User login
Safety and Efficacy of Ezetimibe in Patients With and Without Chronic Kidney Disease at a Pharmacist-Managed Clinic
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
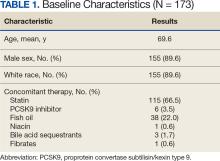
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
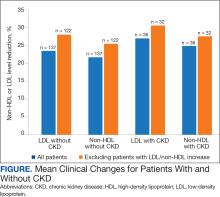
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608