User login
EARLY LIFE STRESS AND DEPRESSION Childhood trauma may lead to neurobiologically unique mood disorders
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
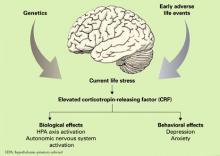
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.