User login
Forming specialized immune cell structures could combat pancreatic cancer
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
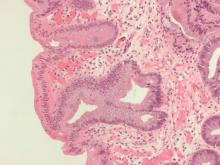
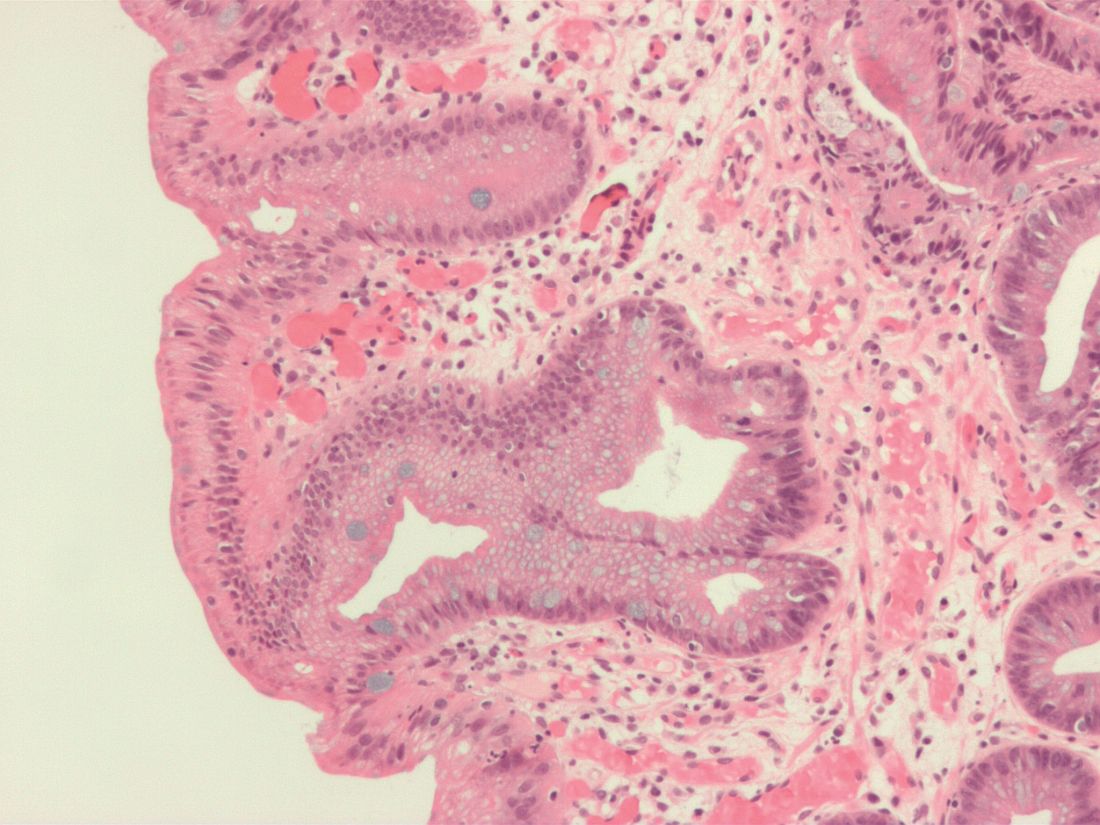
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Noninvasive ELF test identifies risk of advanced fibrosis in NAFLD
A noninvasive enhanced liver fibrosis (ELF) blood test identifies patients with nonalcoholic fatty liver disease (NAFLD) who are at increased risk of advanced fibrosis, according to a new study.
According to the study researchers, when combined with the fibrosis-4 (FIB-4) score the ELF test may be a reliable method that can assess for advanced fibrosis in clinical practice.
Despite the utility of identifying advanced fibrosis in the NAFLD population, significant barriers exist to “risk stratifying patients in clinical practice owing to the need for liver biopsy,” wrote study authors Zobair M. Younossi, MD, of the Inova Health System in Falls Church, Va., and colleagues in JAMA Network Open.
“NASH [nonalcoholic steatohepatitis] can only be diagnosed by biopsy and liver pathology yet validated noninvasive tests that accurately diagnose NASH don’t exist,” said Dr. Younossi in an interview. “Developing noninvasive tests to accurately risk stratify patients with significant fibrosis in NASH is highly desirable in clinical practice. A blood test such as ELF can open the opportunity to order this test anywhere in the country.”
