117 patients who were daily smokers for less than one year between the ages of 18-79 who were scheduled for elective general or orthopedic surgery, this study showed that a smoking-cessation intervention initiated as little as four weeks prior to surgery resulted in fewer postoperative complications. The complication rate was reduced from 41% in the control group to 21% in the intervention group, which received cessation counseling and nicotine-replacement therapy. The relative risk reduction was 49% (95% confidence interval, 3-40) with a number needed to treat of five.
Because this was a randomized controlled trial with a large observed benefit, it appears to be reasonable to endorse perioperative smoking cessation as late as four weeks before an elective surgery. The study was limited in its ability to detect a difference in wound infections by the small sample size and the possibility patients might have unblinded themselves to outcome assessors, causing an overestimation of the effect of the intervention on the primary outcome of all complications.
Bottom line: Perioperative smoking cessation reduces postoperative complications even when started just four weeks prior to surgery.
Citation: Lindstrom D, Azodi OS, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications. Ann Surg. 2008;248(5):739-745.
Does implantable-defibrillator therapy cause deterioration in quality of life for patients with heart failure?
Background: Patients with depressed left-ventricular function are known to have improved survival after receiving implantable cardioverter defibrillators (ICDs). However, there is concern ICD therapy can prolong survival at the expense of a diminished quality of life.
Study design: Randomized placebo-controlled trial.
Setting: Multiple centers in the U.S., Canada, and New Zealand.
Synopsis: Using 2,479 patients from the Sudden Cardiac Death in Heart Failure trial who were 18 and older and had stable heart failure and depressed left-ventricular function, this study demonstrated no significant quality-of-life difference at 30 months when compared with patients who received ICD, amiodarone, and state-of-the-art medical therapy or an amiodarone placebo and state-of-the-art medical therapy. While functional status did not differ at any time between the three groups, psychological well-being was improved in the ICD group at three months (p=0.01) and 12 months (p=0.03) when compared with the placebo group, but at 30 months there was no difference between the groups.
While the trial was randomized and placebo-controlled, the investigators were unable to blind patients or outcome assessors. Nevertheless, the lack of deterioration of quality of life in ICD patients is reassuring.
Bottom line: Placement of ICDs in heart failure patients with a high risk of sudden cardiac death does not appear to decrease quality of life.
Citation: Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999-1008.
Can a simplified, revised Geneva score retain diagnostic accuracy and clinical utility?
Background: The revised Geneva score is a validated and objective clinical decision rule, but has multiple variables with different weights. This can make the tool cumbersome and difficult to remember, and could lead to inaccurate calculations and misjudgments in patient care.
Study design: Retrospective cohort study.
Setting: Four university-affiliated European hospitals.
Synopsis: Using data from two prior prospective trials involving patients with suspected pulmonary embolism (PE), this study showed re-analysis of these patients with a simplified, revised Geneva score, which gives only one point to each clinical factor, resulted in the same level of diagnostic accuracy. Specifically, data from 1,049 patients was used to construct a receiver-operating characteristic curve analysis comparing the standardized and simplified Geneva score, which showed areas under the curve of 0.75 (95% confidence interval 0.71-0.78) and 0.74 (0.70-0.77), respectively. Additionally, the safety of using this clinical tool to rule out PE was demonstrated when using both a three-level (low-intermediate probability) and a dichotomized scheme (PE unlikely) in combination with a negative D-dimer test.
The retrospective nature of the study was its major limitation. The authors suggest a prospective study to complete validation of the simplified, revised Geneva score.
Bottom line: With prospective analysis, it might be possible to further validate a simplified, revised Geneva score.
Citation: Klok FA, Mos ICM, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131-2136.
Is the rate of postoperative major adverse cardiac events (MACEs) inversely related to time after percutaneous coronary intervention (PCI) with a drug-eluting stent (DES)?
Background: The American College of Cardiology and the American Heart Association recently released an advisory that included a recommendation to delay elective noncardiac surgery (NCS) for one year after DES placement. However, no large study addresses the timing of NCS after PCI with DES.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 520 patients who had NCS after DES at the Mayo Clinic, 5.4% experienced MACEs, but the rate of MACEs was not significantly associated with the time after stent placement to surgery (p=0.337). However, observed rates of MACEs were lower after one year. Elderly patients and those going for emergent surgery are at the highest risk for MACE. Bleeding complications were not associated with antiplatelet use.
Although this study does not provide a clear cutoff time for when it is safe to proceed to NCS after DES, it is somewhat reassuring to see the relatively small number of MACEs and the lack of bleeding complications associated with antiplatelet use. However, careful coordination between hospitalists, cardiologists, anesthesiologists, and surgeons is still needed when coordinating NCS after DES, especially in the elderly or during emergent situations.
Bottom line: While time to noncardiac surgery after drug-eluting stent placement is not associated with major adverse cardiac events, observed rates of events are lower after one year.
Citation: Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of non-cardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology.2008;109: 596-604.
Is the risk of MACEs and bleeding events for patients undergoing NCS related to the time interval between PCI with bare-metal stent?
Background: In order to prevent thrombosis of bare-metal stents (BMS) placed during percutaneous coronary intervention (PCI), antiplatelet therapy is used. This poses a risk of bleeding, if surgery is needed during the antiplatelet therapy. The American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS.
Study design: Retrospective observational study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: Looking at 899 patients who had NCS within one year of PCI with BMS at the Mayo Clinic between Jan. 1, 1990, and Jan. 1, 2005, this study found that when NCS was done 30 days or less after PCI with BMS, the MACEs rate was 10.5%, compared with 2.8% when NCS was done 91 or more days after PCI with BMS. After a multivariable analysis, it also was shown bleeding events were not associated with time between PCI with BMS and NCS.
While the American College of Cardiology and the American Heart Association recommends delaying NCS for at least six weeks after PCI with BMS, waiting at least 90 days would permit completion of antiplatelet therapy and re-endothelialization of the stent.
Bottom line: The risk of MACEs with noncardiac surgery is lowest when performed at least 90 days after PCI with bare-metal stent.
Citation: Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent, percutaneous coronary intervention. Anesthesiology. 2008;109: 588-595.
Should we screen extensively for cancer in patients with newly diagnosed venous thromboembolism (VTE)?
Background: It is well known VTE can be the first manifestation of previously undiagnosed cancer. Retrospective studies have suggested “limited” cancer screening, including a history and physical examination, along with basic blood work, adequately identifies malignancy in patients with unexplained VTE. However, more recent prospective studies have suggested more extensive screening, which includes imaging studies or tumor-marker measurement, can increase the rate of cancer detection.
Study design: Systematic review.
Setting: Literature search using MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, and evidence-based medicine reviews.
Synopsis: Thirty-six studies of 9,516 patients with VTE reported the period prevalence of previously undiagnosed cancer from baseline to 12 months was 6.3% (95% confidence interval (CI) of 5.6% to 6.9%) in all patients with VTE, and was even higher in patients with unprovoked VTE, 10% (95% CI 8.6% to 11.3%). Of the 34 articles used for prevalence assessment, an extensive screening strategy using CT scans of the abdomen and pelvis increased the proportion of previously undiagnosed cancer detection from 49.4% (CI, 40.2% to 58.5%; limited screening) to 69.7% (CI, 61.1% to 77.8%) in patients with unprovoked VTE. Ultrasonography of the abdomen and pelvis and tumor-marker screening did not result in a clinically significant increase in the frequency of cancer detection.
Four studies compared the rate of detection of early-stage, previously undiagnosed cancer between the limited and extensive screening strategies. Extensive screening led to an absolute decrease in cancer-related mortality of 1.9%, but this difference was not statistically significant.
In this systematic review, there is a great deal of heterogeneity in the studies. Most of the studies did not look at whether an increase in detection of new malignant conditions resulted in a change in the detection rate of early-stage cancer, or a decrease in cancer-related morbidity, cancer-related mortality, or overall mortality. Furthermore, the studies did not assess the consequences of extensive screening, such as patient anxiety and discomfort, testing complications, burden of additional tests for false-positive results, or cost-effectiveness. However, it is important for hospitalists to recognize undiagnosed cancer is common in unexplained VTE and warrants at least a limited-screening approach with more extensive screening.
Bottom line: Although the prevalence of undiagnosed cancer is common in VTE, extensive screening did not offer a cancer-related mortality benefit. CT of the abdomen and pelvis did, however, lead to a greater number of cancer diagnoses in patients with unexplained VTE.
Citation: Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149: 323-333.
Does the use of preadmission statins decrease the risk of death, bacteremia, and pulmonary complications in patients admitted with pneumonia?
Background: Both experimental and clinical studies have suggested statins improve outcomes in severe infections, such as sepsis. This is thought to be due to the antithrombotic, anti-inflammatory, and immunomodulatory effects of statins. However, previous studies on the effect of statins on pneumonia have conflicting outcomes.
Study design: Population-based cohort study of 29,900 patients.
Setting: Danish Health Registry.
Synopsis: Researchers studied patients ages 15 years and older hospitalized with pneumonia for the first time between January 1997 and December 2004. While statin users had more co-morbidities than nonusers, the 30-day mortality was 10.3% in users, compared with 15.7% in nonusers, corresponding to an adjusted 30-day mortality rate ratio of 0.69 (95% CI of 0.58-0.82). The 90-day mortality ratio was 16.8% in users, compared with 22.4% in nonusers, corresponding to an adjusted 90-day mortality ratio of 0.75 (95% CI of 0.65-0.86). Former use of statins was not associated with a decreased risk of death. The adjusted risk for bacteremia and pulmonary complications was not significantly different between nonusers and users.
Because this was an observational study, a causal relationship cannot be determined. Hospitalists should be alerted to the possibility statins might, in the future, prove to be a standard treatment modality in pneumonia. A randomized, double-blind trial might help further determine the effect of the acute use of statins on pneumonia outcomes.
Bottom line: Preadmission statin use is associated with a decrease in 30- and 90-day mortality in pneumonia.
Citation: Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia. Arch Intern Med. 2008;168(19):2081-2087.
Do outcomes differ when patients with acute myocardial infarction (MI) undergo PCI with drug-eluting stents (DES) compared with bare-metal stents?
Background: Randomized trials comparing drug-eluting stents with bare-metal stents in acute MI have been limited in size and duration. Concern exists regarding higher mortality among patients with ST-elevation MI treated with DES.
Study design: Observational, cohort study.
Setting: Patients were identified from a state-mandated database, in which all PCI performed in Massachusetts are reported.
Synopsis: Between April 2003 and September 2004, 7,217 eligible patients underwent stenting for acute MI. They were assigned to either the DES group or the bare-metal stent (BMS) group using propensity score matching. Patients in the DES group had lower mortality at two years, compared to a matched cohort of patients in the BMS group with MI (10.7% vs. 12.8%; absolute risk difference, -2.1%, CI, -3.8% to -0.4%). A statistically significant difference was noted among patients with or without ST-elevation MI.
The rates of target vessel revascularization at two years with MI were significantly lower among patients receiving DES than among those receiving BMS (9.6% vs. 14.5%; risk difference, -4.9%; CI, -6.1% to -3.1%).
The study is limited by its observational nature and residual confounding bias after matching. Importantly, this study was performed to determine if DESs were harmful, and the finding of reduced mortality was unanticipated.
Bottom line: Although patients with acute MI treated with drug-eluting stents had lower mortality and repeat revascularization rates compared with bare-metal stents, this outcome merits confirmation in randomized trials.
Citation: Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008;359 (13):1330-1342.