User login
Laryngopharyngeal reflux: More questions than answers
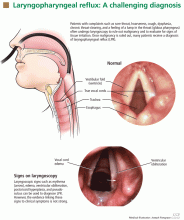
The scenario is common: a patient complains of chronic hoarseness, cough, throat-clearing, sore throat, dysphagia, or a lump in the throat and undergoes laryngoscopy. If this test rules out cancer, the patient is given a diagnosis of laryngopharyngeal reflux (LPR), ie, a form of gastroesophageal reflux disease (GERD) in which the stomach contents get all the way up into the pharynx and down into the larynx. A proton pump inhibitor (PPI) is often prescribed, usually twice daily for 2 months.1–6
In this article, we review the current understanding of the pathophysiology of LPR and evaluate current diagnostic tests and treatment regimens for patients with suspected LPR.
THE PATHOPHYSIOLOGY OF LPR IS POORLY UNDERSTOOD
Transient relaxation of the lower esophageal sphincter
In a study in 10 healthy volunteers, Dent et al7 found that the pressure in the lower esophageal sphincter varies considerably over a 12-hour period. Episodes of reflux were not related to low basal (resting) pressure. Rather, 70% to 100% of reflux episodes occurred during random episodes of transient, complete, and inappropriate relaxation of the sphincter that lasted about 5 to 30 seconds. The mechanism of this relaxation is not known but is thought to be related to activation of the vagus nerve, possibly as a consequence of gastric distention.8
Gastric, not duodenal products seem to cause the damage
In a study in dogs, Adhami et al9 evaluated the possible role of gastric juices (acid and pepsin) vs duodenal juices (bile acids and trypsin) in laryngeal tissue damage. After taking baseline biopsy samples of the larynx, the investigators applied a variety of gastric and duodenal enzymes at varying pH levels (pH 1–7) to the larynxes. After 9 to 12 applications, they took another biopsy and assessed the changes visually and histologically.
At low (ie, acidic) pH levels, pepsin and conjugated bile acids were the most injurious, causing erythema and histologic evidence of inflammation. The authors concluded that gastric and not duodenal substances cause laryngeal injury and that acid-suppressive therapy “should eliminate the injurious potential” of acid reflux.9
The larynx is more sensitive than the esophagus
Monitoring of esophageal pH has shown that healthy people can tolerate as many as 50 episodes a day of acid reflux (pH < 4) in the esophagus. However, Koufman10 found that as few as three episodes of laryngeal reflux per week can cause severe laryngeal inflammation and injury.
Does pepsin deplete buffers, worsening acid damage?
Johnston et al11 took biopsies from a control group of healthy volunteers and from patients diagnosed with LPR. They detected pepsin in the samples from eight of the nine patients with LPR but in none of the controls. Furthermore, the tissue from patients with LPR had low levels of carbonic anhydrase III. The authors hypothesized that pepsin depletes the laryngopharynx of carbonic anhydrase III, and that therefore these tissues cannot produce enough bicarbonate to buffer the gastric acid. Less bicarbonate would mean greater acidity, so the pepsin would remain active and would be more likely to cause cellular damage.11
However, this contention is controversial. What is universally agreed upon is that reflux of gastric or gastroduodenal contents is most likely causing injury, most likely through direct exposure, although indirect effects through vagal mechanisms cannot be ruled out.
CURRENT DIAGNOSTIC TESTS FOR LPR HAVE SHORTCOMINGS
A careful history is important. Many patients report they have sore throat, hoarseness, cough, dysphasia, or chronic throat-clearing.13 Factors that may predispose a patient to esophageal reflux should be discussed, eg:
- Tobacco use
- Diet (eg, soda, spicy foods, fatty foods)
- Alcohol use
- Certain drugs (calcium channel blockers, nitrates, steroids).
Up to 50% of patients presenting with extraesophageal symptoms may not have classic reflux symptoms such as heartburn and regur-gitation.14 However, the existence of “silent reflux” is currently controversial.
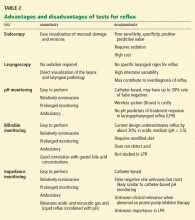
Laryngoscopy is nonspecific and subjective
Because the key symptoms of LPR are nonspecific, many patients who present to an otolaryngologist undergo laryngoscopy, mainly to rule out malignancy. Once cancer is ruled out, many patients are given a diagnosis of LPR.
Furthermore, Branski et al17 performed transoral rigid laryngoscopy with videorecording in 100 consecutive patients presenting with a chief complaint of dysphonia. Five board-certified otolaryngologists individually viewed each recording, scored the degree of erythema and edema, and assessed the likelihood that LPR played a role in dysphonia and the severity of the LPR findings. The physicians’ ratings showed considerable interobserver variability. In other words, this study showed that laryngeal findings are often nonspecific and that the laryngoscopic diagnosis of LPR tends to be subjective.17
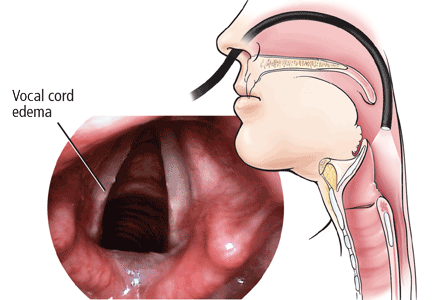
The Reflux Finding Score. Concerned by the lack of consistency in the diagnosis of LPR, Belafsky et al18 created a scoring system for documenting the physical findings and severity of disease on a standardized scale. Their Reflux Finding Score is based on eight laryngoscopic findings: subglottic edema, ventricular edema, erythema, vocal cord edema, diffuse laryngeal edema, hypertrophy of the posterior commissure, granuloma or granulation tissue, and thick endolaryngeal mucus. The total score can range from 0 (best) to 26 (worst).
In 40 patients with LPR confirmed by pH monitoring, the mean score was 11.5, compared with 5.2 in 40 age-matched controls. The authors calculated they could be 95% certain that a person with a score higher than 7 has LPR.18
However, this diagnostic method has not been validated in a large-scale randomized trial and so has yet to be incorporated into routine otolaryngology practice.
Ambulatory pH monitoring is not so golden for diagnosing LPR
Although pH monitoring was once the gold standard for diagnosing reflux, it has since been shown to be unreliable in patients who have laryngeal symptoms.4
How high or low in the esophagus the probe is placed is clearly critical for useful results. 4 But the test is subject to variability: different physicians place the probe in different locations, and the probe may shift. Another problem is that reflux may occur during untested periods.19
A pH of less than 4 in the esophagus had originally been shown to have high sensitivity and specificity,20 but Reichel and Issing21 suggested using a pH of less than 5 as the cutoff, which would identify more patients as having LPR. Further trials are needed to more precisely determine the pH threshold for the diagnosis of LPR.
Enthusiasm is waning for pharyngeal pH monitoring
In LPR, it was initially thought that pH monitoring in the pharynx was more accurate than in the distal or proximal esophagus.
Shaker et al22 monitored the pH in the pharynx, proximal esophagus, and distal esophagus in four groups: 14 patients who had both laryngeal signs and symptoms, 12 patients who had laryngeal symptoms only, 16 patients who had GERD but no laryngeal symptoms, and 12 healthy volunteers. They found that pharyngeal reflux was more frequent and in greater quantity in patients with laryngeal signs and symptoms than in the other groups. This study suggested that pharyngeal pH monitoring may be useful in diagnosing LPR in patients who have laryngeal signs and symptoms.
However, hypopharyngeal pH monitoring has several problems. One issue is that, even in this trial, 2 of 12 healthy volunteers had episodes of pharyngeal reflux.22 In other studies, the rate of false-positive results ranged from 7% to 17%.23,24 Additionally, in 12 previous studies, only 54% of 1,217 patients with suspected LPR had esophageal acid exposure, regardless of where the pH probe was placed.25
More importantly, another study found that patients with pharyngeal reflux documented by pH monitoring were no more likely to respond to acid-suppressive therapy than patients with no documented reflux.26 These findings dampen the enthusiasm for pharyngeal pH monitoring in LPR.
Impedance monitoring on therapy may be useful in refractory cases
Esophageal impedance monitoring, a newer test, uses a catheter that measures electrical resistance (impedance) between different points along the esophagus. Thus, it can detect the reflux of acid and nonacid liquid or gaseous material.
Pritchett et al27 performed esophageal impedance and pH monitoring in 39 patients who were on twice-daily PPI therapy and then evaluated the same patients with wireless pH monitoring while they were off therapy. The most prevalent complaint in the study group was cough (56%), followed by heartburn (18%) and sore throat (10%).
Of the 39 patients, 25 (64%) had normal results on impedance/pH monitoring while on therapy, ruling out reflux. On pH monitoring off therapy, 28 (72%) of the 39 patients had abnormal results; this group included 13 (93%) of the 14 patients who had abnormal results on impedance/pH monitoring while on therapy. The authors recommended on-therapy testing with impedance monitoring in patients with refractory reflux, since it provides more useful clinical information.27 If the results of impedance/pH monitoring are negative in these patients, a diagnosis other than reflux should be considered.
EMPIRIC PPI TREATMENT HAS SHOWN DISAPPOINTING RESULTS
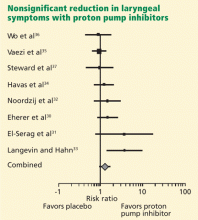
Because laryngoscopy and pH monitoring are not very sensitive or specific for LPR, experts recommend empiric therapy with a PPI twice daily. However, the results have been disappointing when PPIs were compared with placebo in clinical trials.
In a randomized controlled trial,28 we found that patients who had complaints of chronic throat-clearing, cough, globus, sore throat, and hoarseness had a similar response to twice-daily esomeprazole (Nexium) compared with placebo: their primary symptom had resolved by 16 weeks in 14.7% of the esomeprazole group vs 16.0% of the placebo group (P = .799). Similarly, the final findings on laryngoscopy such as edema, erythema, and surface irregularity were not significantly different between groups.
Adding a histamine-2 receptor antagonist is not recommended
Adding a histamine-2 receptor antagonist to PPI therapy has also been considered as a treatment for LPR.
Fackler et al38 studied 16 GERD patients and 18 healthy volunteers to determine if adding ranitidine (Pepcid) to the PPI omeprazole (Prilosec) could improve GERD symptoms. Patients underwent baseline manometry and then gastroesophageal pH monitoring before starting the drugs. They first received omeprazole 20 mg twice daily alone for 2 weeks, and then added ranitidine 300 mg at bedtime. A pH test was done again after the first day of treatment with ranitidine, at the end of 1 week of combination therapy, and after 4 weeks of combination therapy. The combination reduced nocturnal acid breakthrough on day 1; however, due to tolerance to ranitidine, no significant difference in acid suppression was seen after 1 week of therapy. Therefore, this combination is not recommended.
Surgery is not recommended either
Some experts have argued for surgical fundoplication in patients whose symptoms persist despite drug therapy.
Swoger et al39 treated 72 patients who had symptoms consistent with LPR with a PPI for 4 months; 25 patients in this group had less than a 50% improvement despite maximal drug therapy. Ten of these patients underwent surgical fundoplication, and 15 remained on drug therapy alone. At 1 year of follow-up, only one surgical patient (10%) reported improvement in laryngeal symptoms.
In view of this report and prior studies of surgical fundoplication,40 surgery is not recommended for patients whose symptoms do not respond to aggressive PPI therapy.
IF A PPI FAILS, LOOK FOR OTHER CAUSES OF SYMPTOMS
Although gastroesophageal reflux and laryngeal signs and symptoms have been associated with one another, this relation may have been overstated, leading to the overdiagnosis of LPR.
The diagnosis of LPR is difficult, as laryngoscopy has high interrater variability and as the results of pH monitoring do not dependably predict who will respond to treatment.
Because PPI therapy is easy and appears to be safe, patients with extraesophageal symptoms thought to be related to reflux should undergo a trial of twice-daily PPI therapy for at least 2 months. If the patient responds to therapy, then tapering to once-daily therapy initially and then to minimal acid suppression to control symptoms would be prudent.
In patients who show no improvement, other causes of symptoms should be explored. Diseases that can mimic LPR include postnasal drip, allergies, sinus inflammation, and various pulmonary diseases. These patients should also be advised to adopt lifestyle modifications—eg, to stop smoking, lose weight, and decrease activities that cause stress on the voice. Surgery is not likely to provide any benefit in this situation. The patient should be tapered off the PPI to make sure no rebound acid reflux occurs.
- Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976; 21:953–956.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Jonaitis L, Pribuisiene R, Kupcinskas L, Uloza V. Laryngeal examination is superior to endoscopy in the diagnosis of the laryngopharyngeal form of gastroesophageal reflux disease. Scand J Gastroenterol 2006; 41:131–137.
- Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 2003; 1:333–344.
- Jaspersen D, Kulig M, Labenz J, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD study. Aliment Pharmacol Ther 2003; 17:1515–1520.
- Karkos PD, Benton J, Leong SC, et al. Trends in laryngopharyngeal reflux: a British ENT survey. Eur Arch Otorhinolaryngol 2007; 264:513–517.
- Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 1980; 65:256–267.
- Schreiber S, Garten D, Sudhoff H, et al. Pathophysiological mechanisms of extraesophageal reflux in otolaryngeal disorders. Eur Arch Otorhinolaryngol 2009; 266:17–24.
- Adhami T, Goldblum JR, Richter JE, Vaezi MF. The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol 2004; 99:2098–2106.
- Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991; 101(4 pt 2 suppl 53):1–78.
- Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 2004; 114:2129–2134.
- Vaezi MF. Therapy insight: gastroesophageal reflux disease and laryngopharyngeal reflux. Nat Clin Pract Gastroenterol Hepatol 2005; 2:595–603.
- Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis 2007; 13:349–359.
- Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J 2002; 81( 9 suppl 2):7–9.
- Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 2000; 123:385–388.
- Milstein CF, Charbel S, Hicks DM, Abelson TI, Richter JE, Vaezi MF. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs flexible scope). Laryngoscope 2005; 115;2256–2261.
- Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope 2002; 112;1019–1024.
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001; 111:1313–1317.
- Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg 2009; 17:143–148.
- Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992; 87:1102–1111.
- Reichel O, Issing WJ. Impact of different pH thresholds for 24-h dual probe pH monitoring in patients with suspected laryngopharyngeal reflux. J Laryngol Otol 2008; 122:485–489.
- Shaker R, Milbrath M, Ren J, et al. Esophagopharyngeal distribution of refluxed gastric acid in patients with reflux laryngitis. Gastroenterology 1995; 109:1575–1582.
- Jacob P, Kahrilas PJ, Herzon G. Proximal esophageal pH-metry in patients with “reflux laryngitis.” Gastroenterology 1991; 100:305–310.
- Eubanks TR, Omelanczuk PE, Maronian N, Hillel A, Pope CE, Pellegrini CA. Pharyngeal pH monitoring in 222 patients with suspected laryngeal reflux. J Gastrointest Surg 2001; 5:183–190.
- Vaezi MF. Gastroesophageal reflux disease and the larynx. J Clin Gastroenterol 2003; 36:198–203.
- Ulualp SO, Toohill RJ, Shaker R. Outcomes of acid suppressive therapy in patients with posterior laryngitis. Otolaryngol Head Neck Surg 2001; 124:16–22.
- Pritchett JM, Aslam M, Slaughter JC, Ness RM, Garrett CG, Vaezi MF. Efficacy of esophageal impedance/pH monitoring in patients with refractory gastroesophageal reflux disease, on and off therapy. Clin Gastroenterol Hepatol 2009; 7:743–748.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116;254–260.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Eherer AJ, Habermann W, Hammer HF, et al. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 2003; 38:462–467.
- El-Serag HB, Lee P, Buchner A, et al. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 2001; 96:979–983.
- Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 2001; 111:2147–2151.
- Langevin S, Hanh N. GERD-induced ENT symptoms: a prospective placebo controlled study with omeprazole 40 mg a day. Gastroenterology 2001; 120:A-16.
- Havas T, Huang S, Levy M, et al. Posterior pharyngolaryngitis. Double blind randomized placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryng 1999; 3:243–246.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Wo JM, Koopman JI, Harrell SP, et al. Double-blind placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol 2006; 101:1972–1978.
- Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 2004; 131:342–350.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- So JB, Zeitels SM, Rattner DW. Outcome of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery 1998; 124:28–32.
The scenario is common: a patient complains of chronic hoarseness, cough, throat-clearing, sore throat, dysphagia, or a lump in the throat and undergoes laryngoscopy. If this test rules out cancer, the patient is given a diagnosis of laryngopharyngeal reflux (LPR), ie, a form of gastroesophageal reflux disease (GERD) in which the stomach contents get all the way up into the pharynx and down into the larynx. A proton pump inhibitor (PPI) is often prescribed, usually twice daily for 2 months.1–6
In this article, we review the current understanding of the pathophysiology of LPR and evaluate current diagnostic tests and treatment regimens for patients with suspected LPR.
THE PATHOPHYSIOLOGY OF LPR IS POORLY UNDERSTOOD
Transient relaxation of the lower esophageal sphincter
In a study in 10 healthy volunteers, Dent et al7 found that the pressure in the lower esophageal sphincter varies considerably over a 12-hour period. Episodes of reflux were not related to low basal (resting) pressure. Rather, 70% to 100% of reflux episodes occurred during random episodes of transient, complete, and inappropriate relaxation of the sphincter that lasted about 5 to 30 seconds. The mechanism of this relaxation is not known but is thought to be related to activation of the vagus nerve, possibly as a consequence of gastric distention.8
Gastric, not duodenal products seem to cause the damage
In a study in dogs, Adhami et al9 evaluated the possible role of gastric juices (acid and pepsin) vs duodenal juices (bile acids and trypsin) in laryngeal tissue damage. After taking baseline biopsy samples of the larynx, the investigators applied a variety of gastric and duodenal enzymes at varying pH levels (pH 1–7) to the larynxes. After 9 to 12 applications, they took another biopsy and assessed the changes visually and histologically.
At low (ie, acidic) pH levels, pepsin and conjugated bile acids were the most injurious, causing erythema and histologic evidence of inflammation. The authors concluded that gastric and not duodenal substances cause laryngeal injury and that acid-suppressive therapy “should eliminate the injurious potential” of acid reflux.9
The larynx is more sensitive than the esophagus
Monitoring of esophageal pH has shown that healthy people can tolerate as many as 50 episodes a day of acid reflux (pH < 4) in the esophagus. However, Koufman10 found that as few as three episodes of laryngeal reflux per week can cause severe laryngeal inflammation and injury.
Does pepsin deplete buffers, worsening acid damage?
Johnston et al11 took biopsies from a control group of healthy volunteers and from patients diagnosed with LPR. They detected pepsin in the samples from eight of the nine patients with LPR but in none of the controls. Furthermore, the tissue from patients with LPR had low levels of carbonic anhydrase III. The authors hypothesized that pepsin depletes the laryngopharynx of carbonic anhydrase III, and that therefore these tissues cannot produce enough bicarbonate to buffer the gastric acid. Less bicarbonate would mean greater acidity, so the pepsin would remain active and would be more likely to cause cellular damage.11
However, this contention is controversial. What is universally agreed upon is that reflux of gastric or gastroduodenal contents is most likely causing injury, most likely through direct exposure, although indirect effects through vagal mechanisms cannot be ruled out.
CURRENT DIAGNOSTIC TESTS FOR LPR HAVE SHORTCOMINGS
A careful history is important. Many patients report they have sore throat, hoarseness, cough, dysphasia, or chronic throat-clearing.13 Factors that may predispose a patient to esophageal reflux should be discussed, eg:
- Tobacco use
- Diet (eg, soda, spicy foods, fatty foods)
- Alcohol use
- Certain drugs (calcium channel blockers, nitrates, steroids).
Up to 50% of patients presenting with extraesophageal symptoms may not have classic reflux symptoms such as heartburn and regur-gitation.14 However, the existence of “silent reflux” is currently controversial.
Laryngoscopy is nonspecific and subjective
Because the key symptoms of LPR are nonspecific, many patients who present to an otolaryngologist undergo laryngoscopy, mainly to rule out malignancy. Once cancer is ruled out, many patients are given a diagnosis of LPR.
Furthermore, Branski et al17 performed transoral rigid laryngoscopy with videorecording in 100 consecutive patients presenting with a chief complaint of dysphonia. Five board-certified otolaryngologists individually viewed each recording, scored the degree of erythema and edema, and assessed the likelihood that LPR played a role in dysphonia and the severity of the LPR findings. The physicians’ ratings showed considerable interobserver variability. In other words, this study showed that laryngeal findings are often nonspecific and that the laryngoscopic diagnosis of LPR tends to be subjective.17
The Reflux Finding Score. Concerned by the lack of consistency in the diagnosis of LPR, Belafsky et al18 created a scoring system for documenting the physical findings and severity of disease on a standardized scale. Their Reflux Finding Score is based on eight laryngoscopic findings: subglottic edema, ventricular edema, erythema, vocal cord edema, diffuse laryngeal edema, hypertrophy of the posterior commissure, granuloma or granulation tissue, and thick endolaryngeal mucus. The total score can range from 0 (best) to 26 (worst).
In 40 patients with LPR confirmed by pH monitoring, the mean score was 11.5, compared with 5.2 in 40 age-matched controls. The authors calculated they could be 95% certain that a person with a score higher than 7 has LPR.18
However, this diagnostic method has not been validated in a large-scale randomized trial and so has yet to be incorporated into routine otolaryngology practice.
Ambulatory pH monitoring is not so golden for diagnosing LPR
Although pH monitoring was once the gold standard for diagnosing reflux, it has since been shown to be unreliable in patients who have laryngeal symptoms.4
How high or low in the esophagus the probe is placed is clearly critical for useful results. 4 But the test is subject to variability: different physicians place the probe in different locations, and the probe may shift. Another problem is that reflux may occur during untested periods.19
A pH of less than 4 in the esophagus had originally been shown to have high sensitivity and specificity,20 but Reichel and Issing21 suggested using a pH of less than 5 as the cutoff, which would identify more patients as having LPR. Further trials are needed to more precisely determine the pH threshold for the diagnosis of LPR.
Enthusiasm is waning for pharyngeal pH monitoring
In LPR, it was initially thought that pH monitoring in the pharynx was more accurate than in the distal or proximal esophagus.
Shaker et al22 monitored the pH in the pharynx, proximal esophagus, and distal esophagus in four groups: 14 patients who had both laryngeal signs and symptoms, 12 patients who had laryngeal symptoms only, 16 patients who had GERD but no laryngeal symptoms, and 12 healthy volunteers. They found that pharyngeal reflux was more frequent and in greater quantity in patients with laryngeal signs and symptoms than in the other groups. This study suggested that pharyngeal pH monitoring may be useful in diagnosing LPR in patients who have laryngeal signs and symptoms.
However, hypopharyngeal pH monitoring has several problems. One issue is that, even in this trial, 2 of 12 healthy volunteers had episodes of pharyngeal reflux.22 In other studies, the rate of false-positive results ranged from 7% to 17%.23,24 Additionally, in 12 previous studies, only 54% of 1,217 patients with suspected LPR had esophageal acid exposure, regardless of where the pH probe was placed.25
More importantly, another study found that patients with pharyngeal reflux documented by pH monitoring were no more likely to respond to acid-suppressive therapy than patients with no documented reflux.26 These findings dampen the enthusiasm for pharyngeal pH monitoring in LPR.
Impedance monitoring on therapy may be useful in refractory cases
Esophageal impedance monitoring, a newer test, uses a catheter that measures electrical resistance (impedance) between different points along the esophagus. Thus, it can detect the reflux of acid and nonacid liquid or gaseous material.
Pritchett et al27 performed esophageal impedance and pH monitoring in 39 patients who were on twice-daily PPI therapy and then evaluated the same patients with wireless pH monitoring while they were off therapy. The most prevalent complaint in the study group was cough (56%), followed by heartburn (18%) and sore throat (10%).
Of the 39 patients, 25 (64%) had normal results on impedance/pH monitoring while on therapy, ruling out reflux. On pH monitoring off therapy, 28 (72%) of the 39 patients had abnormal results; this group included 13 (93%) of the 14 patients who had abnormal results on impedance/pH monitoring while on therapy. The authors recommended on-therapy testing with impedance monitoring in patients with refractory reflux, since it provides more useful clinical information.27 If the results of impedance/pH monitoring are negative in these patients, a diagnosis other than reflux should be considered.
EMPIRIC PPI TREATMENT HAS SHOWN DISAPPOINTING RESULTS
Because laryngoscopy and pH monitoring are not very sensitive or specific for LPR, experts recommend empiric therapy with a PPI twice daily. However, the results have been disappointing when PPIs were compared with placebo in clinical trials.
In a randomized controlled trial,28 we found that patients who had complaints of chronic throat-clearing, cough, globus, sore throat, and hoarseness had a similar response to twice-daily esomeprazole (Nexium) compared with placebo: their primary symptom had resolved by 16 weeks in 14.7% of the esomeprazole group vs 16.0% of the placebo group (P = .799). Similarly, the final findings on laryngoscopy such as edema, erythema, and surface irregularity were not significantly different between groups.
Adding a histamine-2 receptor antagonist is not recommended
Adding a histamine-2 receptor antagonist to PPI therapy has also been considered as a treatment for LPR.
Fackler et al38 studied 16 GERD patients and 18 healthy volunteers to determine if adding ranitidine (Pepcid) to the PPI omeprazole (Prilosec) could improve GERD symptoms. Patients underwent baseline manometry and then gastroesophageal pH monitoring before starting the drugs. They first received omeprazole 20 mg twice daily alone for 2 weeks, and then added ranitidine 300 mg at bedtime. A pH test was done again after the first day of treatment with ranitidine, at the end of 1 week of combination therapy, and after 4 weeks of combination therapy. The combination reduced nocturnal acid breakthrough on day 1; however, due to tolerance to ranitidine, no significant difference in acid suppression was seen after 1 week of therapy. Therefore, this combination is not recommended.
Surgery is not recommended either
Some experts have argued for surgical fundoplication in patients whose symptoms persist despite drug therapy.
Swoger et al39 treated 72 patients who had symptoms consistent with LPR with a PPI for 4 months; 25 patients in this group had less than a 50% improvement despite maximal drug therapy. Ten of these patients underwent surgical fundoplication, and 15 remained on drug therapy alone. At 1 year of follow-up, only one surgical patient (10%) reported improvement in laryngeal symptoms.
In view of this report and prior studies of surgical fundoplication,40 surgery is not recommended for patients whose symptoms do not respond to aggressive PPI therapy.
IF A PPI FAILS, LOOK FOR OTHER CAUSES OF SYMPTOMS
Although gastroesophageal reflux and laryngeal signs and symptoms have been associated with one another, this relation may have been overstated, leading to the overdiagnosis of LPR.
The diagnosis of LPR is difficult, as laryngoscopy has high interrater variability and as the results of pH monitoring do not dependably predict who will respond to treatment.
Because PPI therapy is easy and appears to be safe, patients with extraesophageal symptoms thought to be related to reflux should undergo a trial of twice-daily PPI therapy for at least 2 months. If the patient responds to therapy, then tapering to once-daily therapy initially and then to minimal acid suppression to control symptoms would be prudent.
In patients who show no improvement, other causes of symptoms should be explored. Diseases that can mimic LPR include postnasal drip, allergies, sinus inflammation, and various pulmonary diseases. These patients should also be advised to adopt lifestyle modifications—eg, to stop smoking, lose weight, and decrease activities that cause stress on the voice. Surgery is not likely to provide any benefit in this situation. The patient should be tapered off the PPI to make sure no rebound acid reflux occurs.
The scenario is common: a patient complains of chronic hoarseness, cough, throat-clearing, sore throat, dysphagia, or a lump in the throat and undergoes laryngoscopy. If this test rules out cancer, the patient is given a diagnosis of laryngopharyngeal reflux (LPR), ie, a form of gastroesophageal reflux disease (GERD) in which the stomach contents get all the way up into the pharynx and down into the larynx. A proton pump inhibitor (PPI) is often prescribed, usually twice daily for 2 months.1–6
In this article, we review the current understanding of the pathophysiology of LPR and evaluate current diagnostic tests and treatment regimens for patients with suspected LPR.
THE PATHOPHYSIOLOGY OF LPR IS POORLY UNDERSTOOD
Transient relaxation of the lower esophageal sphincter
In a study in 10 healthy volunteers, Dent et al7 found that the pressure in the lower esophageal sphincter varies considerably over a 12-hour period. Episodes of reflux were not related to low basal (resting) pressure. Rather, 70% to 100% of reflux episodes occurred during random episodes of transient, complete, and inappropriate relaxation of the sphincter that lasted about 5 to 30 seconds. The mechanism of this relaxation is not known but is thought to be related to activation of the vagus nerve, possibly as a consequence of gastric distention.8
Gastric, not duodenal products seem to cause the damage
In a study in dogs, Adhami et al9 evaluated the possible role of gastric juices (acid and pepsin) vs duodenal juices (bile acids and trypsin) in laryngeal tissue damage. After taking baseline biopsy samples of the larynx, the investigators applied a variety of gastric and duodenal enzymes at varying pH levels (pH 1–7) to the larynxes. After 9 to 12 applications, they took another biopsy and assessed the changes visually and histologically.
At low (ie, acidic) pH levels, pepsin and conjugated bile acids were the most injurious, causing erythema and histologic evidence of inflammation. The authors concluded that gastric and not duodenal substances cause laryngeal injury and that acid-suppressive therapy “should eliminate the injurious potential” of acid reflux.9
The larynx is more sensitive than the esophagus
Monitoring of esophageal pH has shown that healthy people can tolerate as many as 50 episodes a day of acid reflux (pH < 4) in the esophagus. However, Koufman10 found that as few as three episodes of laryngeal reflux per week can cause severe laryngeal inflammation and injury.
Does pepsin deplete buffers, worsening acid damage?
Johnston et al11 took biopsies from a control group of healthy volunteers and from patients diagnosed with LPR. They detected pepsin in the samples from eight of the nine patients with LPR but in none of the controls. Furthermore, the tissue from patients with LPR had low levels of carbonic anhydrase III. The authors hypothesized that pepsin depletes the laryngopharynx of carbonic anhydrase III, and that therefore these tissues cannot produce enough bicarbonate to buffer the gastric acid. Less bicarbonate would mean greater acidity, so the pepsin would remain active and would be more likely to cause cellular damage.11
However, this contention is controversial. What is universally agreed upon is that reflux of gastric or gastroduodenal contents is most likely causing injury, most likely through direct exposure, although indirect effects through vagal mechanisms cannot be ruled out.
CURRENT DIAGNOSTIC TESTS FOR LPR HAVE SHORTCOMINGS
A careful history is important. Many patients report they have sore throat, hoarseness, cough, dysphasia, or chronic throat-clearing.13 Factors that may predispose a patient to esophageal reflux should be discussed, eg:
- Tobacco use
- Diet (eg, soda, spicy foods, fatty foods)
- Alcohol use
- Certain drugs (calcium channel blockers, nitrates, steroids).
Up to 50% of patients presenting with extraesophageal symptoms may not have classic reflux symptoms such as heartburn and regur-gitation.14 However, the existence of “silent reflux” is currently controversial.
Laryngoscopy is nonspecific and subjective
Because the key symptoms of LPR are nonspecific, many patients who present to an otolaryngologist undergo laryngoscopy, mainly to rule out malignancy. Once cancer is ruled out, many patients are given a diagnosis of LPR.
Furthermore, Branski et al17 performed transoral rigid laryngoscopy with videorecording in 100 consecutive patients presenting with a chief complaint of dysphonia. Five board-certified otolaryngologists individually viewed each recording, scored the degree of erythema and edema, and assessed the likelihood that LPR played a role in dysphonia and the severity of the LPR findings. The physicians’ ratings showed considerable interobserver variability. In other words, this study showed that laryngeal findings are often nonspecific and that the laryngoscopic diagnosis of LPR tends to be subjective.17
The Reflux Finding Score. Concerned by the lack of consistency in the diagnosis of LPR, Belafsky et al18 created a scoring system for documenting the physical findings and severity of disease on a standardized scale. Their Reflux Finding Score is based on eight laryngoscopic findings: subglottic edema, ventricular edema, erythema, vocal cord edema, diffuse laryngeal edema, hypertrophy of the posterior commissure, granuloma or granulation tissue, and thick endolaryngeal mucus. The total score can range from 0 (best) to 26 (worst).
In 40 patients with LPR confirmed by pH monitoring, the mean score was 11.5, compared with 5.2 in 40 age-matched controls. The authors calculated they could be 95% certain that a person with a score higher than 7 has LPR.18
However, this diagnostic method has not been validated in a large-scale randomized trial and so has yet to be incorporated into routine otolaryngology practice.
Ambulatory pH monitoring is not so golden for diagnosing LPR
Although pH monitoring was once the gold standard for diagnosing reflux, it has since been shown to be unreliable in patients who have laryngeal symptoms.4
How high or low in the esophagus the probe is placed is clearly critical for useful results. 4 But the test is subject to variability: different physicians place the probe in different locations, and the probe may shift. Another problem is that reflux may occur during untested periods.19
A pH of less than 4 in the esophagus had originally been shown to have high sensitivity and specificity,20 but Reichel and Issing21 suggested using a pH of less than 5 as the cutoff, which would identify more patients as having LPR. Further trials are needed to more precisely determine the pH threshold for the diagnosis of LPR.
Enthusiasm is waning for pharyngeal pH monitoring
In LPR, it was initially thought that pH monitoring in the pharynx was more accurate than in the distal or proximal esophagus.
Shaker et al22 monitored the pH in the pharynx, proximal esophagus, and distal esophagus in four groups: 14 patients who had both laryngeal signs and symptoms, 12 patients who had laryngeal symptoms only, 16 patients who had GERD but no laryngeal symptoms, and 12 healthy volunteers. They found that pharyngeal reflux was more frequent and in greater quantity in patients with laryngeal signs and symptoms than in the other groups. This study suggested that pharyngeal pH monitoring may be useful in diagnosing LPR in patients who have laryngeal signs and symptoms.
However, hypopharyngeal pH monitoring has several problems. One issue is that, even in this trial, 2 of 12 healthy volunteers had episodes of pharyngeal reflux.22 In other studies, the rate of false-positive results ranged from 7% to 17%.23,24 Additionally, in 12 previous studies, only 54% of 1,217 patients with suspected LPR had esophageal acid exposure, regardless of where the pH probe was placed.25
More importantly, another study found that patients with pharyngeal reflux documented by pH monitoring were no more likely to respond to acid-suppressive therapy than patients with no documented reflux.26 These findings dampen the enthusiasm for pharyngeal pH monitoring in LPR.
Impedance monitoring on therapy may be useful in refractory cases
Esophageal impedance monitoring, a newer test, uses a catheter that measures electrical resistance (impedance) between different points along the esophagus. Thus, it can detect the reflux of acid and nonacid liquid or gaseous material.
Pritchett et al27 performed esophageal impedance and pH monitoring in 39 patients who were on twice-daily PPI therapy and then evaluated the same patients with wireless pH monitoring while they were off therapy. The most prevalent complaint in the study group was cough (56%), followed by heartburn (18%) and sore throat (10%).
Of the 39 patients, 25 (64%) had normal results on impedance/pH monitoring while on therapy, ruling out reflux. On pH monitoring off therapy, 28 (72%) of the 39 patients had abnormal results; this group included 13 (93%) of the 14 patients who had abnormal results on impedance/pH monitoring while on therapy. The authors recommended on-therapy testing with impedance monitoring in patients with refractory reflux, since it provides more useful clinical information.27 If the results of impedance/pH monitoring are negative in these patients, a diagnosis other than reflux should be considered.
EMPIRIC PPI TREATMENT HAS SHOWN DISAPPOINTING RESULTS
Because laryngoscopy and pH monitoring are not very sensitive or specific for LPR, experts recommend empiric therapy with a PPI twice daily. However, the results have been disappointing when PPIs were compared with placebo in clinical trials.
In a randomized controlled trial,28 we found that patients who had complaints of chronic throat-clearing, cough, globus, sore throat, and hoarseness had a similar response to twice-daily esomeprazole (Nexium) compared with placebo: their primary symptom had resolved by 16 weeks in 14.7% of the esomeprazole group vs 16.0% of the placebo group (P = .799). Similarly, the final findings on laryngoscopy such as edema, erythema, and surface irregularity were not significantly different between groups.
Adding a histamine-2 receptor antagonist is not recommended
Adding a histamine-2 receptor antagonist to PPI therapy has also been considered as a treatment for LPR.
Fackler et al38 studied 16 GERD patients and 18 healthy volunteers to determine if adding ranitidine (Pepcid) to the PPI omeprazole (Prilosec) could improve GERD symptoms. Patients underwent baseline manometry and then gastroesophageal pH monitoring before starting the drugs. They first received omeprazole 20 mg twice daily alone for 2 weeks, and then added ranitidine 300 mg at bedtime. A pH test was done again after the first day of treatment with ranitidine, at the end of 1 week of combination therapy, and after 4 weeks of combination therapy. The combination reduced nocturnal acid breakthrough on day 1; however, due to tolerance to ranitidine, no significant difference in acid suppression was seen after 1 week of therapy. Therefore, this combination is not recommended.
Surgery is not recommended either
Some experts have argued for surgical fundoplication in patients whose symptoms persist despite drug therapy.
Swoger et al39 treated 72 patients who had symptoms consistent with LPR with a PPI for 4 months; 25 patients in this group had less than a 50% improvement despite maximal drug therapy. Ten of these patients underwent surgical fundoplication, and 15 remained on drug therapy alone. At 1 year of follow-up, only one surgical patient (10%) reported improvement in laryngeal symptoms.
In view of this report and prior studies of surgical fundoplication,40 surgery is not recommended for patients whose symptoms do not respond to aggressive PPI therapy.
IF A PPI FAILS, LOOK FOR OTHER CAUSES OF SYMPTOMS
Although gastroesophageal reflux and laryngeal signs and symptoms have been associated with one another, this relation may have been overstated, leading to the overdiagnosis of LPR.
The diagnosis of LPR is difficult, as laryngoscopy has high interrater variability and as the results of pH monitoring do not dependably predict who will respond to treatment.
Because PPI therapy is easy and appears to be safe, patients with extraesophageal symptoms thought to be related to reflux should undergo a trial of twice-daily PPI therapy for at least 2 months. If the patient responds to therapy, then tapering to once-daily therapy initially and then to minimal acid suppression to control symptoms would be prudent.
In patients who show no improvement, other causes of symptoms should be explored. Diseases that can mimic LPR include postnasal drip, allergies, sinus inflammation, and various pulmonary diseases. These patients should also be advised to adopt lifestyle modifications—eg, to stop smoking, lose weight, and decrease activities that cause stress on the voice. Surgery is not likely to provide any benefit in this situation. The patient should be tapered off the PPI to make sure no rebound acid reflux occurs.
- Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976; 21:953–956.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Jonaitis L, Pribuisiene R, Kupcinskas L, Uloza V. Laryngeal examination is superior to endoscopy in the diagnosis of the laryngopharyngeal form of gastroesophageal reflux disease. Scand J Gastroenterol 2006; 41:131–137.
- Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 2003; 1:333–344.
- Jaspersen D, Kulig M, Labenz J, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD study. Aliment Pharmacol Ther 2003; 17:1515–1520.
- Karkos PD, Benton J, Leong SC, et al. Trends in laryngopharyngeal reflux: a British ENT survey. Eur Arch Otorhinolaryngol 2007; 264:513–517.
- Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 1980; 65:256–267.
- Schreiber S, Garten D, Sudhoff H, et al. Pathophysiological mechanisms of extraesophageal reflux in otolaryngeal disorders. Eur Arch Otorhinolaryngol 2009; 266:17–24.
- Adhami T, Goldblum JR, Richter JE, Vaezi MF. The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol 2004; 99:2098–2106.
- Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991; 101(4 pt 2 suppl 53):1–78.
- Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 2004; 114:2129–2134.
- Vaezi MF. Therapy insight: gastroesophageal reflux disease and laryngopharyngeal reflux. Nat Clin Pract Gastroenterol Hepatol 2005; 2:595–603.
- Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis 2007; 13:349–359.
- Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J 2002; 81( 9 suppl 2):7–9.
- Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 2000; 123:385–388.
- Milstein CF, Charbel S, Hicks DM, Abelson TI, Richter JE, Vaezi MF. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs flexible scope). Laryngoscope 2005; 115;2256–2261.
- Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope 2002; 112;1019–1024.
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001; 111:1313–1317.
- Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg 2009; 17:143–148.
- Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992; 87:1102–1111.
- Reichel O, Issing WJ. Impact of different pH thresholds for 24-h dual probe pH monitoring in patients with suspected laryngopharyngeal reflux. J Laryngol Otol 2008; 122:485–489.
- Shaker R, Milbrath M, Ren J, et al. Esophagopharyngeal distribution of refluxed gastric acid in patients with reflux laryngitis. Gastroenterology 1995; 109:1575–1582.
- Jacob P, Kahrilas PJ, Herzon G. Proximal esophageal pH-metry in patients with “reflux laryngitis.” Gastroenterology 1991; 100:305–310.
- Eubanks TR, Omelanczuk PE, Maronian N, Hillel A, Pope CE, Pellegrini CA. Pharyngeal pH monitoring in 222 patients with suspected laryngeal reflux. J Gastrointest Surg 2001; 5:183–190.
- Vaezi MF. Gastroesophageal reflux disease and the larynx. J Clin Gastroenterol 2003; 36:198–203.
- Ulualp SO, Toohill RJ, Shaker R. Outcomes of acid suppressive therapy in patients with posterior laryngitis. Otolaryngol Head Neck Surg 2001; 124:16–22.
- Pritchett JM, Aslam M, Slaughter JC, Ness RM, Garrett CG, Vaezi MF. Efficacy of esophageal impedance/pH monitoring in patients with refractory gastroesophageal reflux disease, on and off therapy. Clin Gastroenterol Hepatol 2009; 7:743–748.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116;254–260.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Eherer AJ, Habermann W, Hammer HF, et al. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 2003; 38:462–467.
- El-Serag HB, Lee P, Buchner A, et al. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 2001; 96:979–983.
- Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 2001; 111:2147–2151.
- Langevin S, Hanh N. GERD-induced ENT symptoms: a prospective placebo controlled study with omeprazole 40 mg a day. Gastroenterology 2001; 120:A-16.
- Havas T, Huang S, Levy M, et al. Posterior pharyngolaryngitis. Double blind randomized placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryng 1999; 3:243–246.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Wo JM, Koopman JI, Harrell SP, et al. Double-blind placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol 2006; 101:1972–1978.
- Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 2004; 131:342–350.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- So JB, Zeitels SM, Rattner DW. Outcome of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery 1998; 124:28–32.
- Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976; 21:953–956.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Jonaitis L, Pribuisiene R, Kupcinskas L, Uloza V. Laryngeal examination is superior to endoscopy in the diagnosis of the laryngopharyngeal form of gastroesophageal reflux disease. Scand J Gastroenterol 2006; 41:131–137.
- Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 2003; 1:333–344.
- Jaspersen D, Kulig M, Labenz J, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD study. Aliment Pharmacol Ther 2003; 17:1515–1520.
- Karkos PD, Benton J, Leong SC, et al. Trends in laryngopharyngeal reflux: a British ENT survey. Eur Arch Otorhinolaryngol 2007; 264:513–517.
- Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 1980; 65:256–267.
- Schreiber S, Garten D, Sudhoff H, et al. Pathophysiological mechanisms of extraesophageal reflux in otolaryngeal disorders. Eur Arch Otorhinolaryngol 2009; 266:17–24.
- Adhami T, Goldblum JR, Richter JE, Vaezi MF. The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol 2004; 99:2098–2106.
- Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991; 101(4 pt 2 suppl 53):1–78.
- Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 2004; 114:2129–2134.
- Vaezi MF. Therapy insight: gastroesophageal reflux disease and laryngopharyngeal reflux. Nat Clin Pract Gastroenterol Hepatol 2005; 2:595–603.
- Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis 2007; 13:349–359.
- Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J 2002; 81( 9 suppl 2):7–9.
- Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 2000; 123:385–388.
- Milstein CF, Charbel S, Hicks DM, Abelson TI, Richter JE, Vaezi MF. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs flexible scope). Laryngoscope 2005; 115;2256–2261.
- Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope 2002; 112;1019–1024.
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001; 111:1313–1317.
- Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg 2009; 17:143–148.
- Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992; 87:1102–1111.
- Reichel O, Issing WJ. Impact of different pH thresholds for 24-h dual probe pH monitoring in patients with suspected laryngopharyngeal reflux. J Laryngol Otol 2008; 122:485–489.
- Shaker R, Milbrath M, Ren J, et al. Esophagopharyngeal distribution of refluxed gastric acid in patients with reflux laryngitis. Gastroenterology 1995; 109:1575–1582.
- Jacob P, Kahrilas PJ, Herzon G. Proximal esophageal pH-metry in patients with “reflux laryngitis.” Gastroenterology 1991; 100:305–310.
- Eubanks TR, Omelanczuk PE, Maronian N, Hillel A, Pope CE, Pellegrini CA. Pharyngeal pH monitoring in 222 patients with suspected laryngeal reflux. J Gastrointest Surg 2001; 5:183–190.
- Vaezi MF. Gastroesophageal reflux disease and the larynx. J Clin Gastroenterol 2003; 36:198–203.
- Ulualp SO, Toohill RJ, Shaker R. Outcomes of acid suppressive therapy in patients with posterior laryngitis. Otolaryngol Head Neck Surg 2001; 124:16–22.
- Pritchett JM, Aslam M, Slaughter JC, Ness RM, Garrett CG, Vaezi MF. Efficacy of esophageal impedance/pH monitoring in patients with refractory gastroesophageal reflux disease, on and off therapy. Clin Gastroenterol Hepatol 2009; 7:743–748.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116;254–260.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Eherer AJ, Habermann W, Hammer HF, et al. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 2003; 38:462–467.
- El-Serag HB, Lee P, Buchner A, et al. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 2001; 96:979–983.
- Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 2001; 111:2147–2151.
- Langevin S, Hanh N. GERD-induced ENT symptoms: a prospective placebo controlled study with omeprazole 40 mg a day. Gastroenterology 2001; 120:A-16.
- Havas T, Huang S, Levy M, et al. Posterior pharyngolaryngitis. Double blind randomized placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryng 1999; 3:243–246.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Wo JM, Koopman JI, Harrell SP, et al. Double-blind placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol 2006; 101:1972–1978.
- Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 2004; 131:342–350.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- So JB, Zeitels SM, Rattner DW. Outcome of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery 1998; 124:28–32.
KEY POINTS
- Laryngoscopy has high interrater variability, and results of pH monitoring do not reliably predict who will respond to treatment.
- A proton pump inhibitor twice daily for 2 months is currently recommended for patients with laryngeal signs and symptoms. If the condition responds to therapy, tapering to once-daily therapy and then to minimal acid-suppression to control symptoms is prudent.
- Patients whose symptoms do not respond to a proton pump inhibitor are unlikely to benefit from surgery. Other diagnoses should be entertained, while the drug is tapered to prevent rebound acid reflux.