User login
Noninvasive Ventilation A Practical Guide
Overview
Candidates for noninvasive ventilation (NIV) most commonly present to the ED with acute respiratory failure (ARF) secondary to chronic obstructive pulmonary disease (COPD) or congestive heart-failure (CHF) exacerbations. The emergency physician (EP) must select patients appropriately, recognizing which would benefit most from NIV, as well as those with contraindications to this therapy. When indicated, early application confers benefit to the patient and can help avoid endotracheal intubation. Once therapy is initiated, clinical deterioration is still possible, and close monitoring and troubleshooting are imperative. Frequently, the clinician must make adjustments in ventilatory parameters to support the patient.
In this article, the author discusses the evidence supporting the use of NIV in appropriately selected patients with ARF, as well as review the types of NIV commonly used in the ED, the physiologic effects of positive-pressure ventilation (PPV), and how to identify and avoid common pitfalls.
Case Presentation Examples
Case 1
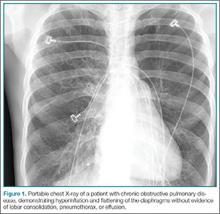
A 72-year-old man with a past medical history of COPD was brought to the ED by emergency medical services for evaluation of shortness of breath and wheezing. The patient’s initial oxygen (O2) saturation was 84%, which responded to bronchodilators and supplemental O2. At the time of arrival, he was somewhat somnolent, but aroused to verbal stimuli. A nonrebreather mask was placed delivering 15 L/minute of O2 with a saturation of 96%. His vital signs were: blood pressure (BP), 142/76 mm Hg, heart rate, 108 beats/minute; and respiratory rate (RR), 13 breaths/minute. A cardiac monitor revealed sinus tachycardia, and a portable chest X-ray was obtained (Figure 1). On lung examination, the patient’s breath sounds were diminished in the bases with suboptimal respiratory effort and expiratory wheezes in all lung fields. Venous blood gas measurement revealed a pH of 7.25; end-tidal carbon dioxide (CO2) was 77.
After the initial assessment, the EP considered NIV as an adjunct to improve ventilation as he suspected the patient was experiencing significant respiratory acidosis secondary to CO2 retention. The respiratory therapist suggested NIV at 12/5 before titrating down the fraction of inspired O2 (FiO2) and sought approval from the EP.
Discussion Questions: Is the above recommendation from the respiratory therapist the most appropriate therapy for this patient? What are the contraindications to this treatment and how should he be monitored to measure improvement?
Case 2
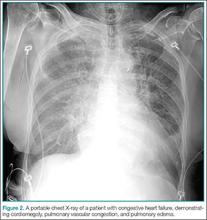
A 54-year-old woman presented to the ED for shortness of breath. On examination, she was diaphoretic and in severe distress with one- to two-word dyspnea and gasping respirations with pink-tinged sputum. Her BP was 236/158 mm Hg. A portable chest X-ray was obtained (Figure 2); rales were present with significant jugular venous distension. An electrocardiogram revealed a left-ventricular hypertrophy strain pattern but no evidence of ST-segment elevation.
During the assessment, the EP considered hypertensive emergency with resulting flash pulmonary edema as the cause of the patient’s condition; as such, he contemplated NIV to decrease the work of breathing and improve oxygenation. However, the EP had concerns regarding the preload and afterload ramifications. Although there was no respiratory therapist in the ED, the EP was able to set up the machine, but was not certain which mode of NIV or initial settings would be appropriate.
Discussion Questions: What is the protocol for proper set up to ensure a good mask fit? Once therapy is initiated, how should the EP monitor the patient? How should the EP explain this therapy to the patient and instruct her on how to work with the ventilator?
Acute-Care Application
Noninvasive ventilation refers to PPV delivered through a device such as a facemask, nasal mask, nasal plugs, or helmet. This modality was first used in the 1940s to treat respiratory failure, and its use has since grown to parallel that of mechanical ventilation.1-3 Although the application of NIV does not represent definitive airway management, this therapy has dramatically changed the care and treatment of both chronic and ARF. It serves as a significant intervention to prevent further respiratory compromise; to reverse either existing physiologic, hemodynamic, or ventilatory derangements; and to avoid endotracheal intubation.
Modes of Delivery
In the acute setting, NIV is typically delivered via two modes. Continuous positive-airway pressure (CPAP) is delivered regardless of the phase of respiration, and noninvasive positive-pressure ventilation (NIPPV; typically referred to as bi-level positive-airway pressure [BiPAP] or BPPV) is delivered in the inspiratory and expiratory phases of the respiratory cycle. Inspiratory positive-airway pressure (IPAP) refers to an inspiratory boost that is triggered by the negative airway pressure on inspiration in a synchronous fashion. This inspiratory pressure is fixed, but the volume delivered fluctuates based on the patient’s inspiratory effort. Expiratory positive-airway pressure (EPAP) is the delivery of constant pressure during exhalation. The difference between the IPAP and EPAP is referred to as pressure support, which serves to decrease the work of breathing and improve ventilation. (A list of commonly used abbreviations, terms, and definitions are outlined in Table 1.)
Etiology of Respiratory Failure and Treatment Decisions
At the time of initial presentation, the exact etiology of a patient’s respiratory failure may not be known, and treatment decisions will be necessary before all relevant data are present. Patients presenting in acute respiratory distress (ARD) are often suffering from shunt physiology, in which alveoli are perfused but not ventilated due to the presence of fluid or collapse, as in pulmonary edema or COPD.4 Regardless of the etiology, patients will benefit from early application of NIV.5 Thus, the clinician must be aggressive in the application of this therapy to identify those patients who will benefit the most from treatment. All patients receiving NIV must be monitored closely as failure of therapy is still a possibility.
Patient Selection
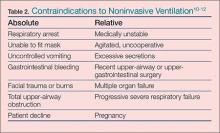
The utilization of NIV has increased in the hospital and ED setting and it is now often initiated in the prehospital setting6-8 with observed improvement in dyspnea scores and oxygenation with early intervention.9 Regarding patient selection, in the absence of contraindications (Table 2), all dyspneic patients should be considered eligible for a trial of NIV. 13 For some patients, this may be their first use of the therapy; as such, they are in effect learning to “swim while drowning.” The agitated and anxious patient will require coaching to provide reassurance and instruction while he or she learns to synchronize and work with the ventilator. The presence and quality of this instruction, though not previously measured, would intuitively be very helpful and an important determinant of success in the application of NIV in the naïve patient.
Common Conditions and NIV
In the ED, NIV is commonly utilized for the treatment of COPD and acute decompensated heart failure. These two conditions have been extensively studied and a robust amount of literature supports the routine use of NIV in these patients.
Chronic Obstructive Pulmonary Disease
For COPD, BiPAP has been shown and is widely accepted as the modality that confers the most benefit, with one study demonstrating a 462% increase in its use and a 42% decline in mechanical intubation rates from 1998 to 2008.14 Multiple studies have demonstrated a reduction in the intubation rate, improvement in the work of breathing, and a more rapid improvement in RR and symptoms.15,16
Acute Decompensated Heart Failure and Pulmonary Edema
Noninvasive ventilation is used commonly for decompensated heart failure and acute cardiogenic pulmonary edema (ACPE). The rapid patient improvement with its use when compared to standard O2 therapy is well documented. A successful trial and application of NIV demonstrated benefit in a recent retrospective analysis of 2,430 acutely decompensated heart-failure patients in the United States. The study found that the patients who were treated with NIV, but not immediately intubated, had better outcomes.17 (In these types of patients, pulmonary edema is typically not related to volume overload, but the result of imbalanced hemodynamics with markedly increased cardiac afterload and systemic vascular resistance.)
With respect to type of NIV, the use of CPAP is widely accepted as the primary modality of choice to confer the most benefit in ACPE.18 Although theoretical advantages do exist for the use of BiPAP over CPAP, this benefit has been noted in smaller studies19 but not clearly demonstrated in large reviews.20,21 In addition, patients suffering from long-term CHF develop the syndrome of cardiac cachexia, characterized by the loss of quantity and quality of skeletal muscle.22 This reduction in muscle mass can produce a significant deficit in inspiratory muscle strength and ability, providing an opportunity for benefit with the use of BiPAP.
Previously, BiPAP was considered unsafe in the setting of ACPE due to an increase in myocardial infarction.23 These results have not been reproduced in larger studies, and it is widely accepted that although BiPAP may not confer any benefit, it also does not increase harm.
Asthma
Because the underlying pathology of asthma differs from COPD, the current evidence for NIV use in patients presenting with an asthmatic episode is not very strong. Chronic obstructive pulmonary disease is characterized by collapse of terminal airways, with destruction of pulmonary architecture, and decreased compliance of the chest wall. In contrast, the airway obstruction in asthma progresses as the severity of the attack increases, and NIV may offer potential benefit in high-risk patients to avoid intubation.24 Several small studies suggest the application of NIV for severe asthma exacerbations is reasonable, with some demonstration of improvement in the work of breathing and ventilatory status.25-27
The Critically Ill Patient
Critically ill patients represent a high-risk group for desaturation during endotracheal intubation, and NIV should be considered for preoxygenation unless contraindications exist (Table 2). If standard high-flow O2 without positive pressure does not improve oxygenation, the application of NIV may overcome shunt physiology, improve oxygenation, and lessen peri-intubation time with dangerous desaturation events.4,28-30
Interfaces, Mask Leaks, Patient-Ventilator Interaction, and Respiratory Failure
Interfaces
Patient interfaces (mask types) for NIV include nasal prongs, full facial mask, or most commonly, an oronasal mask.31 For successful delivery of positive pressure, there must be an adequate fit or seal with minimal air leak to establish a ventilator circuit. Even though there is no perfect interface, patient comfort and treatment efficacy should be balanced. The interface chosen should minimize skin damage, maximize seal, and optimize patient-ventilator interface. The interfaces have straps that are used to secure the mask in place and balance the tension and stress on the skin to ensure a good seal and to avoid excess focal pressure that may result in complications such as skin breakdown, necrosis, or discomfort. Multiple interfaces and mask types have been evaluated in different acute-care situations, and it is important the clinician be familiar with the various options available for NIV interface and delivery.
Mask Leaks
Unintentional leaks are an unavoidable reality with NIV use. The ventilators designed for NIV typically use a single-limb circuit with an intentional leak port close to the patient. This port provides resistance and, as the ventilator produces airflow, it can subsequently generate pressure. Because this leak port is incorporated into the interface, it is important to utilize the same manufacturer of the ventilator and interface to avoid interface-ventilator mismatch.32
In some cases, unintentional leaks have been linked to asynchrony leading to increased work of breathing, ineffective delivery of breaths, and missed triggering events.33 The goal of any chosen interface is the lowest measurable unintentional leak rate as higher values demonstrate significant variability (and inaccuracy) of measured tidal volumes (VT).34 Overtightening the mask should be avoided since it can compromise both patient comfort and increase the chance of skin necrosis or breakdown.
Patient-Ventilator Interaction
The importance of the patient-ventilator interaction and the development of synchrony between the two cannot be overstated. After initial application, the patient should be closely monitored as he or she begins to work with the ventilator. This is especially important in BiPAP.
Optimal patient-ventilator synchrony can be difficult to achieve, especially in the NIV-naïve patient with critical respiratory distress. Of note, approximately 20% to 30% of patients with ARD cannot be managed by NIV,11 and asynchrony, though difficult to quantify in the acute-care setting, may contribute to this number.
Respiratory Failure
Acute respiratory failure is caused by a change in the patient’s baseline gas exchange, resulting in an inability to provide sufficient levels of O2 or to ventilate adequately. The etiologies of ARF are characterized into four types.
Type I. Also referred to as hypoxemic respiratory failure, type I is the most common and is characterized by an arterial oxygen tension (PaO2) of less than 60 mm Hg, with either normal or low levels of arterial CO2 that is not responsive to supplemental O2.
Type II. This type of respiratory failure is characterized by alveolar hypoventilation, with a PaCO2 level greater than 45 mm Hg, although hypoxemia may also be present due to concomitant loss of central nervous system drive.
Type III. Failure primarily occurs in the perioperative setting where the functional residual capacity is reduced in combination with increasing atelectasis.
Type IV. Type IV ARF is secondary to circulatory failure and resolves when shock is corrected.35,36
Regardless of the respiratory failure etiology, the patient is at risk of further deterioration and the need for endotracheal intubation.
Physiologic effects of NIV
Once the interface is secured, NIV has several important effects on both the cardiac and pulmonary systems. For this discussion, intrathoracic pressure (PIT) is considered synonymous with mean airway pressure (Paw).
Noninvasive ventilation improves airflow, lung volumes, and subsequent VT while overcoming pulmonary atelectasis. The increase in lung volume is directly proportional to an increase in Paw. This effect is only seen after overcoming airway resistance and chest wall and lung compliance. There is also an improvement in alveolar recruitment and redistribution of pulmonary blood flow37 with decreased work of breathing.
With the increase in PIT, there is decreased venous return to the right heart and a resulting decrease in cardiac preload.38 In the setting of acute cardiogenic pulmonary edema (ACPE), this effect is highly favorable. However, in the volume-depleted or hemodynamically unstable patient, this may result in a drop in cardiac output and hypotension. The “normal” heart is more sensitive to preload, and the application of positive pressure can cause a significant decrease in cardiac output. Cardiac afterload is reduced through multiple mechanisms, including directly from a decreased left-ventricular (LV) preload and also from a decrease in the LV transmural pressure (referred to as PTM).
The effects of positive pressure on the ventricles are opposite in the normal heart, with a decrease in both right and LV preload, increased right ventricular afterload and decreased LV afterload,39,40 as well as an overall decrease in cardiac chamber size that is directly proportional to the level of PPV.41 For the decompensated CHF patient, this can produce an increase in cardiac output simply by shifting the patient to a more favorable (leftward) position on the Frank-Starling curve.42-44
Troubleshooting
Once NIV is initiated, it is imperative, at least initially, to remain at bedside to monitor progress and improvement. Even though NIV is beneficial in the acute setting, it should always be viewed as a temporary bridging measure. With improvement, NIV may be discontinued, but in cases of failure, it is necessary to proceed with endotracheal intubation.
As the patient synchronizes with the ventilator, changes should be seen rather quickly, including improvement in the work of breathing, a restoration of mental status (if significant hypercapnia is present), and improved oxygenation. In the patient with severe uncontrolled hypertension and resulting flash pulmonary edema, the reduction in preload and afterload should contribute to a decrease in systolic BP (in addition to medical therapy). There should be a low threshold for obtaining an initial arterial blood gas (or a venous sample coupled with end-tidal CO2 data) as it may be helpful to guide therapy.
Noninvasive ventilation is similar to mechanical ventilation in that the clinician should not view it is as a static therapy, but rather as a dynamic process. For application of NIV in the acute setting, it should be recognized that the patient’s physiology is deranged (albeit transiently); as physiology eventually returns to preexisting levels, changes in NIV-pressure levels (or modes) are therefore necessary. Moreover, initial starting pressures may not be adequate to either overcome deficits in oxygenation, ventilation, or provide significant preload/afterload reduction. Knowledge of which parameters or values to adjust contribute to increased patient comfort, patient safety, improved cardiopulmonary dynamics, and a faster restoration of ventilatory status. In essence, the EP at the bedside should always ask himself or herself “what am I trying to fix?”
When the patient begins to develop synchrony with the ventilator, improvement and stabilization in the measured VT should be observed. The goal of delivered VT should be 6 to 10 mg/kg of ideal body weight. An increase in the IPAP value will improve the VT and decrease the work of breathing, and it should be the first value increased to reduce PaCO2. The use of EPAP will help to reduce intrinsic positive-end expiratory pressure and atelectasis and reduce upper airway obstruction. Increasing EPAP will improve oxygenation. Table 3 lists the common starting values for both modes of NIV and provides troubleshooting suggestions.
To date, no clinical trials have addressed the optimal initiation strategy or application settings for NIV. It should be understood, however, that the initial settings will typically be lower pressures to ensure patient comfort and development of familiarity with the device and interaction. For BiPAP, it is common to start with settings of 10/5 (IPAP/EPAP), and then titrate up (not exceeding 25 cm H2O) and maintaining minimum pressure support of 4 to 5 cm H2O. For CPAP, initial settings of 5 to 10 cm H2O are reasonable. Increased pressures can lead to patient discomfort, unintentional leak, and the development of patient-ventilator associated asynchrony.12 The goal is to balance therapeutic effect(s) with patient comfort. Higher pressures, even though they may be optimal, must be balanced with patient comfort as long as it is physiologically acceptable.
With increasing support, there may be an increase in mask leak; despite this, increasing levels of pressure or volume ventilation have been shown to increase minute ventilation (referred to as VE).45 In cases such as acute pulmonary edema or significant hypercapnia, initial higher-pressure settings may only be necessary for a brief time to reverse the pathology present and restore normal ventilation and hemodynamics. After the initial application, IPAP, EPAP, and FiO2 all may require titration.
Patients who fail to show improvement (either clinically or based on ventilatory parameters) or those with persistent mental status abnormalities, agitation, excessive secretions, or ventilator asynchrony after 1 hour of NIV are at high risk for NIV failure.46,47
Interpreting the Literature
Sizeable and sometimes conflicting literature exists on the subject of NIV. Despite a lack of clear and consistently reproducible benefit in morbidity, NIV use continues to increase. There are multiple factors that make interpretation of the results difficult and at times seemingly contradictory. Careful examination of the literature therefore must be undertaken before applying NIV to daily practice. Inconsistency of therapy type delivered, NIV pressure settings, pressure adjustments, patient monitoring, differing mask types, ventilator designs, endpoints, patient populations and the influence of cotreatments can all influence outcomes and potential benefit. To further complicate the data, unmeasured factors such as patient tolerance, interface fit, mask leak, and patient-ventilator asynchrony may be grouped as “NIV failure.”
For a patient suffering from ARF, the point in time of NIV application may have more to do with study enrollment and study group assignment (NIV or intubation) than the underlying pathology. Specifically, in some cases if NIV had been initiated hours prior, a clear benefit may have been demonstrated. One must also remember that at many institutions, the threshold for intubation (or intensive care unit admission) may be different, as well as the treating provider’s expertise and experience with NIV. In addition, well-established and consistent criteria for NIV failure have not been clearly defined and vary significantly study to study, making generalizations difficult. A comparison of patient groups with equal possible clinical outcomes is necessary to compare the findings “on a level playing field” and determine external validity.
Conclusion
Noninvasive ventilation represents a critically important intervention—one that should be applied early and aggressively in the ED to patients presenting with ARD in whom there are no contraindications to treatment. The EP should recognize the patient at high risk and, at the time of application, continue to closely monitor him or her for signs of improvement or deterioration.
As NIV use continues to increase, it is important that the clinician have a good working knowledge of its setup, modes of operation, and potential complications. A comfort level should exist for troubleshooting at the bedside. As provider competence increases, standardized quality of care is improved.
Dr Burns is an associate professor, residency director, and vice chair of academic affairs, department of emergency medicine, The University of Oklahoma School of Community Medicine, Tulsa.
- Pierson DJ. History and epidemiology of noninvasive ventilation in the acute-care setting. Resp Care. 2009;54(1):40-52.
- Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014; 40(4):582-591.
- Ozsancak Ugurlu A, Sidhom SS, Khodabandeh A, et al. Use and outcomes of noninvasive positive pressure ventilation in acute care hospitals in Massachusetts. Chest. 2014;145(5):964-971.
- Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165-175.
- Williams JW Jr, Cox CE, Hargett CW, et al. Noninvasive positive-pressure ventilation (NPPV) for acute respiratory failure. Rockville, MD: Agency for Healthcare Research and Quality. US Department of Health and Human Services. Comparative Effectiveness Reviews, No. 68. AHRQ publication 12-EHC089-EFJuly 2012. http://www.ncbi.nlm.nih.gov/books/NBK99179/. Published July 2012. Accessed January 7, 2015.
- Williams TA, Finn J, Perkins GD, Jacobs IG. Prehospital continuous positive airway pressure for acute respiratory failure: a systematic review and meta-analysis. Prehosp Emerg Care. 2013;17(2):261-273.
- Williams B, Boyle M, Robertson N, Giddings C. When pressure is positive: a literature review of the prehospital use of continuous positive airway pressure. Prehosp Disaster Med. 2013;28(1):52-60.
- Mal S, McLeod S, Iansavichene A, Dukelow A, Lewell M. Effect of out-of-hospital noninvasive positive-pressure support ventilation in adult patients with severe respiratory distress: a systemic review and meta-analysis. Annals of Em Med. 2014;63(5):600-607.
- Plaisance P, Pirracchio R, Berton C, Vicaut E, Paven D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur Heart J. 2007;28(23):2895-2901.
- Roberts CM, Brown JL, Reinhardt AK, et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med. 2008;8(5):517-521.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192-211.
- Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852.
- Tomii K, Seo R, Tachikawa R, et al. Impact of noninvasive ventilation (NIV) trial for various types of acute respiratory failure in the emergency department; decreased mortality and use of the ICU. Respir Med. 2009;103(1):67-73.
- Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004(3):CD004104.
- Royal College of Physicians, British Thoracic Society, Intensive Care Society. Chronic obstructive pulmonary disease: non-invasive ventilation with bi-phasic positive airways pressure in the management of patients with actute type 2 respiratory failure. Concise Guidance to Good Practice series, No. 11. London: RCP, 2008. https://www.rcplondon.ac.uk/sites/default/files/concise-niv-in-copd-2008.pdf. Published October 2008. Accessed January 7, 2015.
- Tallman TA, Peacock WF, Emerman CL, et al; Acute Decompensated Heart Failure National Registry (ADHERE). Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad Emerg Med. 2008;15(4):355-362.
- Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600.
- Liesching T, Nelson DL, Cormier KL, et al. Randomized trial of bilevel versus continuous positive airway pressure for acute pulmonary edema. J Emerg Med. 2014;46(1):130-140.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142-151.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systemic review and meta-analysis. JAMA. 2005;294(24)3124-3130.
- Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85(1):51-66.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620-628.
- Soroksky A, Klinowski E, Ilgyev E, et al. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur Respir Rev. 2010;19(115):39-45.
- Meduri GU, Cook TR, Turner RE, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767-774.
- Soma T, Hino M, Kida K, Kudoh S. A prospective and randomized study for improvement of acute asthma by non-invasive positive pressure ventilation (NPPV). Intern Med. 2008;47(6):493-501.
- Lim WJ, Mohammed Akram R, Carson KV, et al Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171-177.
- Weingart SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2011;40(6):661-667.
- Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114(6):1354-1363.
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71-84.
- Hess DR. Patient-ventilator interaction during noninvasive ventilation. Respir Care. 2011;56(2):153-165.
- Vignaux L, Vargas F, Roeseler et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35(5):840-846.
- Luján M, Sogo A, Pomares X, Monsó E, Sales B, Blanch L. Effect of leak and breathing pattern on the accuracy of tidal volume estimation by commercial home ventilators: a bench study. Respir Care. 2013;58(5):770-777.
- 11.35. Wood LDH. The pathophysiology and differential diagnosis of acute respiratory failure. In: Hall JB, Schmidt GA, Wood LDH. eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005. http://accessmedicine.mhmedical.com/content.aspx?bookid=361&Sectionid=39866399. Accessed January 7, 2015.
- Kemp WL, Burns DK, Brown TG. Pulmonary pathology. In: Kemp WL, Burns DK, Brown TG. eds. Pathology: The Big Picture. New York, NY: McGraw-Hill; 2008. http://accessmedicine.mhmedical.com/content.aspx?bookid=499&Sectionid=41568296. Accessed January 7, 2015.
- Carvalho AR, Spieth PM, Pelosi P, et al. Pressure support ventilation and biphasic positive airway pressure improve oxygenation by redistribution of pulmonary blood flow. Anesth Analg. 2009;109(3):858-865.
- Bersten AD, Holt AW, Vedig AE, Skowronski GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325(26):1825-1830.
- Leucke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607-621.
- Mitaka C, Naguara T, Sakanishi N, Tsunoda Y, Amaha K. Two-dimensional echocardiographic evaluation of inferior vena cava, right ventricle, and left ventricle during positive-pressure ventilation with varying levels of positive end-expiratory pressure. Crit Care Med. 1989;17(3):205-210.
- Kyhl K, Ahtarovski KA, Iversen K, et al. The decrease of cardiac chamber volumes and output during positive-pressure ventilation. Am J Physiol Heart Circ Physiol. 2013;305(7):H1004-H1009.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102(5):1397-1401.
- Chadda K, Annane D, Hart N, Gajdos P, Paphaël JC, Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30(11):2457-2461.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725-1731.
- 12.45. Tuggey JM, Elliott MW. Titration of non-invasive positive pressure ventilation in chronic respiratory failure. Respir Med. 2006;100(7):1262-1269.
- Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med. 2014;14:19.
- Merlani PG, Pasquina P, Granier JM, Treggiari M, Rutschmann O, Ricou B. Factors associated with failure of noninvasive positive pressure ventilation in the emergency department. Acad Emerg Med. 2005;12(12)1206-1215.
Overview
Candidates for noninvasive ventilation (NIV) most commonly present to the ED with acute respiratory failure (ARF) secondary to chronic obstructive pulmonary disease (COPD) or congestive heart-failure (CHF) exacerbations. The emergency physician (EP) must select patients appropriately, recognizing which would benefit most from NIV, as well as those with contraindications to this therapy. When indicated, early application confers benefit to the patient and can help avoid endotracheal intubation. Once therapy is initiated, clinical deterioration is still possible, and close monitoring and troubleshooting are imperative. Frequently, the clinician must make adjustments in ventilatory parameters to support the patient.
In this article, the author discusses the evidence supporting the use of NIV in appropriately selected patients with ARF, as well as review the types of NIV commonly used in the ED, the physiologic effects of positive-pressure ventilation (PPV), and how to identify and avoid common pitfalls.
Case Presentation Examples
Case 1
A 72-year-old man with a past medical history of COPD was brought to the ED by emergency medical services for evaluation of shortness of breath and wheezing. The patient’s initial oxygen (O2) saturation was 84%, which responded to bronchodilators and supplemental O2. At the time of arrival, he was somewhat somnolent, but aroused to verbal stimuli. A nonrebreather mask was placed delivering 15 L/minute of O2 with a saturation of 96%. His vital signs were: blood pressure (BP), 142/76 mm Hg, heart rate, 108 beats/minute; and respiratory rate (RR), 13 breaths/minute. A cardiac monitor revealed sinus tachycardia, and a portable chest X-ray was obtained (Figure 1). On lung examination, the patient’s breath sounds were diminished in the bases with suboptimal respiratory effort and expiratory wheezes in all lung fields. Venous blood gas measurement revealed a pH of 7.25; end-tidal carbon dioxide (CO2) was 77.
After the initial assessment, the EP considered NIV as an adjunct to improve ventilation as he suspected the patient was experiencing significant respiratory acidosis secondary to CO2 retention. The respiratory therapist suggested NIV at 12/5 before titrating down the fraction of inspired O2 (FiO2) and sought approval from the EP.
Discussion Questions: Is the above recommendation from the respiratory therapist the most appropriate therapy for this patient? What are the contraindications to this treatment and how should he be monitored to measure improvement?
Case 2
A 54-year-old woman presented to the ED for shortness of breath. On examination, she was diaphoretic and in severe distress with one- to two-word dyspnea and gasping respirations with pink-tinged sputum. Her BP was 236/158 mm Hg. A portable chest X-ray was obtained (Figure 2); rales were present with significant jugular venous distension. An electrocardiogram revealed a left-ventricular hypertrophy strain pattern but no evidence of ST-segment elevation.
During the assessment, the EP considered hypertensive emergency with resulting flash pulmonary edema as the cause of the patient’s condition; as such, he contemplated NIV to decrease the work of breathing and improve oxygenation. However, the EP had concerns regarding the preload and afterload ramifications. Although there was no respiratory therapist in the ED, the EP was able to set up the machine, but was not certain which mode of NIV or initial settings would be appropriate.
Discussion Questions: What is the protocol for proper set up to ensure a good mask fit? Once therapy is initiated, how should the EP monitor the patient? How should the EP explain this therapy to the patient and instruct her on how to work with the ventilator?
Acute-Care Application
Noninvasive ventilation refers to PPV delivered through a device such as a facemask, nasal mask, nasal plugs, or helmet. This modality was first used in the 1940s to treat respiratory failure, and its use has since grown to parallel that of mechanical ventilation.1-3 Although the application of NIV does not represent definitive airway management, this therapy has dramatically changed the care and treatment of both chronic and ARF. It serves as a significant intervention to prevent further respiratory compromise; to reverse either existing physiologic, hemodynamic, or ventilatory derangements; and to avoid endotracheal intubation.
Modes of Delivery
In the acute setting, NIV is typically delivered via two modes. Continuous positive-airway pressure (CPAP) is delivered regardless of the phase of respiration, and noninvasive positive-pressure ventilation (NIPPV; typically referred to as bi-level positive-airway pressure [BiPAP] or BPPV) is delivered in the inspiratory and expiratory phases of the respiratory cycle. Inspiratory positive-airway pressure (IPAP) refers to an inspiratory boost that is triggered by the negative airway pressure on inspiration in a synchronous fashion. This inspiratory pressure is fixed, but the volume delivered fluctuates based on the patient’s inspiratory effort. Expiratory positive-airway pressure (EPAP) is the delivery of constant pressure during exhalation. The difference between the IPAP and EPAP is referred to as pressure support, which serves to decrease the work of breathing and improve ventilation. (A list of commonly used abbreviations, terms, and definitions are outlined in Table 1.)
Etiology of Respiratory Failure and Treatment Decisions
At the time of initial presentation, the exact etiology of a patient’s respiratory failure may not be known, and treatment decisions will be necessary before all relevant data are present. Patients presenting in acute respiratory distress (ARD) are often suffering from shunt physiology, in which alveoli are perfused but not ventilated due to the presence of fluid or collapse, as in pulmonary edema or COPD.4 Regardless of the etiology, patients will benefit from early application of NIV.5 Thus, the clinician must be aggressive in the application of this therapy to identify those patients who will benefit the most from treatment. All patients receiving NIV must be monitored closely as failure of therapy is still a possibility.
Patient Selection
The utilization of NIV has increased in the hospital and ED setting and it is now often initiated in the prehospital setting6-8 with observed improvement in dyspnea scores and oxygenation with early intervention.9 Regarding patient selection, in the absence of contraindications (Table 2), all dyspneic patients should be considered eligible for a trial of NIV. 13 For some patients, this may be their first use of the therapy; as such, they are in effect learning to “swim while drowning.” The agitated and anxious patient will require coaching to provide reassurance and instruction while he or she learns to synchronize and work with the ventilator. The presence and quality of this instruction, though not previously measured, would intuitively be very helpful and an important determinant of success in the application of NIV in the naïve patient.
Common Conditions and NIV
In the ED, NIV is commonly utilized for the treatment of COPD and acute decompensated heart failure. These two conditions have been extensively studied and a robust amount of literature supports the routine use of NIV in these patients.
Chronic Obstructive Pulmonary Disease
For COPD, BiPAP has been shown and is widely accepted as the modality that confers the most benefit, with one study demonstrating a 462% increase in its use and a 42% decline in mechanical intubation rates from 1998 to 2008.14 Multiple studies have demonstrated a reduction in the intubation rate, improvement in the work of breathing, and a more rapid improvement in RR and symptoms.15,16
Acute Decompensated Heart Failure and Pulmonary Edema
Noninvasive ventilation is used commonly for decompensated heart failure and acute cardiogenic pulmonary edema (ACPE). The rapid patient improvement with its use when compared to standard O2 therapy is well documented. A successful trial and application of NIV demonstrated benefit in a recent retrospective analysis of 2,430 acutely decompensated heart-failure patients in the United States. The study found that the patients who were treated with NIV, but not immediately intubated, had better outcomes.17 (In these types of patients, pulmonary edema is typically not related to volume overload, but the result of imbalanced hemodynamics with markedly increased cardiac afterload and systemic vascular resistance.)
With respect to type of NIV, the use of CPAP is widely accepted as the primary modality of choice to confer the most benefit in ACPE.18 Although theoretical advantages do exist for the use of BiPAP over CPAP, this benefit has been noted in smaller studies19 but not clearly demonstrated in large reviews.20,21 In addition, patients suffering from long-term CHF develop the syndrome of cardiac cachexia, characterized by the loss of quantity and quality of skeletal muscle.22 This reduction in muscle mass can produce a significant deficit in inspiratory muscle strength and ability, providing an opportunity for benefit with the use of BiPAP.
Previously, BiPAP was considered unsafe in the setting of ACPE due to an increase in myocardial infarction.23 These results have not been reproduced in larger studies, and it is widely accepted that although BiPAP may not confer any benefit, it also does not increase harm.
Asthma
Because the underlying pathology of asthma differs from COPD, the current evidence for NIV use in patients presenting with an asthmatic episode is not very strong. Chronic obstructive pulmonary disease is characterized by collapse of terminal airways, with destruction of pulmonary architecture, and decreased compliance of the chest wall. In contrast, the airway obstruction in asthma progresses as the severity of the attack increases, and NIV may offer potential benefit in high-risk patients to avoid intubation.24 Several small studies suggest the application of NIV for severe asthma exacerbations is reasonable, with some demonstration of improvement in the work of breathing and ventilatory status.25-27
The Critically Ill Patient
Critically ill patients represent a high-risk group for desaturation during endotracheal intubation, and NIV should be considered for preoxygenation unless contraindications exist (Table 2). If standard high-flow O2 without positive pressure does not improve oxygenation, the application of NIV may overcome shunt physiology, improve oxygenation, and lessen peri-intubation time with dangerous desaturation events.4,28-30
Interfaces, Mask Leaks, Patient-Ventilator Interaction, and Respiratory Failure
Interfaces
Patient interfaces (mask types) for NIV include nasal prongs, full facial mask, or most commonly, an oronasal mask.31 For successful delivery of positive pressure, there must be an adequate fit or seal with minimal air leak to establish a ventilator circuit. Even though there is no perfect interface, patient comfort and treatment efficacy should be balanced. The interface chosen should minimize skin damage, maximize seal, and optimize patient-ventilator interface. The interfaces have straps that are used to secure the mask in place and balance the tension and stress on the skin to ensure a good seal and to avoid excess focal pressure that may result in complications such as skin breakdown, necrosis, or discomfort. Multiple interfaces and mask types have been evaluated in different acute-care situations, and it is important the clinician be familiar with the various options available for NIV interface and delivery.
Mask Leaks
Unintentional leaks are an unavoidable reality with NIV use. The ventilators designed for NIV typically use a single-limb circuit with an intentional leak port close to the patient. This port provides resistance and, as the ventilator produces airflow, it can subsequently generate pressure. Because this leak port is incorporated into the interface, it is important to utilize the same manufacturer of the ventilator and interface to avoid interface-ventilator mismatch.32
In some cases, unintentional leaks have been linked to asynchrony leading to increased work of breathing, ineffective delivery of breaths, and missed triggering events.33 The goal of any chosen interface is the lowest measurable unintentional leak rate as higher values demonstrate significant variability (and inaccuracy) of measured tidal volumes (VT).34 Overtightening the mask should be avoided since it can compromise both patient comfort and increase the chance of skin necrosis or breakdown.
Patient-Ventilator Interaction
The importance of the patient-ventilator interaction and the development of synchrony between the two cannot be overstated. After initial application, the patient should be closely monitored as he or she begins to work with the ventilator. This is especially important in BiPAP.
Optimal patient-ventilator synchrony can be difficult to achieve, especially in the NIV-naïve patient with critical respiratory distress. Of note, approximately 20% to 30% of patients with ARD cannot be managed by NIV,11 and asynchrony, though difficult to quantify in the acute-care setting, may contribute to this number.
Respiratory Failure
Acute respiratory failure is caused by a change in the patient’s baseline gas exchange, resulting in an inability to provide sufficient levels of O2 or to ventilate adequately. The etiologies of ARF are characterized into four types.
Type I. Also referred to as hypoxemic respiratory failure, type I is the most common and is characterized by an arterial oxygen tension (PaO2) of less than 60 mm Hg, with either normal or low levels of arterial CO2 that is not responsive to supplemental O2.
Type II. This type of respiratory failure is characterized by alveolar hypoventilation, with a PaCO2 level greater than 45 mm Hg, although hypoxemia may also be present due to concomitant loss of central nervous system drive.
Type III. Failure primarily occurs in the perioperative setting where the functional residual capacity is reduced in combination with increasing atelectasis.
Type IV. Type IV ARF is secondary to circulatory failure and resolves when shock is corrected.35,36
Regardless of the respiratory failure etiology, the patient is at risk of further deterioration and the need for endotracheal intubation.
Physiologic effects of NIV
Once the interface is secured, NIV has several important effects on both the cardiac and pulmonary systems. For this discussion, intrathoracic pressure (PIT) is considered synonymous with mean airway pressure (Paw).
Noninvasive ventilation improves airflow, lung volumes, and subsequent VT while overcoming pulmonary atelectasis. The increase in lung volume is directly proportional to an increase in Paw. This effect is only seen after overcoming airway resistance and chest wall and lung compliance. There is also an improvement in alveolar recruitment and redistribution of pulmonary blood flow37 with decreased work of breathing.
With the increase in PIT, there is decreased venous return to the right heart and a resulting decrease in cardiac preload.38 In the setting of acute cardiogenic pulmonary edema (ACPE), this effect is highly favorable. However, in the volume-depleted or hemodynamically unstable patient, this may result in a drop in cardiac output and hypotension. The “normal” heart is more sensitive to preload, and the application of positive pressure can cause a significant decrease in cardiac output. Cardiac afterload is reduced through multiple mechanisms, including directly from a decreased left-ventricular (LV) preload and also from a decrease in the LV transmural pressure (referred to as PTM).
The effects of positive pressure on the ventricles are opposite in the normal heart, with a decrease in both right and LV preload, increased right ventricular afterload and decreased LV afterload,39,40 as well as an overall decrease in cardiac chamber size that is directly proportional to the level of PPV.41 For the decompensated CHF patient, this can produce an increase in cardiac output simply by shifting the patient to a more favorable (leftward) position on the Frank-Starling curve.42-44
Troubleshooting
Once NIV is initiated, it is imperative, at least initially, to remain at bedside to monitor progress and improvement. Even though NIV is beneficial in the acute setting, it should always be viewed as a temporary bridging measure. With improvement, NIV may be discontinued, but in cases of failure, it is necessary to proceed with endotracheal intubation.
As the patient synchronizes with the ventilator, changes should be seen rather quickly, including improvement in the work of breathing, a restoration of mental status (if significant hypercapnia is present), and improved oxygenation. In the patient with severe uncontrolled hypertension and resulting flash pulmonary edema, the reduction in preload and afterload should contribute to a decrease in systolic BP (in addition to medical therapy). There should be a low threshold for obtaining an initial arterial blood gas (or a venous sample coupled with end-tidal CO2 data) as it may be helpful to guide therapy.
Noninvasive ventilation is similar to mechanical ventilation in that the clinician should not view it is as a static therapy, but rather as a dynamic process. For application of NIV in the acute setting, it should be recognized that the patient’s physiology is deranged (albeit transiently); as physiology eventually returns to preexisting levels, changes in NIV-pressure levels (or modes) are therefore necessary. Moreover, initial starting pressures may not be adequate to either overcome deficits in oxygenation, ventilation, or provide significant preload/afterload reduction. Knowledge of which parameters or values to adjust contribute to increased patient comfort, patient safety, improved cardiopulmonary dynamics, and a faster restoration of ventilatory status. In essence, the EP at the bedside should always ask himself or herself “what am I trying to fix?”
When the patient begins to develop synchrony with the ventilator, improvement and stabilization in the measured VT should be observed. The goal of delivered VT should be 6 to 10 mg/kg of ideal body weight. An increase in the IPAP value will improve the VT and decrease the work of breathing, and it should be the first value increased to reduce PaCO2. The use of EPAP will help to reduce intrinsic positive-end expiratory pressure and atelectasis and reduce upper airway obstruction. Increasing EPAP will improve oxygenation. Table 3 lists the common starting values for both modes of NIV and provides troubleshooting suggestions.
To date, no clinical trials have addressed the optimal initiation strategy or application settings for NIV. It should be understood, however, that the initial settings will typically be lower pressures to ensure patient comfort and development of familiarity with the device and interaction. For BiPAP, it is common to start with settings of 10/5 (IPAP/EPAP), and then titrate up (not exceeding 25 cm H2O) and maintaining minimum pressure support of 4 to 5 cm H2O. For CPAP, initial settings of 5 to 10 cm H2O are reasonable. Increased pressures can lead to patient discomfort, unintentional leak, and the development of patient-ventilator associated asynchrony.12 The goal is to balance therapeutic effect(s) with patient comfort. Higher pressures, even though they may be optimal, must be balanced with patient comfort as long as it is physiologically acceptable.
With increasing support, there may be an increase in mask leak; despite this, increasing levels of pressure or volume ventilation have been shown to increase minute ventilation (referred to as VE).45 In cases such as acute pulmonary edema or significant hypercapnia, initial higher-pressure settings may only be necessary for a brief time to reverse the pathology present and restore normal ventilation and hemodynamics. After the initial application, IPAP, EPAP, and FiO2 all may require titration.
Patients who fail to show improvement (either clinically or based on ventilatory parameters) or those with persistent mental status abnormalities, agitation, excessive secretions, or ventilator asynchrony after 1 hour of NIV are at high risk for NIV failure.46,47
Interpreting the Literature
Sizeable and sometimes conflicting literature exists on the subject of NIV. Despite a lack of clear and consistently reproducible benefit in morbidity, NIV use continues to increase. There are multiple factors that make interpretation of the results difficult and at times seemingly contradictory. Careful examination of the literature therefore must be undertaken before applying NIV to daily practice. Inconsistency of therapy type delivered, NIV pressure settings, pressure adjustments, patient monitoring, differing mask types, ventilator designs, endpoints, patient populations and the influence of cotreatments can all influence outcomes and potential benefit. To further complicate the data, unmeasured factors such as patient tolerance, interface fit, mask leak, and patient-ventilator asynchrony may be grouped as “NIV failure.”
For a patient suffering from ARF, the point in time of NIV application may have more to do with study enrollment and study group assignment (NIV or intubation) than the underlying pathology. Specifically, in some cases if NIV had been initiated hours prior, a clear benefit may have been demonstrated. One must also remember that at many institutions, the threshold for intubation (or intensive care unit admission) may be different, as well as the treating provider’s expertise and experience with NIV. In addition, well-established and consistent criteria for NIV failure have not been clearly defined and vary significantly study to study, making generalizations difficult. A comparison of patient groups with equal possible clinical outcomes is necessary to compare the findings “on a level playing field” and determine external validity.
Conclusion
Noninvasive ventilation represents a critically important intervention—one that should be applied early and aggressively in the ED to patients presenting with ARD in whom there are no contraindications to treatment. The EP should recognize the patient at high risk and, at the time of application, continue to closely monitor him or her for signs of improvement or deterioration.
As NIV use continues to increase, it is important that the clinician have a good working knowledge of its setup, modes of operation, and potential complications. A comfort level should exist for troubleshooting at the bedside. As provider competence increases, standardized quality of care is improved.
Dr Burns is an associate professor, residency director, and vice chair of academic affairs, department of emergency medicine, The University of Oklahoma School of Community Medicine, Tulsa.
Overview
Candidates for noninvasive ventilation (NIV) most commonly present to the ED with acute respiratory failure (ARF) secondary to chronic obstructive pulmonary disease (COPD) or congestive heart-failure (CHF) exacerbations. The emergency physician (EP) must select patients appropriately, recognizing which would benefit most from NIV, as well as those with contraindications to this therapy. When indicated, early application confers benefit to the patient and can help avoid endotracheal intubation. Once therapy is initiated, clinical deterioration is still possible, and close monitoring and troubleshooting are imperative. Frequently, the clinician must make adjustments in ventilatory parameters to support the patient.
In this article, the author discusses the evidence supporting the use of NIV in appropriately selected patients with ARF, as well as review the types of NIV commonly used in the ED, the physiologic effects of positive-pressure ventilation (PPV), and how to identify and avoid common pitfalls.
Case Presentation Examples
Case 1
A 72-year-old man with a past medical history of COPD was brought to the ED by emergency medical services for evaluation of shortness of breath and wheezing. The patient’s initial oxygen (O2) saturation was 84%, which responded to bronchodilators and supplemental O2. At the time of arrival, he was somewhat somnolent, but aroused to verbal stimuli. A nonrebreather mask was placed delivering 15 L/minute of O2 with a saturation of 96%. His vital signs were: blood pressure (BP), 142/76 mm Hg, heart rate, 108 beats/minute; and respiratory rate (RR), 13 breaths/minute. A cardiac monitor revealed sinus tachycardia, and a portable chest X-ray was obtained (Figure 1). On lung examination, the patient’s breath sounds were diminished in the bases with suboptimal respiratory effort and expiratory wheezes in all lung fields. Venous blood gas measurement revealed a pH of 7.25; end-tidal carbon dioxide (CO2) was 77.
After the initial assessment, the EP considered NIV as an adjunct to improve ventilation as he suspected the patient was experiencing significant respiratory acidosis secondary to CO2 retention. The respiratory therapist suggested NIV at 12/5 before titrating down the fraction of inspired O2 (FiO2) and sought approval from the EP.
Discussion Questions: Is the above recommendation from the respiratory therapist the most appropriate therapy for this patient? What are the contraindications to this treatment and how should he be monitored to measure improvement?
Case 2
A 54-year-old woman presented to the ED for shortness of breath. On examination, she was diaphoretic and in severe distress with one- to two-word dyspnea and gasping respirations with pink-tinged sputum. Her BP was 236/158 mm Hg. A portable chest X-ray was obtained (Figure 2); rales were present with significant jugular venous distension. An electrocardiogram revealed a left-ventricular hypertrophy strain pattern but no evidence of ST-segment elevation.
During the assessment, the EP considered hypertensive emergency with resulting flash pulmonary edema as the cause of the patient’s condition; as such, he contemplated NIV to decrease the work of breathing and improve oxygenation. However, the EP had concerns regarding the preload and afterload ramifications. Although there was no respiratory therapist in the ED, the EP was able to set up the machine, but was not certain which mode of NIV or initial settings would be appropriate.
Discussion Questions: What is the protocol for proper set up to ensure a good mask fit? Once therapy is initiated, how should the EP monitor the patient? How should the EP explain this therapy to the patient and instruct her on how to work with the ventilator?
Acute-Care Application
Noninvasive ventilation refers to PPV delivered through a device such as a facemask, nasal mask, nasal plugs, or helmet. This modality was first used in the 1940s to treat respiratory failure, and its use has since grown to parallel that of mechanical ventilation.1-3 Although the application of NIV does not represent definitive airway management, this therapy has dramatically changed the care and treatment of both chronic and ARF. It serves as a significant intervention to prevent further respiratory compromise; to reverse either existing physiologic, hemodynamic, or ventilatory derangements; and to avoid endotracheal intubation.
Modes of Delivery
In the acute setting, NIV is typically delivered via two modes. Continuous positive-airway pressure (CPAP) is delivered regardless of the phase of respiration, and noninvasive positive-pressure ventilation (NIPPV; typically referred to as bi-level positive-airway pressure [BiPAP] or BPPV) is delivered in the inspiratory and expiratory phases of the respiratory cycle. Inspiratory positive-airway pressure (IPAP) refers to an inspiratory boost that is triggered by the negative airway pressure on inspiration in a synchronous fashion. This inspiratory pressure is fixed, but the volume delivered fluctuates based on the patient’s inspiratory effort. Expiratory positive-airway pressure (EPAP) is the delivery of constant pressure during exhalation. The difference between the IPAP and EPAP is referred to as pressure support, which serves to decrease the work of breathing and improve ventilation. (A list of commonly used abbreviations, terms, and definitions are outlined in Table 1.)
Etiology of Respiratory Failure and Treatment Decisions
At the time of initial presentation, the exact etiology of a patient’s respiratory failure may not be known, and treatment decisions will be necessary before all relevant data are present. Patients presenting in acute respiratory distress (ARD) are often suffering from shunt physiology, in which alveoli are perfused but not ventilated due to the presence of fluid or collapse, as in pulmonary edema or COPD.4 Regardless of the etiology, patients will benefit from early application of NIV.5 Thus, the clinician must be aggressive in the application of this therapy to identify those patients who will benefit the most from treatment. All patients receiving NIV must be monitored closely as failure of therapy is still a possibility.
Patient Selection
The utilization of NIV has increased in the hospital and ED setting and it is now often initiated in the prehospital setting6-8 with observed improvement in dyspnea scores and oxygenation with early intervention.9 Regarding patient selection, in the absence of contraindications (Table 2), all dyspneic patients should be considered eligible for a trial of NIV. 13 For some patients, this may be their first use of the therapy; as such, they are in effect learning to “swim while drowning.” The agitated and anxious patient will require coaching to provide reassurance and instruction while he or she learns to synchronize and work with the ventilator. The presence and quality of this instruction, though not previously measured, would intuitively be very helpful and an important determinant of success in the application of NIV in the naïve patient.
Common Conditions and NIV
In the ED, NIV is commonly utilized for the treatment of COPD and acute decompensated heart failure. These two conditions have been extensively studied and a robust amount of literature supports the routine use of NIV in these patients.
Chronic Obstructive Pulmonary Disease
For COPD, BiPAP has been shown and is widely accepted as the modality that confers the most benefit, with one study demonstrating a 462% increase in its use and a 42% decline in mechanical intubation rates from 1998 to 2008.14 Multiple studies have demonstrated a reduction in the intubation rate, improvement in the work of breathing, and a more rapid improvement in RR and symptoms.15,16
Acute Decompensated Heart Failure and Pulmonary Edema
Noninvasive ventilation is used commonly for decompensated heart failure and acute cardiogenic pulmonary edema (ACPE). The rapid patient improvement with its use when compared to standard O2 therapy is well documented. A successful trial and application of NIV demonstrated benefit in a recent retrospective analysis of 2,430 acutely decompensated heart-failure patients in the United States. The study found that the patients who were treated with NIV, but not immediately intubated, had better outcomes.17 (In these types of patients, pulmonary edema is typically not related to volume overload, but the result of imbalanced hemodynamics with markedly increased cardiac afterload and systemic vascular resistance.)
With respect to type of NIV, the use of CPAP is widely accepted as the primary modality of choice to confer the most benefit in ACPE.18 Although theoretical advantages do exist for the use of BiPAP over CPAP, this benefit has been noted in smaller studies19 but not clearly demonstrated in large reviews.20,21 In addition, patients suffering from long-term CHF develop the syndrome of cardiac cachexia, characterized by the loss of quantity and quality of skeletal muscle.22 This reduction in muscle mass can produce a significant deficit in inspiratory muscle strength and ability, providing an opportunity for benefit with the use of BiPAP.
Previously, BiPAP was considered unsafe in the setting of ACPE due to an increase in myocardial infarction.23 These results have not been reproduced in larger studies, and it is widely accepted that although BiPAP may not confer any benefit, it also does not increase harm.
Asthma
Because the underlying pathology of asthma differs from COPD, the current evidence for NIV use in patients presenting with an asthmatic episode is not very strong. Chronic obstructive pulmonary disease is characterized by collapse of terminal airways, with destruction of pulmonary architecture, and decreased compliance of the chest wall. In contrast, the airway obstruction in asthma progresses as the severity of the attack increases, and NIV may offer potential benefit in high-risk patients to avoid intubation.24 Several small studies suggest the application of NIV for severe asthma exacerbations is reasonable, with some demonstration of improvement in the work of breathing and ventilatory status.25-27
The Critically Ill Patient
Critically ill patients represent a high-risk group for desaturation during endotracheal intubation, and NIV should be considered for preoxygenation unless contraindications exist (Table 2). If standard high-flow O2 without positive pressure does not improve oxygenation, the application of NIV may overcome shunt physiology, improve oxygenation, and lessen peri-intubation time with dangerous desaturation events.4,28-30
Interfaces, Mask Leaks, Patient-Ventilator Interaction, and Respiratory Failure
Interfaces
Patient interfaces (mask types) for NIV include nasal prongs, full facial mask, or most commonly, an oronasal mask.31 For successful delivery of positive pressure, there must be an adequate fit or seal with minimal air leak to establish a ventilator circuit. Even though there is no perfect interface, patient comfort and treatment efficacy should be balanced. The interface chosen should minimize skin damage, maximize seal, and optimize patient-ventilator interface. The interfaces have straps that are used to secure the mask in place and balance the tension and stress on the skin to ensure a good seal and to avoid excess focal pressure that may result in complications such as skin breakdown, necrosis, or discomfort. Multiple interfaces and mask types have been evaluated in different acute-care situations, and it is important the clinician be familiar with the various options available for NIV interface and delivery.
Mask Leaks
Unintentional leaks are an unavoidable reality with NIV use. The ventilators designed for NIV typically use a single-limb circuit with an intentional leak port close to the patient. This port provides resistance and, as the ventilator produces airflow, it can subsequently generate pressure. Because this leak port is incorporated into the interface, it is important to utilize the same manufacturer of the ventilator and interface to avoid interface-ventilator mismatch.32
In some cases, unintentional leaks have been linked to asynchrony leading to increased work of breathing, ineffective delivery of breaths, and missed triggering events.33 The goal of any chosen interface is the lowest measurable unintentional leak rate as higher values demonstrate significant variability (and inaccuracy) of measured tidal volumes (VT).34 Overtightening the mask should be avoided since it can compromise both patient comfort and increase the chance of skin necrosis or breakdown.
Patient-Ventilator Interaction
The importance of the patient-ventilator interaction and the development of synchrony between the two cannot be overstated. After initial application, the patient should be closely monitored as he or she begins to work with the ventilator. This is especially important in BiPAP.
Optimal patient-ventilator synchrony can be difficult to achieve, especially in the NIV-naïve patient with critical respiratory distress. Of note, approximately 20% to 30% of patients with ARD cannot be managed by NIV,11 and asynchrony, though difficult to quantify in the acute-care setting, may contribute to this number.
Respiratory Failure
Acute respiratory failure is caused by a change in the patient’s baseline gas exchange, resulting in an inability to provide sufficient levels of O2 or to ventilate adequately. The etiologies of ARF are characterized into four types.
Type I. Also referred to as hypoxemic respiratory failure, type I is the most common and is characterized by an arterial oxygen tension (PaO2) of less than 60 mm Hg, with either normal or low levels of arterial CO2 that is not responsive to supplemental O2.
Type II. This type of respiratory failure is characterized by alveolar hypoventilation, with a PaCO2 level greater than 45 mm Hg, although hypoxemia may also be present due to concomitant loss of central nervous system drive.
Type III. Failure primarily occurs in the perioperative setting where the functional residual capacity is reduced in combination with increasing atelectasis.
Type IV. Type IV ARF is secondary to circulatory failure and resolves when shock is corrected.35,36
Regardless of the respiratory failure etiology, the patient is at risk of further deterioration and the need for endotracheal intubation.
Physiologic effects of NIV
Once the interface is secured, NIV has several important effects on both the cardiac and pulmonary systems. For this discussion, intrathoracic pressure (PIT) is considered synonymous with mean airway pressure (Paw).
Noninvasive ventilation improves airflow, lung volumes, and subsequent VT while overcoming pulmonary atelectasis. The increase in lung volume is directly proportional to an increase in Paw. This effect is only seen after overcoming airway resistance and chest wall and lung compliance. There is also an improvement in alveolar recruitment and redistribution of pulmonary blood flow37 with decreased work of breathing.
With the increase in PIT, there is decreased venous return to the right heart and a resulting decrease in cardiac preload.38 In the setting of acute cardiogenic pulmonary edema (ACPE), this effect is highly favorable. However, in the volume-depleted or hemodynamically unstable patient, this may result in a drop in cardiac output and hypotension. The “normal” heart is more sensitive to preload, and the application of positive pressure can cause a significant decrease in cardiac output. Cardiac afterload is reduced through multiple mechanisms, including directly from a decreased left-ventricular (LV) preload and also from a decrease in the LV transmural pressure (referred to as PTM).
The effects of positive pressure on the ventricles are opposite in the normal heart, with a decrease in both right and LV preload, increased right ventricular afterload and decreased LV afterload,39,40 as well as an overall decrease in cardiac chamber size that is directly proportional to the level of PPV.41 For the decompensated CHF patient, this can produce an increase in cardiac output simply by shifting the patient to a more favorable (leftward) position on the Frank-Starling curve.42-44
Troubleshooting
Once NIV is initiated, it is imperative, at least initially, to remain at bedside to monitor progress and improvement. Even though NIV is beneficial in the acute setting, it should always be viewed as a temporary bridging measure. With improvement, NIV may be discontinued, but in cases of failure, it is necessary to proceed with endotracheal intubation.
As the patient synchronizes with the ventilator, changes should be seen rather quickly, including improvement in the work of breathing, a restoration of mental status (if significant hypercapnia is present), and improved oxygenation. In the patient with severe uncontrolled hypertension and resulting flash pulmonary edema, the reduction in preload and afterload should contribute to a decrease in systolic BP (in addition to medical therapy). There should be a low threshold for obtaining an initial arterial blood gas (or a venous sample coupled with end-tidal CO2 data) as it may be helpful to guide therapy.
Noninvasive ventilation is similar to mechanical ventilation in that the clinician should not view it is as a static therapy, but rather as a dynamic process. For application of NIV in the acute setting, it should be recognized that the patient’s physiology is deranged (albeit transiently); as physiology eventually returns to preexisting levels, changes in NIV-pressure levels (or modes) are therefore necessary. Moreover, initial starting pressures may not be adequate to either overcome deficits in oxygenation, ventilation, or provide significant preload/afterload reduction. Knowledge of which parameters or values to adjust contribute to increased patient comfort, patient safety, improved cardiopulmonary dynamics, and a faster restoration of ventilatory status. In essence, the EP at the bedside should always ask himself or herself “what am I trying to fix?”
When the patient begins to develop synchrony with the ventilator, improvement and stabilization in the measured VT should be observed. The goal of delivered VT should be 6 to 10 mg/kg of ideal body weight. An increase in the IPAP value will improve the VT and decrease the work of breathing, and it should be the first value increased to reduce PaCO2. The use of EPAP will help to reduce intrinsic positive-end expiratory pressure and atelectasis and reduce upper airway obstruction. Increasing EPAP will improve oxygenation. Table 3 lists the common starting values for both modes of NIV and provides troubleshooting suggestions.
To date, no clinical trials have addressed the optimal initiation strategy or application settings for NIV. It should be understood, however, that the initial settings will typically be lower pressures to ensure patient comfort and development of familiarity with the device and interaction. For BiPAP, it is common to start with settings of 10/5 (IPAP/EPAP), and then titrate up (not exceeding 25 cm H2O) and maintaining minimum pressure support of 4 to 5 cm H2O. For CPAP, initial settings of 5 to 10 cm H2O are reasonable. Increased pressures can lead to patient discomfort, unintentional leak, and the development of patient-ventilator associated asynchrony.12 The goal is to balance therapeutic effect(s) with patient comfort. Higher pressures, even though they may be optimal, must be balanced with patient comfort as long as it is physiologically acceptable.
With increasing support, there may be an increase in mask leak; despite this, increasing levels of pressure or volume ventilation have been shown to increase minute ventilation (referred to as VE).45 In cases such as acute pulmonary edema or significant hypercapnia, initial higher-pressure settings may only be necessary for a brief time to reverse the pathology present and restore normal ventilation and hemodynamics. After the initial application, IPAP, EPAP, and FiO2 all may require titration.
Patients who fail to show improvement (either clinically or based on ventilatory parameters) or those with persistent mental status abnormalities, agitation, excessive secretions, or ventilator asynchrony after 1 hour of NIV are at high risk for NIV failure.46,47
Interpreting the Literature
Sizeable and sometimes conflicting literature exists on the subject of NIV. Despite a lack of clear and consistently reproducible benefit in morbidity, NIV use continues to increase. There are multiple factors that make interpretation of the results difficult and at times seemingly contradictory. Careful examination of the literature therefore must be undertaken before applying NIV to daily practice. Inconsistency of therapy type delivered, NIV pressure settings, pressure adjustments, patient monitoring, differing mask types, ventilator designs, endpoints, patient populations and the influence of cotreatments can all influence outcomes and potential benefit. To further complicate the data, unmeasured factors such as patient tolerance, interface fit, mask leak, and patient-ventilator asynchrony may be grouped as “NIV failure.”
For a patient suffering from ARF, the point in time of NIV application may have more to do with study enrollment and study group assignment (NIV or intubation) than the underlying pathology. Specifically, in some cases if NIV had been initiated hours prior, a clear benefit may have been demonstrated. One must also remember that at many institutions, the threshold for intubation (or intensive care unit admission) may be different, as well as the treating provider’s expertise and experience with NIV. In addition, well-established and consistent criteria for NIV failure have not been clearly defined and vary significantly study to study, making generalizations difficult. A comparison of patient groups with equal possible clinical outcomes is necessary to compare the findings “on a level playing field” and determine external validity.
Conclusion
Noninvasive ventilation represents a critically important intervention—one that should be applied early and aggressively in the ED to patients presenting with ARD in whom there are no contraindications to treatment. The EP should recognize the patient at high risk and, at the time of application, continue to closely monitor him or her for signs of improvement or deterioration.
As NIV use continues to increase, it is important that the clinician have a good working knowledge of its setup, modes of operation, and potential complications. A comfort level should exist for troubleshooting at the bedside. As provider competence increases, standardized quality of care is improved.
Dr Burns is an associate professor, residency director, and vice chair of academic affairs, department of emergency medicine, The University of Oklahoma School of Community Medicine, Tulsa.
- Pierson DJ. History and epidemiology of noninvasive ventilation in the acute-care setting. Resp Care. 2009;54(1):40-52.
- Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014; 40(4):582-591.
- Ozsancak Ugurlu A, Sidhom SS, Khodabandeh A, et al. Use and outcomes of noninvasive positive pressure ventilation in acute care hospitals in Massachusetts. Chest. 2014;145(5):964-971.
- Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165-175.
- Williams JW Jr, Cox CE, Hargett CW, et al. Noninvasive positive-pressure ventilation (NPPV) for acute respiratory failure. Rockville, MD: Agency for Healthcare Research and Quality. US Department of Health and Human Services. Comparative Effectiveness Reviews, No. 68. AHRQ publication 12-EHC089-EFJuly 2012. http://www.ncbi.nlm.nih.gov/books/NBK99179/. Published July 2012. Accessed January 7, 2015.
- Williams TA, Finn J, Perkins GD, Jacobs IG. Prehospital continuous positive airway pressure for acute respiratory failure: a systematic review and meta-analysis. Prehosp Emerg Care. 2013;17(2):261-273.
- Williams B, Boyle M, Robertson N, Giddings C. When pressure is positive: a literature review of the prehospital use of continuous positive airway pressure. Prehosp Disaster Med. 2013;28(1):52-60.
- Mal S, McLeod S, Iansavichene A, Dukelow A, Lewell M. Effect of out-of-hospital noninvasive positive-pressure support ventilation in adult patients with severe respiratory distress: a systemic review and meta-analysis. Annals of Em Med. 2014;63(5):600-607.
- Plaisance P, Pirracchio R, Berton C, Vicaut E, Paven D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur Heart J. 2007;28(23):2895-2901.
- Roberts CM, Brown JL, Reinhardt AK, et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med. 2008;8(5):517-521.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192-211.
- Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852.
- Tomii K, Seo R, Tachikawa R, et al. Impact of noninvasive ventilation (NIV) trial for various types of acute respiratory failure in the emergency department; decreased mortality and use of the ICU. Respir Med. 2009;103(1):67-73.
- Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004(3):CD004104.
- Royal College of Physicians, British Thoracic Society, Intensive Care Society. Chronic obstructive pulmonary disease: non-invasive ventilation with bi-phasic positive airways pressure in the management of patients with actute type 2 respiratory failure. Concise Guidance to Good Practice series, No. 11. London: RCP, 2008. https://www.rcplondon.ac.uk/sites/default/files/concise-niv-in-copd-2008.pdf. Published October 2008. Accessed January 7, 2015.
- Tallman TA, Peacock WF, Emerman CL, et al; Acute Decompensated Heart Failure National Registry (ADHERE). Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad Emerg Med. 2008;15(4):355-362.
- Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600.
- Liesching T, Nelson DL, Cormier KL, et al. Randomized trial of bilevel versus continuous positive airway pressure for acute pulmonary edema. J Emerg Med. 2014;46(1):130-140.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142-151.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systemic review and meta-analysis. JAMA. 2005;294(24)3124-3130.
- Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85(1):51-66.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620-628.
- Soroksky A, Klinowski E, Ilgyev E, et al. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur Respir Rev. 2010;19(115):39-45.
- Meduri GU, Cook TR, Turner RE, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767-774.
- Soma T, Hino M, Kida K, Kudoh S. A prospective and randomized study for improvement of acute asthma by non-invasive positive pressure ventilation (NPPV). Intern Med. 2008;47(6):493-501.
- Lim WJ, Mohammed Akram R, Carson KV, et al Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171-177.
- Weingart SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2011;40(6):661-667.
- Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114(6):1354-1363.
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71-84.
- Hess DR. Patient-ventilator interaction during noninvasive ventilation. Respir Care. 2011;56(2):153-165.
- Vignaux L, Vargas F, Roeseler et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35(5):840-846.
- Luján M, Sogo A, Pomares X, Monsó E, Sales B, Blanch L. Effect of leak and breathing pattern on the accuracy of tidal volume estimation by commercial home ventilators: a bench study. Respir Care. 2013;58(5):770-777.
- 11.35. Wood LDH. The pathophysiology and differential diagnosis of acute respiratory failure. In: Hall JB, Schmidt GA, Wood LDH. eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005. http://accessmedicine.mhmedical.com/content.aspx?bookid=361&Sectionid=39866399. Accessed January 7, 2015.
- Kemp WL, Burns DK, Brown TG. Pulmonary pathology. In: Kemp WL, Burns DK, Brown TG. eds. Pathology: The Big Picture. New York, NY: McGraw-Hill; 2008. http://accessmedicine.mhmedical.com/content.aspx?bookid=499&Sectionid=41568296. Accessed January 7, 2015.
- Carvalho AR, Spieth PM, Pelosi P, et al. Pressure support ventilation and biphasic positive airway pressure improve oxygenation by redistribution of pulmonary blood flow. Anesth Analg. 2009;109(3):858-865.
- Bersten AD, Holt AW, Vedig AE, Skowronski GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325(26):1825-1830.
- Leucke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607-621.
- Mitaka C, Naguara T, Sakanishi N, Tsunoda Y, Amaha K. Two-dimensional echocardiographic evaluation of inferior vena cava, right ventricle, and left ventricle during positive-pressure ventilation with varying levels of positive end-expiratory pressure. Crit Care Med. 1989;17(3):205-210.
- Kyhl K, Ahtarovski KA, Iversen K, et al. The decrease of cardiac chamber volumes and output during positive-pressure ventilation. Am J Physiol Heart Circ Physiol. 2013;305(7):H1004-H1009.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102(5):1397-1401.
- Chadda K, Annane D, Hart N, Gajdos P, Paphaël JC, Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30(11):2457-2461.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725-1731.
- 12.45. Tuggey JM, Elliott MW. Titration of non-invasive positive pressure ventilation in chronic respiratory failure. Respir Med. 2006;100(7):1262-1269.
- Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med. 2014;14:19.
- Merlani PG, Pasquina P, Granier JM, Treggiari M, Rutschmann O, Ricou B. Factors associated with failure of noninvasive positive pressure ventilation in the emergency department. Acad Emerg Med. 2005;12(12)1206-1215.
- Pierson DJ. History and epidemiology of noninvasive ventilation in the acute-care setting. Resp Care. 2009;54(1):40-52.
- Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014; 40(4):582-591.
- Ozsancak Ugurlu A, Sidhom SS, Khodabandeh A, et al. Use and outcomes of noninvasive positive pressure ventilation in acute care hospitals in Massachusetts. Chest. 2014;145(5):964-971.
- Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165-175.
- Williams JW Jr, Cox CE, Hargett CW, et al. Noninvasive positive-pressure ventilation (NPPV) for acute respiratory failure. Rockville, MD: Agency for Healthcare Research and Quality. US Department of Health and Human Services. Comparative Effectiveness Reviews, No. 68. AHRQ publication 12-EHC089-EFJuly 2012. http://www.ncbi.nlm.nih.gov/books/NBK99179/. Published July 2012. Accessed January 7, 2015.
- Williams TA, Finn J, Perkins GD, Jacobs IG. Prehospital continuous positive airway pressure for acute respiratory failure: a systematic review and meta-analysis. Prehosp Emerg Care. 2013;17(2):261-273.
- Williams B, Boyle M, Robertson N, Giddings C. When pressure is positive: a literature review of the prehospital use of continuous positive airway pressure. Prehosp Disaster Med. 2013;28(1):52-60.
- Mal S, McLeod S, Iansavichene A, Dukelow A, Lewell M. Effect of out-of-hospital noninvasive positive-pressure support ventilation in adult patients with severe respiratory distress: a systemic review and meta-analysis. Annals of Em Med. 2014;63(5):600-607.
- Plaisance P, Pirracchio R, Berton C, Vicaut E, Paven D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur Heart J. 2007;28(23):2895-2901.
- Roberts CM, Brown JL, Reinhardt AK, et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med. 2008;8(5):517-521.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192-211.
- Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852.
- Tomii K, Seo R, Tachikawa R, et al. Impact of noninvasive ventilation (NIV) trial for various types of acute respiratory failure in the emergency department; decreased mortality and use of the ICU. Respir Med. 2009;103(1):67-73.
- Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004(3):CD004104.
- Royal College of Physicians, British Thoracic Society, Intensive Care Society. Chronic obstructive pulmonary disease: non-invasive ventilation with bi-phasic positive airways pressure in the management of patients with actute type 2 respiratory failure. Concise Guidance to Good Practice series, No. 11. London: RCP, 2008. https://www.rcplondon.ac.uk/sites/default/files/concise-niv-in-copd-2008.pdf. Published October 2008. Accessed January 7, 2015.
- Tallman TA, Peacock WF, Emerman CL, et al; Acute Decompensated Heart Failure National Registry (ADHERE). Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad Emerg Med. 2008;15(4):355-362.
- Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600.
- Liesching T, Nelson DL, Cormier KL, et al. Randomized trial of bilevel versus continuous positive airway pressure for acute pulmonary edema. J Emerg Med. 2014;46(1):130-140.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142-151.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systemic review and meta-analysis. JAMA. 2005;294(24)3124-3130.
- Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85(1):51-66.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620-628.
- Soroksky A, Klinowski E, Ilgyev E, et al. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur Respir Rev. 2010;19(115):39-45.
- Meduri GU, Cook TR, Turner RE, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767-774.
- Soma T, Hino M, Kida K, Kudoh S. A prospective and randomized study for improvement of acute asthma by non-invasive positive pressure ventilation (NPPV). Intern Med. 2008;47(6):493-501.
- Lim WJ, Mohammed Akram R, Carson KV, et al Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171-177.
- Weingart SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2011;40(6):661-667.
- Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114(6):1354-1363.
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71-84.
- Hess DR. Patient-ventilator interaction during noninvasive ventilation. Respir Care. 2011;56(2):153-165.
- Vignaux L, Vargas F, Roeseler et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35(5):840-846.
- Luján M, Sogo A, Pomares X, Monsó E, Sales B, Blanch L. Effect of leak and breathing pattern on the accuracy of tidal volume estimation by commercial home ventilators: a bench study. Respir Care. 2013;58(5):770-777.
- 11.35. Wood LDH. The pathophysiology and differential diagnosis of acute respiratory failure. In: Hall JB, Schmidt GA, Wood LDH. eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005. http://accessmedicine.mhmedical.com/content.aspx?bookid=361&Sectionid=39866399. Accessed January 7, 2015.
- Kemp WL, Burns DK, Brown TG. Pulmonary pathology. In: Kemp WL, Burns DK, Brown TG. eds. Pathology: The Big Picture. New York, NY: McGraw-Hill; 2008. http://accessmedicine.mhmedical.com/content.aspx?bookid=499&Sectionid=41568296. Accessed January 7, 2015.
- Carvalho AR, Spieth PM, Pelosi P, et al. Pressure support ventilation and biphasic positive airway pressure improve oxygenation by redistribution of pulmonary blood flow. Anesth Analg. 2009;109(3):858-865.
- Bersten AD, Holt AW, Vedig AE, Skowronski GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325(26):1825-1830.
- Leucke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607-621.
- Mitaka C, Naguara T, Sakanishi N, Tsunoda Y, Amaha K. Two-dimensional echocardiographic evaluation of inferior vena cava, right ventricle, and left ventricle during positive-pressure ventilation with varying levels of positive end-expiratory pressure. Crit Care Med. 1989;17(3):205-210.
- Kyhl K, Ahtarovski KA, Iversen K, et al. The decrease of cardiac chamber volumes and output during positive-pressure ventilation. Am J Physiol Heart Circ Physiol. 2013;305(7):H1004-H1009.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102(5):1397-1401.
- Chadda K, Annane D, Hart N, Gajdos P, Paphaël JC, Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30(11):2457-2461.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725-1731.
- 12.45. Tuggey JM, Elliott MW. Titration of non-invasive positive pressure ventilation in chronic respiratory failure. Respir Med. 2006;100(7):1262-1269.
- Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med. 2014;14:19.
- Merlani PG, Pasquina P, Granier JM, Treggiari M, Rutschmann O, Ricou B. Factors associated with failure of noninvasive positive pressure ventilation in the emergency department. Acad Emerg Med. 2005;12(12)1206-1215.