The ELF test reflects extracellular matrix metabolism as opposed to tests that assess alterations in hepatic function. The study authors explain that the noninvasive ELF test is a “blood-derived panel of biomarkers consisting of three components: type III procollagen peptide, hyaluronic acid, and tissue inhibitor of metalloproteinase-1.”
To gain a further understanding of the role of ELF in predicting the risk of nonalcoholic steatohepatitis (NASH) in NAFLD, Dr. Younossi and colleagues performed a retrospective, cross-sectional analysis of outpatients within a community-based liver clinic from 2001 in 2020. The study cohort included 829 patients (mean age, 53.1 years) with NAFLD, which was characterized by steatosis greater than 5% without any other liver disease or excessive alcohol use.
In the overall study population, the mean FIB-4 score was 1.34. In the 463 patients with liver biopsy, approximately 24.4% presented with bridging fibrosis or cirrhosis. A total of 79 (17.1%) of the 462 patients with transient elastography data presented with liver stiffness results of 9.6 kPa or greater, which according to the researchers was indicative of advanced fibrosis.
Biopsy determined that those with advanced fibrosis in the study had significantly increased ELF scores versus patients without advanced fibrosis (10.1 vs. 8.6, respectively; P <.001). Moreover, patients with advanced fibrosis had significantly greater liver stiffness as determined by transient elastography (10.0 vs. 9.0; P <.001).
In the NAFLD population, the ELF test demonstrated excellent performance in identifying patients with advanced fibrosis, as reflected by an area under the receiver operating characteristic curve of 0.81 (95% confidence interval, 0.77-0.85) for those whose fibrosis was diagnosed by biopsy as well as 0.79 (95% CI, 0.75-0.82) for fibrosis diagnosed by transient elastography. Similar performances of the ELF score were reported among those with NAFLD who were aged 65 years or older (AUROC, 0.74; 95% CI, 0.58-0.87) or patients who had type 2 diabetes (AUROC, 0.78; 95% CI, 0.71-0.84).
The researchers regarded the combination of an ELF score of 7.2 or greater with a FIB-4 score of 0.74 or greater, as indicative of a negative predictive value of 95.1% (95% CI, 91.8%-98.4%) and a sensitivity of 92.5% (95% CI, 87.4%-97.5%). According to the investigators, these values “can reliably rule out advanced fibrosis.”
Additionally, the combination of an ELF score of 9.8 or greater with a FIB-4 score of 2.9 or greater, was associated with a positive predictive value of 95.0% (95% CI, 85.5%-100%) and a specificity of 99.7% (95% CI, 99.1%-100%), suggesting this combination can conversely “be used to rule in advanced fibrosis,” the researchers wrote.
Serologic approaches for predicting the risk of NASH are more widely available and “easier” to use than radiologic approaches, but the former may not be as reliable or accurate as the latter, explained Tibor Krisko, MD, a gastroenterologist at Weill Cornell Medicine and New York-Presbyterian, in an interview. “The choice of which serologic test is utilized is often guided by what is available in a region/practice, what the provider is familiar with, and what a given patient’s insurance covers,” said Dr. Krisko, who wasn’t involved in the study.
“The study by Dr. Younossi et al. perhaps confirms that ELF alone is unlikely to be the future standard of care, and the authors weave this to a conclusion and strength, highlighting that the combination had excellent accuracy,” commented Dr. Krisko. “This is an exciting and important area of research and clinical practice advancement, but all of these serological tests have limitations, such as their lack in liver specificity, their risk of being affected by clearance rate, and the fact that they are not biomarkers but rather surrogate markers.”
Therefore, added Dr. Krisko, clinicians should continue to consider each patient’s clinical picture carefully and utilize radiographic methods liberally, particularly when serologic results are deemed ambiguous.
Dr. Younossi reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Krisko had no relevant conflicts to disclose.
A noninvasive enhanced liver fibrosis (ELF) blood test identifies patients with nonalcoholic fatty liver disease (NAFLD) who are at increased risk of advanced fibrosis, according to a new study.
According to the study researchers, when combined with the fibrosis-4 (FIB-4) score the ELF test may be a reliable method that can assess for advanced fibrosis in clinical practice.
Despite the utility of identifying advanced fibrosis in the NAFLD population, significant barriers exist to “risk stratifying patients in clinical practice owing to the need for liver biopsy,” wrote study authors Zobair M. Younossi, MD, of the Inova Health System in Falls Church, Va., and colleagues in JAMA Network Open.
“NASH [nonalcoholic steatohepatitis] can only be diagnosed by biopsy and liver pathology yet validated noninvasive tests that accurately diagnose NASH don’t exist,” said Dr. Younossi in an interview. “Developing noninvasive tests to accurately risk stratify patients with significant fibrosis in NASH is highly desirable in clinical practice. A blood test such as ELF can open the opportunity to order this test anywhere in the country.”
The ELF test reflects extracellular matrix metabolism as opposed to tests that assess alterations in hepatic function. The study authors explain that the noninvasive ELF test is a “blood-derived panel of biomarkers consisting of three components: type III procollagen peptide, hyaluronic acid, and tissue inhibitor of metalloproteinase-1.”
To gain a further understanding of the role of ELF in predicting the risk of nonalcoholic steatohepatitis (NASH) in NAFLD, Dr. Younossi and colleagues performed a retrospective, cross-sectional analysis of outpatients within a community-based liver clinic from 2001 in 2020. The study cohort included 829 patients (mean age, 53.1 years) with NAFLD, which was characterized by steatosis greater than 5% without any other liver disease or excessive alcohol use.
In the overall study population, the mean FIB-4 score was 1.34. In the 463 patients with liver biopsy, approximately 24.4% presented with bridging fibrosis or cirrhosis. A total of 79 (17.1%) of the 462 patients with transient elastography data presented with liver stiffness results of 9.6 kPa or greater, which according to the researchers was indicative of advanced fibrosis.
Biopsy determined that those with advanced fibrosis in the study had significantly increased ELF scores versus patients without advanced fibrosis (10.1 vs. 8.6, respectively; P <.001). Moreover, patients with advanced fibrosis had significantly greater liver stiffness as determined by transient elastography (10.0 vs. 9.0; P <.001).
In the NAFLD population, the ELF test demonstrated excellent performance in identifying patients with advanced fibrosis, as reflected by an area under the receiver operating characteristic curve of 0.81 (95% confidence interval, 0.77-0.85) for those whose fibrosis was diagnosed by biopsy as well as 0.79 (95% CI, 0.75-0.82) for fibrosis diagnosed by transient elastography. Similar performances of the ELF score were reported among those with NAFLD who were aged 65 years or older (AUROC, 0.74; 95% CI, 0.58-0.87) or patients who had type 2 diabetes (AUROC, 0.78; 95% CI, 0.71-0.84).
The researchers regarded the combination of an ELF score of 7.2 or greater with a FIB-4 score of 0.74 or greater, as indicative of a negative predictive value of 95.1% (95% CI, 91.8%-98.4%) and a sensitivity of 92.5% (95% CI, 87.4%-97.5%). According to the investigators, these values “can reliably rule out advanced fibrosis.”
Additionally, the combination of an ELF score of 9.8 or greater with a FIB-4 score of 2.9 or greater, was associated with a positive predictive value of 95.0% (95% CI, 85.5%-100%) and a specificity of 99.7% (95% CI, 99.1%-100%), suggesting this combination can conversely “be used to rule in advanced fibrosis,” the researchers wrote.
Serologic approaches for predicting the risk of NASH are more widely available and “easier” to use than radiologic approaches, but the former may not be as reliable or accurate as the latter, explained Tibor Krisko, MD, a gastroenterologist at Weill Cornell Medicine and New York-Presbyterian, in an interview. “The choice of which serologic test is utilized is often guided by what is available in a region/practice, what the provider is familiar with, and what a given patient’s insurance covers,” said Dr. Krisko, who wasn’t involved in the study.
“The study by Dr. Younossi et al. perhaps confirms that ELF alone is unlikely to be the future standard of care, and the authors weave this to a conclusion and strength, highlighting that the combination had excellent accuracy,” commented Dr. Krisko. “This is an exciting and important area of research and clinical practice advancement, but all of these serological tests have limitations, such as their lack in liver specificity, their risk of being affected by clearance rate, and the fact that they are not biomarkers but rather surrogate markers.”
Therefore, added Dr. Krisko, clinicians should continue to consider each patient’s clinical picture carefully and utilize radiographic methods liberally, particularly when serologic results are deemed ambiguous.
Dr. Younossi reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Krisko had no relevant conflicts to disclose.
A noninvasive enhanced liver fibrosis (ELF) blood test identifies patients with nonalcoholic fatty liver disease (NAFLD) who are at increased risk of advanced fibrosis, according to a new study.
According to the study researchers, when combined with the fibrosis-4 (FIB-4) score the ELF test may be a reliable method that can assess for advanced fibrosis in clinical practice.
Despite the utility of identifying advanced fibrosis in the NAFLD population, significant barriers exist to “risk stratifying patients in clinical practice owing to the need for liver biopsy,” wrote study authors Zobair M. Younossi, MD, of the Inova Health System in Falls Church, Va., and colleagues in JAMA Network Open.
“NASH [nonalcoholic steatohepatitis] can only be diagnosed by biopsy and liver pathology yet validated noninvasive tests that accurately diagnose NASH don’t exist,” said Dr. Younossi in an interview. “Developing noninvasive tests to accurately risk stratify patients with significant fibrosis in NASH is highly desirable in clinical practice. A blood test such as ELF can open the opportunity to order this test anywhere in the country.”
The ELF test reflects extracellular matrix metabolism as opposed to tests that assess alterations in hepatic function. The study authors explain that the noninvasive ELF test is a “blood-derived panel of biomarkers consisting of three components: type III procollagen peptide, hyaluronic acid, and tissue inhibitor of metalloproteinase-1.”
To gain a further understanding of the role of ELF in predicting the risk of nonalcoholic steatohepatitis (NASH) in NAFLD, Dr. Younossi and colleagues performed a retrospective, cross-sectional analysis of outpatients within a community-based liver clinic from 2001 in 2020. The study cohort included 829 patients (mean age, 53.1 years) with NAFLD, which was characterized by steatosis greater than 5% without any other liver disease or excessive alcohol use.
In the overall study population, the mean FIB-4 score was 1.34. In the 463 patients with liver biopsy, approximately 24.4% presented with bridging fibrosis or cirrhosis. A total of 79 (17.1%) of the 462 patients with transient elastography data presented with liver stiffness results of 9.6 kPa or greater, which according to the researchers was indicative of advanced fibrosis.
Biopsy determined that those with advanced fibrosis in the study had significantly increased ELF scores versus patients without advanced fibrosis (10.1 vs. 8.6, respectively; P <.001). Moreover, patients with advanced fibrosis had significantly greater liver stiffness as determined by transient elastography (10.0 vs. 9.0; P <.001).
In the NAFLD population, the ELF test demonstrated excellent performance in identifying patients with advanced fibrosis, as reflected by an area under the receiver operating characteristic curve of 0.81 (95% confidence interval, 0.77-0.85) for those whose fibrosis was diagnosed by biopsy as well as 0.79 (95% CI, 0.75-0.82) for fibrosis diagnosed by transient elastography. Similar performances of the ELF score were reported among those with NAFLD who were aged 65 years or older (AUROC, 0.74; 95% CI, 0.58-0.87) or patients who had type 2 diabetes (AUROC, 0.78; 95% CI, 0.71-0.84).
The researchers regarded the combination of an ELF score of 7.2 or greater with a FIB-4 score of 0.74 or greater, as indicative of a negative predictive value of 95.1% (95% CI, 91.8%-98.4%) and a sensitivity of 92.5% (95% CI, 87.4%-97.5%). According to the investigators, these values “can reliably rule out advanced fibrosis.”
Additionally, the combination of an ELF score of 9.8 or greater with a FIB-4 score of 2.9 or greater, was associated with a positive predictive value of 95.0% (95% CI, 85.5%-100%) and a specificity of 99.7% (95% CI, 99.1%-100%), suggesting this combination can conversely “be used to rule in advanced fibrosis,” the researchers wrote.
Serologic approaches for predicting the risk of NASH are more widely available and “easier” to use than radiologic approaches, but the former may not be as reliable or accurate as the latter, explained Tibor Krisko, MD, a gastroenterologist at Weill Cornell Medicine and New York-Presbyterian, in an interview. “The choice of which serologic test is utilized is often guided by what is available in a region/practice, what the provider is familiar with, and what a given patient’s insurance covers,” said Dr. Krisko, who wasn’t involved in the study.
“The study by Dr. Younossi et al. perhaps confirms that ELF alone is unlikely to be the future standard of care, and the authors weave this to a conclusion and strength, highlighting that the combination had excellent accuracy,” commented Dr. Krisko. “This is an exciting and important area of research and clinical practice advancement, but all of these serological tests have limitations, such as their lack in liver specificity, their risk of being affected by clearance rate, and the fact that they are not biomarkers but rather surrogate markers.”
Therefore, added Dr. Krisko, clinicians should continue to consider each patient’s clinical picture carefully and utilize radiographic methods liberally, particularly when serologic results are deemed ambiguous.
Dr. Younossi reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Krisko had no relevant conflicts to disclose.
FROM JAMA NETWORK OPEN
Updated MELD score adds serum albumin, female sex
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
FROM GASTROENTEROLOGY
UC relapse associated with impaired luminal control of macrophage maturation
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
Current therapies for ulcerative colitis, including anti-inflammatory drugs and biologics such as anti–tumor necrosis factor therapies and anti-interleukin-12/23 antibodies, are aimed at inducing and maintaining remission. However, approximately 20%-40% of patients are primary nonresponders and about 23%-46% patients lose response within 12 months of treatment, which suggests an unmet need to look for new therapeutic targets.
Intestinal macrophages are essential in the maintenance of intestinal immune homeostasis by acquiring a hyporesponsive state in response to microbial stimuli. This study by Maasfeh and colleagues highlights the role of luminal factors in shaping the macrophage response. The authors show that human monocyte-derived macrophage treated with fecal luminal factors derived from patients with ulcerative colitis in remission are less hyporesponsive to lipopolysaccharide stimulation. They are also less efficient in modulating cytokine and Toll-like receptor signaling pathway genes and have a dampened ability to suppress CD4+ T-cell activation and interferon gamma secretion compared to controls.
Luminal factors derived from gut microbiota (short-chain fatty acids, indole derivatives, polyamines, and bile acids) can shape macrophage differentiation and antibacterial response. This study points toward key luminal factors, which might be playing pivotal roles in maintaining homeostasis. However, the current study needs further validation in a larger cohort of patients and in the lamina propria macrophages. In addition, it will be important to know the physiologically relevant concentration to achieve functional effect. The identification of specific metabolites responsible for inducing hyporesponsiveness in macrophages could be an approach for mining potential therapeutic targets.
Dr. Sumeet Pandey is in the Translational Gastroenterology Unit at John Radcliffe Hospital at the University of Oxford (England). He has no conflicts of interest.
Current therapies for ulcerative colitis, including anti-inflammatory drugs and biologics such as anti–tumor necrosis factor therapies and anti-interleukin-12/23 antibodies, are aimed at inducing and maintaining remission. However, approximately 20%-40% of patients are primary nonresponders and about 23%-46% patients lose response within 12 months of treatment, which suggests an unmet need to look for new therapeutic targets.
Intestinal macrophages are essential in the maintenance of intestinal immune homeostasis by acquiring a hyporesponsive state in response to microbial stimuli. This study by Maasfeh and colleagues highlights the role of luminal factors in shaping the macrophage response. The authors show that human monocyte-derived macrophage treated with fecal luminal factors derived from patients with ulcerative colitis in remission are less hyporesponsive to lipopolysaccharide stimulation. They are also less efficient in modulating cytokine and Toll-like receptor signaling pathway genes and have a dampened ability to suppress CD4+ T-cell activation and interferon gamma secretion compared to controls.
Luminal factors derived from gut microbiota (short-chain fatty acids, indole derivatives, polyamines, and bile acids) can shape macrophage differentiation and antibacterial response. This study points toward key luminal factors, which might be playing pivotal roles in maintaining homeostasis. However, the current study needs further validation in a larger cohort of patients and in the lamina propria macrophages. In addition, it will be important to know the physiologically relevant concentration to achieve functional effect. The identification of specific metabolites responsible for inducing hyporesponsiveness in macrophages could be an approach for mining potential therapeutic targets.
Dr. Sumeet Pandey is in the Translational Gastroenterology Unit at John Radcliffe Hospital at the University of Oxford (England). He has no conflicts of interest.
Current therapies for ulcerative colitis, including anti-inflammatory drugs and biologics such as anti–tumor necrosis factor therapies and anti-interleukin-12/23 antibodies, are aimed at inducing and maintaining remission. However, approximately 20%-40% of patients are primary nonresponders and about 23%-46% patients lose response within 12 months of treatment, which suggests an unmet need to look for new therapeutic targets.
Intestinal macrophages are essential in the maintenance of intestinal immune homeostasis by acquiring a hyporesponsive state in response to microbial stimuli. This study by Maasfeh and colleagues highlights the role of luminal factors in shaping the macrophage response. The authors show that human monocyte-derived macrophage treated with fecal luminal factors derived from patients with ulcerative colitis in remission are less hyporesponsive to lipopolysaccharide stimulation. They are also less efficient in modulating cytokine and Toll-like receptor signaling pathway genes and have a dampened ability to suppress CD4+ T-cell activation and interferon gamma secretion compared to controls.
Luminal factors derived from gut microbiota (short-chain fatty acids, indole derivatives, polyamines, and bile acids) can shape macrophage differentiation and antibacterial response. This study points toward key luminal factors, which might be playing pivotal roles in maintaining homeostasis. However, the current study needs further validation in a larger cohort of patients and in the lamina propria macrophages. In addition, it will be important to know the physiologically relevant concentration to achieve functional effect. The identification of specific metabolites responsible for inducing hyporesponsiveness in macrophages could be an approach for mining potential therapeutic targets.
Dr. Sumeet Pandey is in the Translational Gastroenterology Unit at John Radcliffe Hospital at the University of Oxford (England). He has no conflicts of interest.
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
WATS-3D plus Seattle protocol increases dysplasia detection in Barrett’s esophagus
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
FROM GASTROINTESTINAL ENDOSCOPY
WATS-3D plus Seattle protocol increases dysplasia detection in Barrett’s esophagus
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
FROM GASTROINTESTINAL ENDOSCOPY
GERD: Composite pH impedance monitoring better identifies treatment escalation need
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
FROM GASTROENTEROLOGY
Morphology drives optical evaluation’s accuracy for predicting SMIC
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
GERD: Composite pH impedance monitoring better identifies treatment escalation need
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
FROM GASTROENTEROLOGY
UC relapse associated with impaired luminal control of macrophage maturation
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
Patients with ulcerative colitis (UC) who are in remission from their disease lack luminal signals capable of inducing macrophage hyporesponsiveness, which may contribute to a patient’s persistent vulnerability to relapse, according to a new study.
“Together with the distinct fecal metabolomic profile of UC patients in remission, our data suggest that UC patients may lack the signals required for proper macrophage education, rendering them vulnerable to relapse,” wrote study authors Lujain Maasfeh, PhD, of the University of Gothenburg (Sweden) and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
Macrophages found in the lamina propria play a role in sustaining intestinal homeostasis. Through education by local signals, intestinal macrophages adopt a hyporesponsive phenotype and tolerogenic nature and are replenished constantly from monocytes. In patients with UC who are in remission, however, the lack of proper macrophage maturation may result in gut inflammation.
Current evidence has yet to define fully the immunomodulating determinants in the education of intestinal macrophages; however, Dr. Maasfeh and associates wrote that “intestinal microbiota and microbiota-derived metabolites increasingly are recognized for their role in imprinting tissue-specific features of intestinal macrophages.”
The researchers added that previous evidence has established that patients with UC demonstrate dysbiosis, which may impact maturation of intestinal macrophages. As such, the hyporesponsive state of intestinal lamina propria macrophages induced by the microbiota may be lost in patients with UC who are in remission, ultimately resulting in disease relapse.
To gauge the effects of fecal luminal factors on macrophage phenotype and function, the researchers extracted fecal supernatants (FS) from the fecal samples of 10 healthy volunteers and 17 patients with UC who were in remission. Following maturation of monocytes to macrophages in the presence of granulocyte-macrophage colony-stimulating factor without and with FS, the researchers assessed macrophage phenotype and function. The investigators also used gas chromatography and mass spectrometry to analyze fecal metabolomic profiles.
In healthy donors, fecal luminal factors effectively downregulated Toll-like receptor signaling, cytokine signaling, as well as antigen presentation in macrophages. In contrast, the fecal luminal factors in patients with UC demonstrated less potency in their ability to induce lipopolysaccharide hyporesponsiveness. An immune pathway scoring analysis also showed a consistently higher reaction potential among UC remission FS-treated macrophages vs. healthy FS-treated macrophages.
While FS treatment did not seem to affect the phagocytic and bactericidal abilities of macrophages, the researchers observed that the healthy FS-treated macrophages better suppressed a cluster of differentiation 4+ T-cell activation as well as interferon gamma secretion vs. FS-treated macrophages from patients with UC in remission. The FS-treated macrophages in the UC remission population also featured less potency in their ability to suppress CD4+ T-cell activation and cytokine secretion.
The authors acknowledged a few limitations, including the small sample size and the effects from using in vitro system.
“Identification of the factors involved in intestinal macrophage education is important to maintain/reestablish gut homeostasis in patients with UC,” they concluded.
The study received financial support from Swedish Research Council-Medicine, in addition to funding from a Region Västra Götaland ALF-agreement, the Knut och Alice Wallenberg Foundation Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Ruth and Richard Julin’s foundation, Adlerbertska Foundation, Wilhelm and Martina Lundgren Foundation, and Apotekare Hedberg’s Foundation. The authors disclose no conflicts.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY