User login
Clostridium difficile: Not just for adults
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
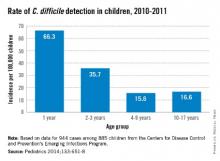
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
Sepsis-like syndrome: Enteroviruses vs. human parechovirus
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.
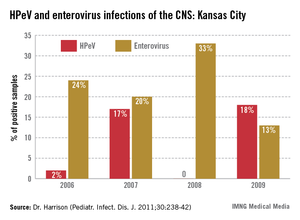
Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.

Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Not infrequently, less than 90 day old infants have fever and irritability and are more sleepy than usual, but have no apparent focus of infection. The sepsis evaluation is usually negative. It is frustrating for parents and providers when we report that we don’t really know what caused the febrile illness.
In summer/autumn season, some infants have enterovirus, with the predominate serotypes varying year to year. The enterovirus genus has several species, i.e., polio, enterovirus, echovirus, and Coxsackie A and Coxsackie B viruses. Echovirus is an acronym for enteric, cytopathic, human, orphan virus. Coxsackie is named from the city where it was first reported. Recently discovered enteroviruses have numbers starting at 68, and include a strain causing severe disease in Asia, enterovirus 71.

Sometimes clinicians can tell that enterovirus is in the community without laboratory tests because children present with hand, foot, and mouth (and sometimes buttock) disease. or herpangina. Enteroviruses also cause pericarditis, myocarditis, or pleurodynia (a.k.a. the "devil’s grippe").
But enteroviruses also cause aseptic meningitis. A modest pleocytosis with a mononuclear predominance and near-normal CSF glucose/protein values, plus negative bacterial cultures, is commonly called "aseptic meningitis."
Enteroviruses cause modest CSF pleocytosis (50-400 WBC), usually with mononuclear predominance and relatively normal CSF chemistries. While there can initially be a CSF neutrophil predominance, the differential usually shifts to mostly mononuclear cells less than 24 hours later. In the 1970s and 1980s (before polymerase chain reaction [PCR]), we used a "double tap" strategy to allow early discontinuation of antibiotics and hospital discharge. If the second CSF obtained within 24 hours of the first CSF had reasonably normal chemistries plus fewer WBCs or shifted to almost all mononuclear cells, children were discharged before final culture results. While hypoglycorrhachia is seen rarely with enterovirus (as low as 10 mg/dL), low CSF glucose values are usually due to bacterial or tuberculous meningitis. CSF protein concentrations with enteroviral meningitis are rarely greater than 80 mg/dL, the usual values for bacterial meningitis.
But consider this caveat: When "aseptic meningitis" seems present but CSF chemistries are abnormal (elevated protein or low glucose), check for tuberculosis risk factors and/or indolent neurological findings that could indicate tuberculous meningitis. In infants less than 2 months of age, consider neonatal herpes simplex virus (HSV) disease, particularly if the CSF protein is elevated.
These days "double taps" are not routine. Instead, CSF PCR is used. HSV and enterovirus PCR on CSF is available at most institutions with results available before bacteria cultures are final. A positive CSF enterovirus PCR (J. Pediatr. 1997;131:393-7) allows discontinuing antibacterials and acyclovir, if it was started empirically, plus early discharge. A positive HSV PCR also clarifies management: Continue acyclovir but discontinue antibacterial drugs. Keep in mind that enteroviral meningitis outbreaks are quite seasonal, with the majority of disease noted in the summer and early fall.
So we know the answer if the enterovirus or HSV PCR is positive. But what if these PCRs and bacterial cultures are negative in a child not pretreated with antibiotics? Well, the new kid on the block for aseptic meningitis is human parechovirus (HPeV). The first viruses classified as HPeV (HPeV1 and HPeV2) were previously called echovirus 22 and echovirus 23. But clinical and genome differences from enteroviruses led to reclassification as HPeVs. Now there are 16 HPeV serotypes. So why do we care?
In the past 6 years, HPeV3 emerged as the most common definable cause of sepsis-like syndrome in young infants with negative bacterial cultures (J. Clin. Virol. 2011;52:187-91; Pediatr. Infect. Dis. J. 2013;32:213-6). Interestingly, HPeV3-infected infants have more frequent peripheral leukopenia and lymphopenia plus more febrile days and higher fevers than those with enteroviruses. HPeV3 has a nearly every-other-year cycle (May-November). HPeV was as frequent or more frequent than all enteroviruses combined.
HPEV is not detected by enterovirus PCR, but is confirmed by HPeV-specific PCR. Like enteroviruses, PCR of blood is usually positive in HPeV-infected infants.
An important difference from HSV or enteroviruses is that almost no HPeV3 CNS-infected infants have CSF pleocytosis. That’s right. CSF in HPeV CNS infection is like HHV-6 (minimal CSF WBCs despite CNS infection). At our institution, HPeV3 PCR is performed routinely on CSF from all infants less than 90 days of age undergoing sepsis evaluations in summer/autumn.
If cultures and PCRs are negative in young infants with sepsis-like syndrome, your laboratory can likely perform or send out HPeV3 PCR. When HPeV3 CSF PCRs are positive, antibacterials can be stopped and patients discharged. Clinicians may be reluctant to discharge before final negative bacterial cultures because these infants can still "look ill," and providers are just learning about HPeV3. But based on our multiyear experience, it appears safe. We saw only three concurrent bacterial infections when HPeV3 was detected in CSF – all urinary tract infections that were easily detected during the sepsis evaluation and treated as such.
There have been no defined sequelae of HPeV CNS infection to date in the United States, although long-term follow-up is lacking for this emerging pathogen. There have been rare CNS sequelae, including white matter changes or seizures outside the United States, but these were apparent during the acute illness. We recommend outpatient follow-up soon after discharge, particularly if fever persists at discharge.
If we add HPeV3 PCR to our testing for infant sepsis-like syndrome during summer/fall, particularly when there is no or minimal CSF pleocytosis plus peripheral leuko/lymphopenia, there will fewer times when we lack a confirmed cause.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Outpatient antibiotics ABRS vs. AOM
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
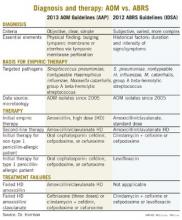
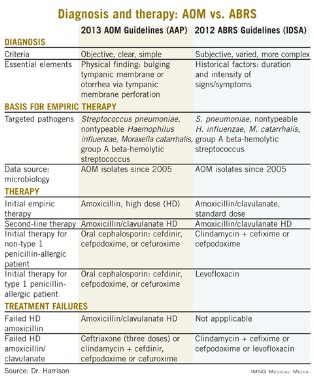
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
CDC Seeks Public Input on Tough Vaccine Decisions
At the next meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP), the group will need to decide whether to recommend routine meningococcal conjugate vaccine (MCV) use in infants. Because other federal committees recently suggested that the CDC engage the public before such decisions, the agency developed a new information-gathering process involving consumers. This took the form of focus group meetings designed to be open and to elicit input from those who might hold differing opinions about vaccines.
Over the summer, four pilot group meetings were held in Concord N.H., Chicago, Seattle, and Denver. There were about 100 attendees in Chicago and Seattle, and about half that number each in New Hampshire and Denver. Participants included provaccine groups such as the Meningitis Angels, who passionately support routine meningococcal immunization for young infants, representatives of the antivaccine group, who call themselves the National Vaccine Information Center, as well as more or less "neutral" individuals recruited through local health departments. They were asked a series of hypothetical questions about their opinions on use of new/future vaccines and the extent that they’d be willing to support and pay for such vaccines. The answers would likely apply to MCV.
Insights from these focus groups will likely play a role in ACIP’s October discussions of routine infant immunization against meningococcal disease. The decision is complicated because of three potentially competing vaccine formulations that are not currently interchangeable and with potentially different schedules:
• Sanofi-Pasteur’s MenACWY-D (Menactra) is currently licensed starting at 9 months of age in select high-risk groups, but could be expanded to all infants.
• Novartis’ MenACWY-CRM (Menveo) was recently approved for children ages 2 years and above, but is before the U.S. Food and Drug Administration (FDA) for licensure at 2, 4, and 6 months of age.
• A not yet licensed GlaxoSmithKline’s HibMenCY (MenHibRix) is a combination vaccine containing Haemophilus influenzae type b and meningococcal type A and C antigens. GSK is seeking licensure for use down to 2 months of age.
In June, ACIP recommended Sanofi-Pasteur’s quadrivalent MenACWY-D for specific high-risk children aged 9-23 months, such as travelers to meningococcal disease-endemic areas, infants with complement deficiencies, those in outbreak situations, and HIV-infected infants with other indications for vaccination. The committee postponed voting on whether 9- to 23-month-olds with functional or anatomic asplenia, including sickle cell disease, should receive this vaccine. This was due to data that pneumococcal geometric mean titers were reduced when MenACWY-D was given at the same time as pneumococcal conjugate vaccine (PCV-7). The clinical impact of such lower titers (that are still in the protective range) are unclear, but pneumococcus is a higher threat than meningococcus for these groups.
So what is the rationale for waiting until 9 months of age to begin MCV? For one, it avoids the need for a potential extra injection at 2, 4, and 6 months, and there are only two, not four doses. Also, none of the candidate MCVs contains meningococcal serotype B, the most common serotype affecting children less than 3 years of age. Several manufacturers are working on a B serogroup vaccine, which is years away from release. Lastly, the absolute number of annually prevented deaths (about five per year) would be relatively small among those less than 9 months of age.
What is the rationale for giving MCV at 2 months of age? Because children aged less than 3 years have the highest attack rate for invasive meningococcal disease (about 50% serogroup B and 50% of the other serogroups combined), it also makes sense to start MCV as early as possible to maximize reduction of nonserogroup-B invasive meningococcal disease. And if one used the GSK combo DTaP-Hep B- IPV vaccine plus Hib MenCY, there would not be additional injections at 2, 4, and 6 months.
So if the licensures for use in 2-month-olds come through soon as is expected, ACIP will need to address routine infant MCV immunization. Consider the complexity. It’s unlikely that Sanofi’s MenACWY-D will be licensed for this age soon, so it remains a candidate only for routine first dosing at 9 months of age. It would become almost obsolete if ACIP recommends routine 2, 4, and 6 month MCV dosing. However, a 2, 4, and 6 month recommendation raises added questions. Should the GSK HibMenCY be preferred over the Novartis MenACWY-CRM (which does not contain Hib) if both are FDA approved for 2-month-olds? And what impact would use of HibMenCY have on the use of Sanofi’s combo DTaP-Hib-IPV vaccine? You wouldn’t use HibMenCY with DTaP-Hib-IPV, because that would be a double dose of Hib vaccine.
So which approach would you use in your practice? It is something to consider. If ACIP recommends both for initial dosing at 2 months, vaccine buying groups may be making decisions for clinicians based on price and package deals. Regardless, it will further complicate the vaccine schedule. And if it is complicated for us, parents are going to be even less likely to understand if their child is "up to date."
The CDC organized the focus groups to help inform those decisions. One question was "Which type of disease do you think should be given priority when it comes to developing new vaccines?" Choices were those diseases that affect many people but aren’t severe; those that are relatively rare but if contracted can cause severe disability or death; or equal priority for both. The attendees had varied opinions, but 26% in Concord and 59% in Seattle chose the rare/severe option, while 34% in Chicago to 48% in Concord chose "equal priority for both."
They also asked, "How many children need to get a severe illness in a typical year in the United States to make it a good idea that children be vaccinated?" Options were 1 in 100, 1 in 1,000, 1 in 10,000, 1 in 100,000, or 1 in 1 million. While each option was picked by at least some participants, the 1 in 100 option was a frequent choice, ranging from 23% in Denver to 57% in Chicago.
When asked what they believed ACIP should recommend for MCV, most – ranging from 53% in Seattle to 86% in Chicago – chose "add the meningococcal vaccine to the schedule for infants/children, and recommend all children be vaccinated." The other choices were less popular. The "no ACIP or CDC recommendation, but add to the Vaccines for Children program" was chosen by 8%-31%, and "no ACIP/CDC recommendation and do not add to the VFC program" was chosen by 7%-21%.
And as for what they would be willing to pay out of pocket, the largest subset in Chicago (44%) and Concord (53%) agreed to pay the highest priced option for those cities (more than $150), while in Denver and Seattle, 31% and 34%, respectively, actually appeared willing to pay more than $500.
Of course, these are very small samples of likely biased data from subsets of the public having high interest in vaccines (positive or negative attitudes). Still, I think it’s a very interesting new direction the CDC has taken to elicit this kind of information when planning to discuss difficult vaccine policy decisions. At the very least, it improves transparency, which should hopefully help us when talking with our patients about new vaccine recommendations and the complicated schedules, whatever they may be.
Dr. Christopher J. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison receives grant funding from GlaxoSmithKline for research trials of MMR.
At the next meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP), the group will need to decide whether to recommend routine meningococcal conjugate vaccine (MCV) use in infants. Because other federal committees recently suggested that the CDC engage the public before such decisions, the agency developed a new information-gathering process involving consumers. This took the form of focus group meetings designed to be open and to elicit input from those who might hold differing opinions about vaccines.
Over the summer, four pilot group meetings were held in Concord N.H., Chicago, Seattle, and Denver. There were about 100 attendees in Chicago and Seattle, and about half that number each in New Hampshire and Denver. Participants included provaccine groups such as the Meningitis Angels, who passionately support routine meningococcal immunization for young infants, representatives of the antivaccine group, who call themselves the National Vaccine Information Center, as well as more or less "neutral" individuals recruited through local health departments. They were asked a series of hypothetical questions about their opinions on use of new/future vaccines and the extent that they’d be willing to support and pay for such vaccines. The answers would likely apply to MCV.
Insights from these focus groups will likely play a role in ACIP’s October discussions of routine infant immunization against meningococcal disease. The decision is complicated because of three potentially competing vaccine formulations that are not currently interchangeable and with potentially different schedules:
• Sanofi-Pasteur’s MenACWY-D (Menactra) is currently licensed starting at 9 months of age in select high-risk groups, but could be expanded to all infants.
• Novartis’ MenACWY-CRM (Menveo) was recently approved for children ages 2 years and above, but is before the U.S. Food and Drug Administration (FDA) for licensure at 2, 4, and 6 months of age.
• A not yet licensed GlaxoSmithKline’s HibMenCY (MenHibRix) is a combination vaccine containing Haemophilus influenzae type b and meningococcal type A and C antigens. GSK is seeking licensure for use down to 2 months of age.
In June, ACIP recommended Sanofi-Pasteur’s quadrivalent MenACWY-D for specific high-risk children aged 9-23 months, such as travelers to meningococcal disease-endemic areas, infants with complement deficiencies, those in outbreak situations, and HIV-infected infants with other indications for vaccination. The committee postponed voting on whether 9- to 23-month-olds with functional or anatomic asplenia, including sickle cell disease, should receive this vaccine. This was due to data that pneumococcal geometric mean titers were reduced when MenACWY-D was given at the same time as pneumococcal conjugate vaccine (PCV-7). The clinical impact of such lower titers (that are still in the protective range) are unclear, but pneumococcus is a higher threat than meningococcus for these groups.
So what is the rationale for waiting until 9 months of age to begin MCV? For one, it avoids the need for a potential extra injection at 2, 4, and 6 months, and there are only two, not four doses. Also, none of the candidate MCVs contains meningococcal serotype B, the most common serotype affecting children less than 3 years of age. Several manufacturers are working on a B serogroup vaccine, which is years away from release. Lastly, the absolute number of annually prevented deaths (about five per year) would be relatively small among those less than 9 months of age.
What is the rationale for giving MCV at 2 months of age? Because children aged less than 3 years have the highest attack rate for invasive meningococcal disease (about 50% serogroup B and 50% of the other serogroups combined), it also makes sense to start MCV as early as possible to maximize reduction of nonserogroup-B invasive meningococcal disease. And if one used the GSK combo DTaP-Hep B- IPV vaccine plus Hib MenCY, there would not be additional injections at 2, 4, and 6 months.
So if the licensures for use in 2-month-olds come through soon as is expected, ACIP will need to address routine infant MCV immunization. Consider the complexity. It’s unlikely that Sanofi’s MenACWY-D will be licensed for this age soon, so it remains a candidate only for routine first dosing at 9 months of age. It would become almost obsolete if ACIP recommends routine 2, 4, and 6 month MCV dosing. However, a 2, 4, and 6 month recommendation raises added questions. Should the GSK HibMenCY be preferred over the Novartis MenACWY-CRM (which does not contain Hib) if both are FDA approved for 2-month-olds? And what impact would use of HibMenCY have on the use of Sanofi’s combo DTaP-Hib-IPV vaccine? You wouldn’t use HibMenCY with DTaP-Hib-IPV, because that would be a double dose of Hib vaccine.
So which approach would you use in your practice? It is something to consider. If ACIP recommends both for initial dosing at 2 months, vaccine buying groups may be making decisions for clinicians based on price and package deals. Regardless, it will further complicate the vaccine schedule. And if it is complicated for us, parents are going to be even less likely to understand if their child is "up to date."
The CDC organized the focus groups to help inform those decisions. One question was "Which type of disease do you think should be given priority when it comes to developing new vaccines?" Choices were those diseases that affect many people but aren’t severe; those that are relatively rare but if contracted can cause severe disability or death; or equal priority for both. The attendees had varied opinions, but 26% in Concord and 59% in Seattle chose the rare/severe option, while 34% in Chicago to 48% in Concord chose "equal priority for both."
They also asked, "How many children need to get a severe illness in a typical year in the United States to make it a good idea that children be vaccinated?" Options were 1 in 100, 1 in 1,000, 1 in 10,000, 1 in 100,000, or 1 in 1 million. While each option was picked by at least some participants, the 1 in 100 option was a frequent choice, ranging from 23% in Denver to 57% in Chicago.
When asked what they believed ACIP should recommend for MCV, most – ranging from 53% in Seattle to 86% in Chicago – chose "add the meningococcal vaccine to the schedule for infants/children, and recommend all children be vaccinated." The other choices were less popular. The "no ACIP or CDC recommendation, but add to the Vaccines for Children program" was chosen by 8%-31%, and "no ACIP/CDC recommendation and do not add to the VFC program" was chosen by 7%-21%.
And as for what they would be willing to pay out of pocket, the largest subset in Chicago (44%) and Concord (53%) agreed to pay the highest priced option for those cities (more than $150), while in Denver and Seattle, 31% and 34%, respectively, actually appeared willing to pay more than $500.
Of course, these are very small samples of likely biased data from subsets of the public having high interest in vaccines (positive or negative attitudes). Still, I think it’s a very interesting new direction the CDC has taken to elicit this kind of information when planning to discuss difficult vaccine policy decisions. At the very least, it improves transparency, which should hopefully help us when talking with our patients about new vaccine recommendations and the complicated schedules, whatever they may be.
Dr. Christopher J. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison receives grant funding from GlaxoSmithKline for research trials of MMR.
At the next meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP), the group will need to decide whether to recommend routine meningococcal conjugate vaccine (MCV) use in infants. Because other federal committees recently suggested that the CDC engage the public before such decisions, the agency developed a new information-gathering process involving consumers. This took the form of focus group meetings designed to be open and to elicit input from those who might hold differing opinions about vaccines.
Over the summer, four pilot group meetings were held in Concord N.H., Chicago, Seattle, and Denver. There were about 100 attendees in Chicago and Seattle, and about half that number each in New Hampshire and Denver. Participants included provaccine groups such as the Meningitis Angels, who passionately support routine meningococcal immunization for young infants, representatives of the antivaccine group, who call themselves the National Vaccine Information Center, as well as more or less "neutral" individuals recruited through local health departments. They were asked a series of hypothetical questions about their opinions on use of new/future vaccines and the extent that they’d be willing to support and pay for such vaccines. The answers would likely apply to MCV.
Insights from these focus groups will likely play a role in ACIP’s October discussions of routine infant immunization against meningococcal disease. The decision is complicated because of three potentially competing vaccine formulations that are not currently interchangeable and with potentially different schedules:
• Sanofi-Pasteur’s MenACWY-D (Menactra) is currently licensed starting at 9 months of age in select high-risk groups, but could be expanded to all infants.
• Novartis’ MenACWY-CRM (Menveo) was recently approved for children ages 2 years and above, but is before the U.S. Food and Drug Administration (FDA) for licensure at 2, 4, and 6 months of age.
• A not yet licensed GlaxoSmithKline’s HibMenCY (MenHibRix) is a combination vaccine containing Haemophilus influenzae type b and meningococcal type A and C antigens. GSK is seeking licensure for use down to 2 months of age.
In June, ACIP recommended Sanofi-Pasteur’s quadrivalent MenACWY-D for specific high-risk children aged 9-23 months, such as travelers to meningococcal disease-endemic areas, infants with complement deficiencies, those in outbreak situations, and HIV-infected infants with other indications for vaccination. The committee postponed voting on whether 9- to 23-month-olds with functional or anatomic asplenia, including sickle cell disease, should receive this vaccine. This was due to data that pneumococcal geometric mean titers were reduced when MenACWY-D was given at the same time as pneumococcal conjugate vaccine (PCV-7). The clinical impact of such lower titers (that are still in the protective range) are unclear, but pneumococcus is a higher threat than meningococcus for these groups.
So what is the rationale for waiting until 9 months of age to begin MCV? For one, it avoids the need for a potential extra injection at 2, 4, and 6 months, and there are only two, not four doses. Also, none of the candidate MCVs contains meningococcal serotype B, the most common serotype affecting children less than 3 years of age. Several manufacturers are working on a B serogroup vaccine, which is years away from release. Lastly, the absolute number of annually prevented deaths (about five per year) would be relatively small among those less than 9 months of age.
What is the rationale for giving MCV at 2 months of age? Because children aged less than 3 years have the highest attack rate for invasive meningococcal disease (about 50% serogroup B and 50% of the other serogroups combined), it also makes sense to start MCV as early as possible to maximize reduction of nonserogroup-B invasive meningococcal disease. And if one used the GSK combo DTaP-Hep B- IPV vaccine plus Hib MenCY, there would not be additional injections at 2, 4, and 6 months.
So if the licensures for use in 2-month-olds come through soon as is expected, ACIP will need to address routine infant MCV immunization. Consider the complexity. It’s unlikely that Sanofi’s MenACWY-D will be licensed for this age soon, so it remains a candidate only for routine first dosing at 9 months of age. It would become almost obsolete if ACIP recommends routine 2, 4, and 6 month MCV dosing. However, a 2, 4, and 6 month recommendation raises added questions. Should the GSK HibMenCY be preferred over the Novartis MenACWY-CRM (which does not contain Hib) if both are FDA approved for 2-month-olds? And what impact would use of HibMenCY have on the use of Sanofi’s combo DTaP-Hib-IPV vaccine? You wouldn’t use HibMenCY with DTaP-Hib-IPV, because that would be a double dose of Hib vaccine.
So which approach would you use in your practice? It is something to consider. If ACIP recommends both for initial dosing at 2 months, vaccine buying groups may be making decisions for clinicians based on price and package deals. Regardless, it will further complicate the vaccine schedule. And if it is complicated for us, parents are going to be even less likely to understand if their child is "up to date."
The CDC organized the focus groups to help inform those decisions. One question was "Which type of disease do you think should be given priority when it comes to developing new vaccines?" Choices were those diseases that affect many people but aren’t severe; those that are relatively rare but if contracted can cause severe disability or death; or equal priority for both. The attendees had varied opinions, but 26% in Concord and 59% in Seattle chose the rare/severe option, while 34% in Chicago to 48% in Concord chose "equal priority for both."
They also asked, "How many children need to get a severe illness in a typical year in the United States to make it a good idea that children be vaccinated?" Options were 1 in 100, 1 in 1,000, 1 in 10,000, 1 in 100,000, or 1 in 1 million. While each option was picked by at least some participants, the 1 in 100 option was a frequent choice, ranging from 23% in Denver to 57% in Chicago.
When asked what they believed ACIP should recommend for MCV, most – ranging from 53% in Seattle to 86% in Chicago – chose "add the meningococcal vaccine to the schedule for infants/children, and recommend all children be vaccinated." The other choices were less popular. The "no ACIP or CDC recommendation, but add to the Vaccines for Children program" was chosen by 8%-31%, and "no ACIP/CDC recommendation and do not add to the VFC program" was chosen by 7%-21%.
And as for what they would be willing to pay out of pocket, the largest subset in Chicago (44%) and Concord (53%) agreed to pay the highest priced option for those cities (more than $150), while in Denver and Seattle, 31% and 34%, respectively, actually appeared willing to pay more than $500.
Of course, these are very small samples of likely biased data from subsets of the public having high interest in vaccines (positive or negative attitudes). Still, I think it’s a very interesting new direction the CDC has taken to elicit this kind of information when planning to discuss difficult vaccine policy decisions. At the very least, it improves transparency, which should hopefully help us when talking with our patients about new vaccine recommendations and the complicated schedules, whatever they may be.
Dr. Christopher J. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison receives grant funding from GlaxoSmithKline for research trials of MMR.