User login
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
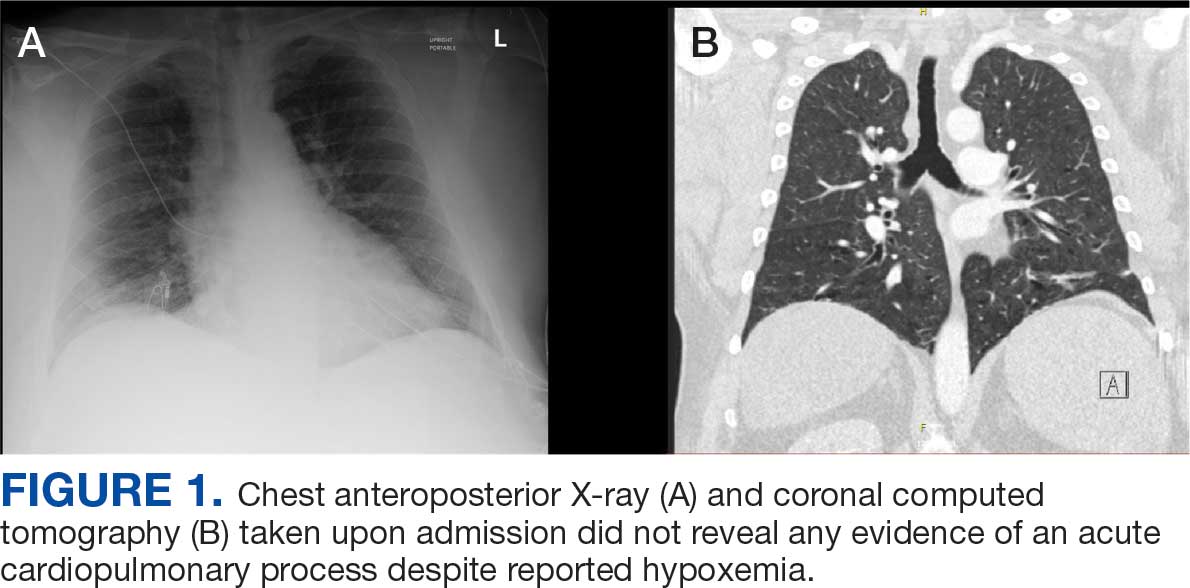
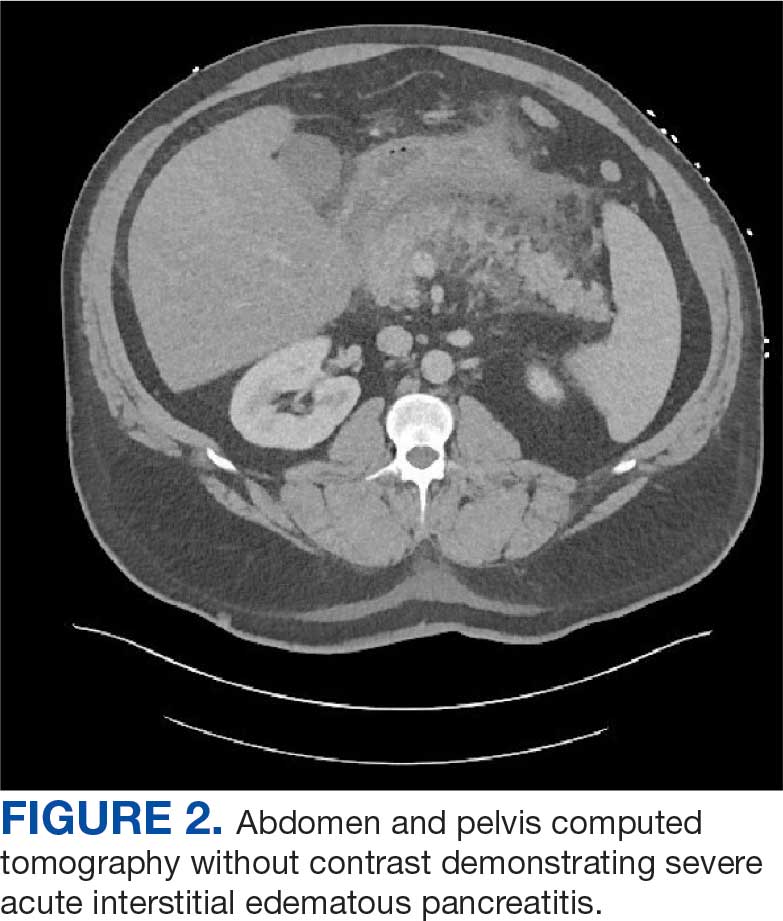
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
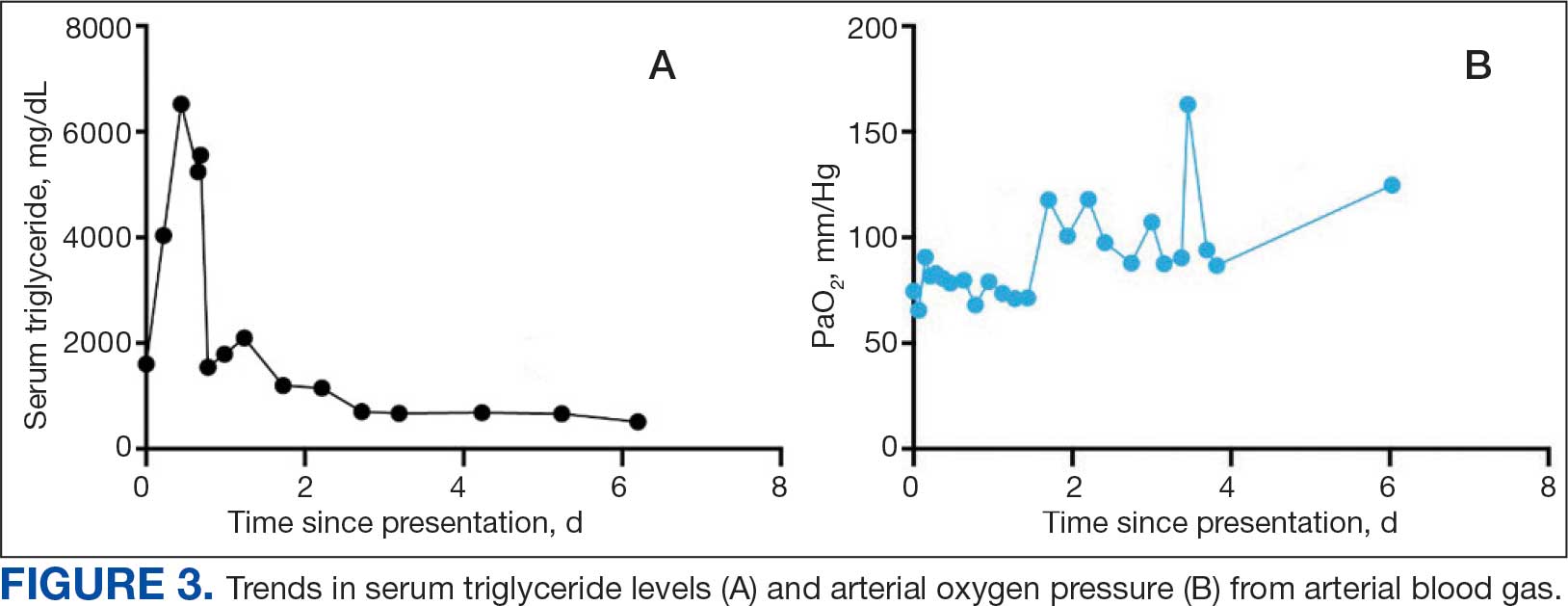
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
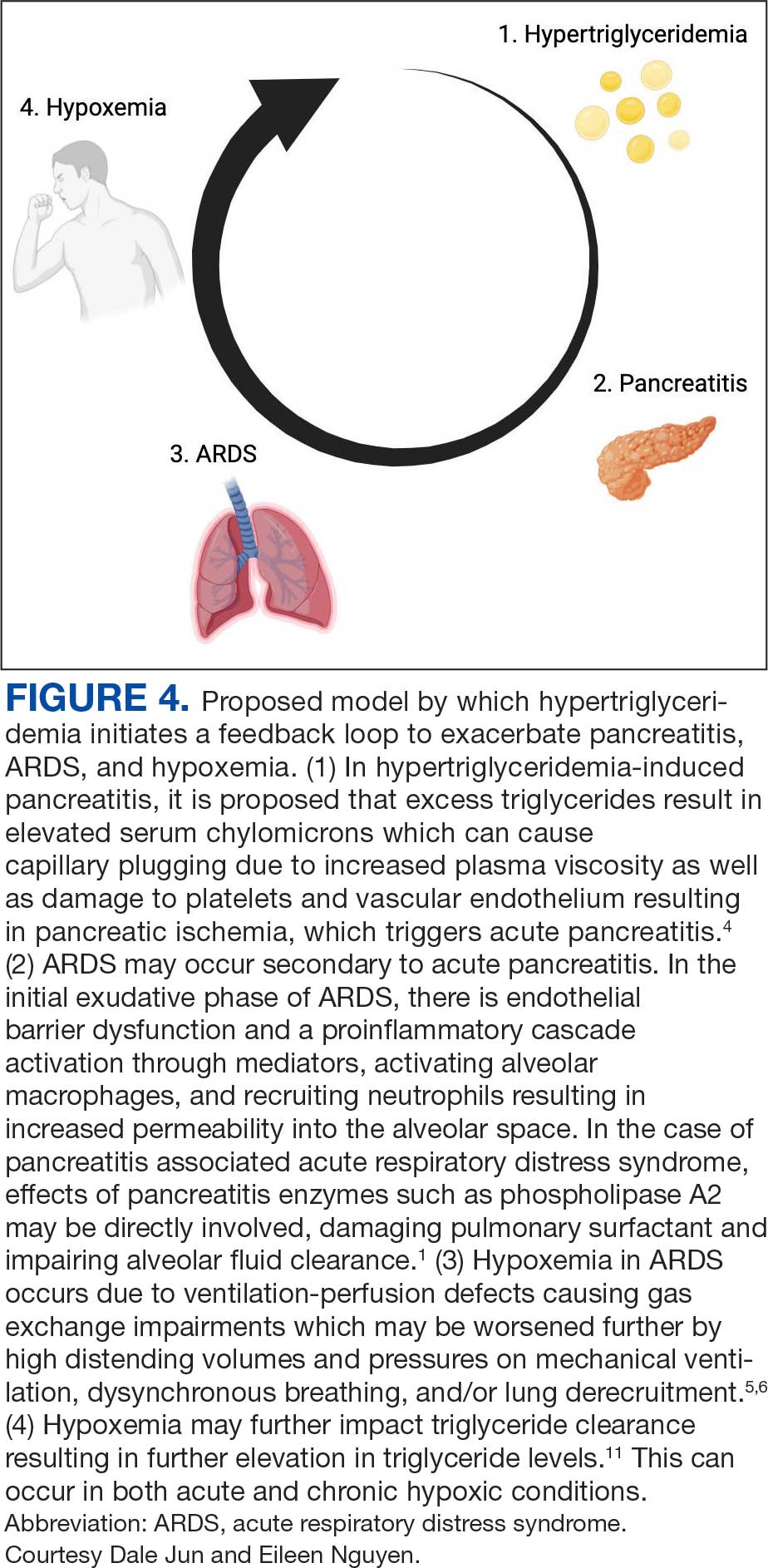
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis