User login
‘Can’t believe we won! (The AGA Shark Tank)’: Building sustainable careers in clinical and translational GI research
Tell us about your recent experience at the AGA Tech Summit.
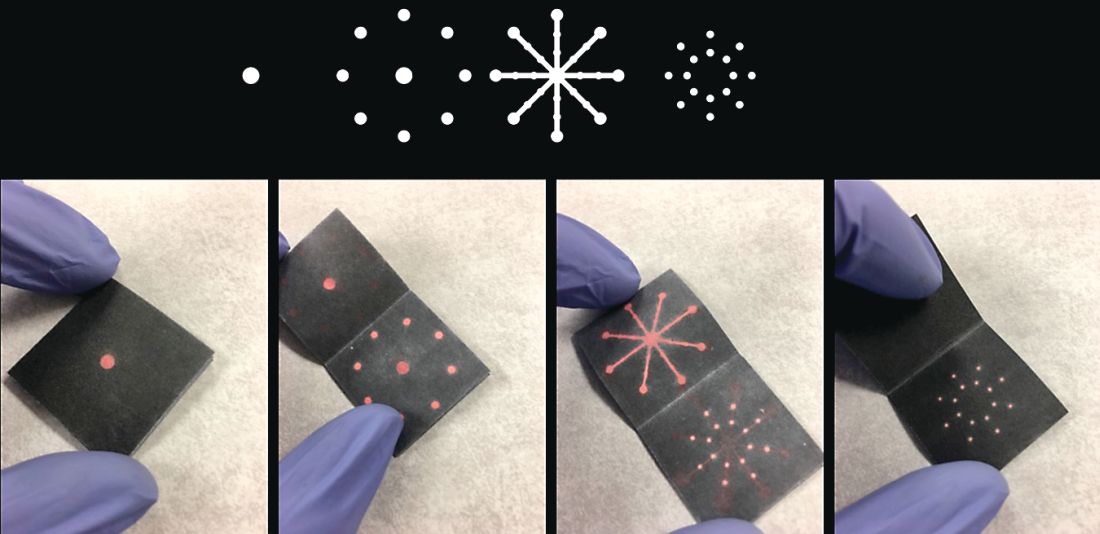
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.
Tell us about your recent experience at the AGA Tech Summit.
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.
Tell us about your recent experience at the AGA Tech Summit.
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.