User login
Antipsychotics for patients with dementia: The road less traveled
As psychiatrists treating an aging population, we frequently face the daunting challenges of managing medically complex and behaviorally unstable patients whose fragile condition tests the brightest among us. As our population enters late life, not only are physicians confronted with aging patients whose bodies have decreased renal and hepatic function, but we also face the challenges of the aging brain, severed neuronal networks, and neurotransmitter diminution. These physiological changes can alter treatment response, increase the frequency of adverse effects, and increase the likelihood of emergence of behavioral and psychological symptoms.
During the past decade, the number of people reaching age 65 has dramatically increased. As life expectancy improves, the “oldest old”—those age 85 and older—are the fastest-growing segment of the population. The prevalence of cognitive impairment, including mild cognitive impairment and dementia, in this cohort is >40%.1 Roughly 90% of patients with dementia will develop clinically significant behavioral problems at some point in the course of their illness.2
Behavioral and psychological symptoms of dementia (BPSD) have a tremendous impact on the quality of life for both patients and their caregivers. We are experts in understanding these behaviors and crafting nonpharmacologic treatment plans to manage them. Understanding the context in which behaviors emerge allows us to modify the environment, communication strategies, and other potential triggers, in turn reducing the need for pharmacologic intervention.
However, when nonpharmacologic interventions have been exhausted, what are the options? Antipsychotics have been one of the approaches used to address the challenges of behavioral disturbances and psychosis occurring in dementia. Unfortunately, there is conflicting evidence regarding the risks and benefits associated with the use of antipsychotics in this population. In this article, we provide a roadmap for the judicious use of antipsychotics for patients with dementia.
Weighing the risks and benefits of antipsychotics
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important but limited role in the treatment of behavioral disturbances in dementia. Although safety risks exist, they can be minimized through the careful selection of appropriate patients for treatment, close monitoring, and effective communication with patients and caregivers before and during treatment.
Several studies examining the efficacy of antipsychotics in the treatment of BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.3 This evidence prompted the FDA to issue a “black-box” warning in 2005 to highlight the increased risk of mortality for patients with dementia who are treated with SGAs.4 Both first-generation antipsychotics (FGAs) and SGAs have been associated with higher rates of mortality than most other psychotropic classes, except anticonvulsants. This increased mortality risk has been shown to persist for at least 6 to 12 months.5,6 FGAs appear to be associated with a greater mortality risk compared with SGAs. As a result, if antipsychotic treatment is necessary, the use of FGAs in this population is not recommended.
The potential mechanisms leading to stroke and death remain unclear. They could include orthostatic hypotension, anticholinergic adverse effects, QT prolongation, platelet aggregation effects, and venous thromboembolism. The presence of cardiovascular and vascular risk factors, electrolyte imbalances, cardiac arrhythmias, and concomitant use of medications that prolong the QTc interval may confer additional risks.
Continued to: Although the use of antipsychotics for patients with dementia...
Although the use of antipsychotics for patients with dementia may increase the risk of mortality, the absolute increased risk to a given individual, at least with short-term treatment, is likely small. The risk may also vary depending on the choice of SGA. Patients who were treated with quetiapine had a slightly lower risk of death than those who were treated risperidone.5 Death rates among patients prescribed aripiprazole, olanzapine, and ziprasidone were similar to the death rates of patients who were treated with risperidone. Compared with patients who were treated with risperidone, patients who were treated with the FGA haloperidol were twice as likely to die during a subsequent 6-month observation period. The largest number of deaths occurred during the first 40 days of treatment.5
While this increased risk of mortality is an important factor to discuss with patients and caregivers when deciding whether to initiate antipsychotic treatment, it is also important to put it into perspective. For example, the risk of suddenly dying from a stroke or heart attack for a person with dementia who is not taking an antipsychotic is approximately 2%. When an individual is started on one of these agents, that risk increases to approximately 4%. While the mortality risk is doubled, it remains relatively small.4 When faced with verbal or physical assaults, hostility, paranoid ideations, or other psychotic symptoms, many families feel that this relatively low risk does not outweigh the potential benefits of reducing caregiver and patient distress. If nonpharmacologic and/or other pharmacologic interventions have failed, the treatment has reached a point of no good alternatives and therapy should then focus on minimizing risk.
Informed consent is essential. A discussion of risks and benefits with the patient, family, or other decision-makers should focus on the risk of stroke, potential metabolic effects, and mortality, as well as potential worsening of cognitive decline associated with antipsychotic treatment. This should be weighed together with the evidence that suggests psychosis and agitation are associated with earlier nursing home admission and death.7,8 Families should be given ample time and opportunity to ask questions. Alternatives to immediate initiation of antipsychotics should be thoroughly reviewed.
Despite the above-noted risks, expert consensus suggests that the use of antipsychotics in the treatment of individuals with dementia can be appropriate, particularly in individuals with dangerous agitation or psychosis.9 These agents can minimize the risk of violence, reduce patient distress, improve the patient’s quality of life, and reduce caregiver burden. In clinical trials, the benefits of antipsychotics have been modest. Nevertheless, evidence has shown that these agents can reduce psychosis, agitation, aggression, hostility, and suspiciousness, which makes them a valid option when other interventions have proven insufficient.
Target specific symptoms
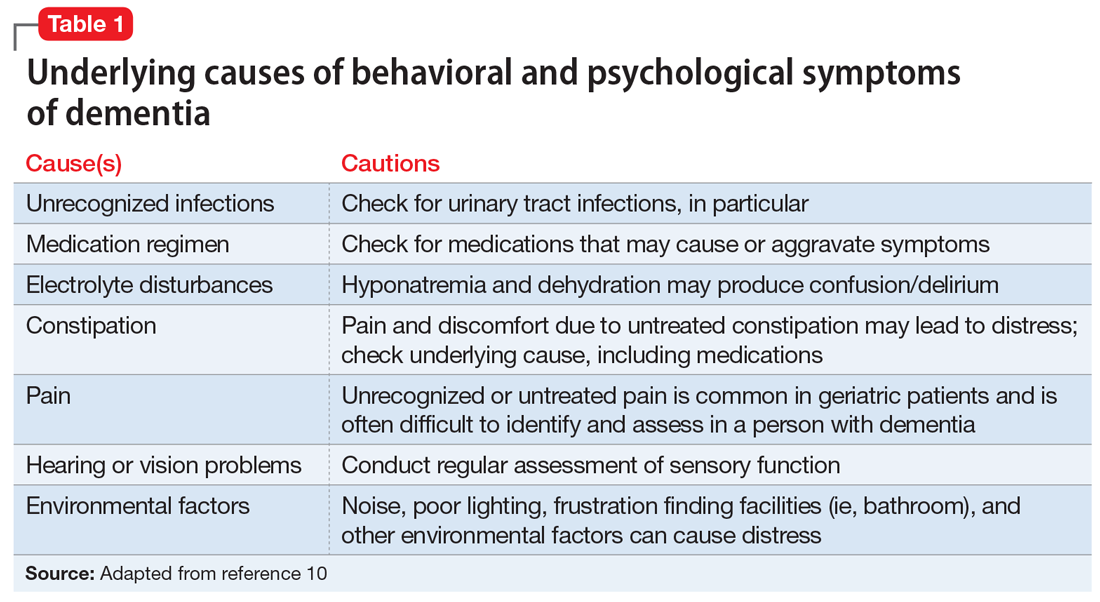
Despite this article’s focus on the appropriate use of antipsychotics for patients with BPSD, it is important to emphasize that the first-line approach to the management of BPSD in this population should always be a person-centered, psychosocial, multidisciplinary, nonpharmacologic approach that focuses on identifying triggers and treating potentially modifiable contributors to behavioral symptoms. Table 110 outlines common underlying causes of BPSD in dementia that should be assessed before prescribing an antipsychotic.
Continued to: Alternative psychopharmacologic treatments...
Alternative psychopharmacologic treatments based on a psychobehavioral metaphor should also be considered (Table 211). This approach matches the dominant target symptoms to the most relevant medication class.11 For example, in the case of a verbally and physically agitated patient who is also irritable, negative, socially withdrawn, and appears dysphoric, we might first undertake a trial of an antidepressant. Conversely, if the patient shows agitation in the context of increased motor activity, loud and rapid speech, and affective lability, we might consider the use of a mood stabilizer. Pharmacologic treatment should be aimed at the modification of clearly identified and documented target behaviors.

Indications to use antipsychotics for patients with dementia include:
- severe agitation and aggression associated with risk of harm
- delusions and hallucinations
- comorbid preexisting mental health conditions (eg, bipolar disorder, schizophrenia, treatment-resistant depression, etc.).
Symptoms that do not usually respond to an antipsychotic include wandering, social withdrawal, shouting, pacing, touching, cognitive defects, and incontinence.12 These symptoms may respond to interventions such as changes to the environment.
Continued to: Choosing an antipsychotic
Choosing an antipsychotic
Once you have identified that an antipsychotic is truly indicated, the choice of an agent will focus on patient-related factors. Considerations such as frailty, comorbid medical conditions including diabetes, history of falls, hepatic insufficiency, cardiac arrhythmias, and cerebrovascular risk factors, should all be analyzed prior to initiating an antipsychotic. The presence of these conditions will increase the likelihood that adverse effects may occur. It will also guide the dose trajectory and the target dose for discontinuation. Antipsychotics differ with respect to their efficacy and adverse effect profile. For practical purposes, adverse effects typically guide the selection of these agents when used for patients with dementia.
Continued to: Gradual structural changes occur...
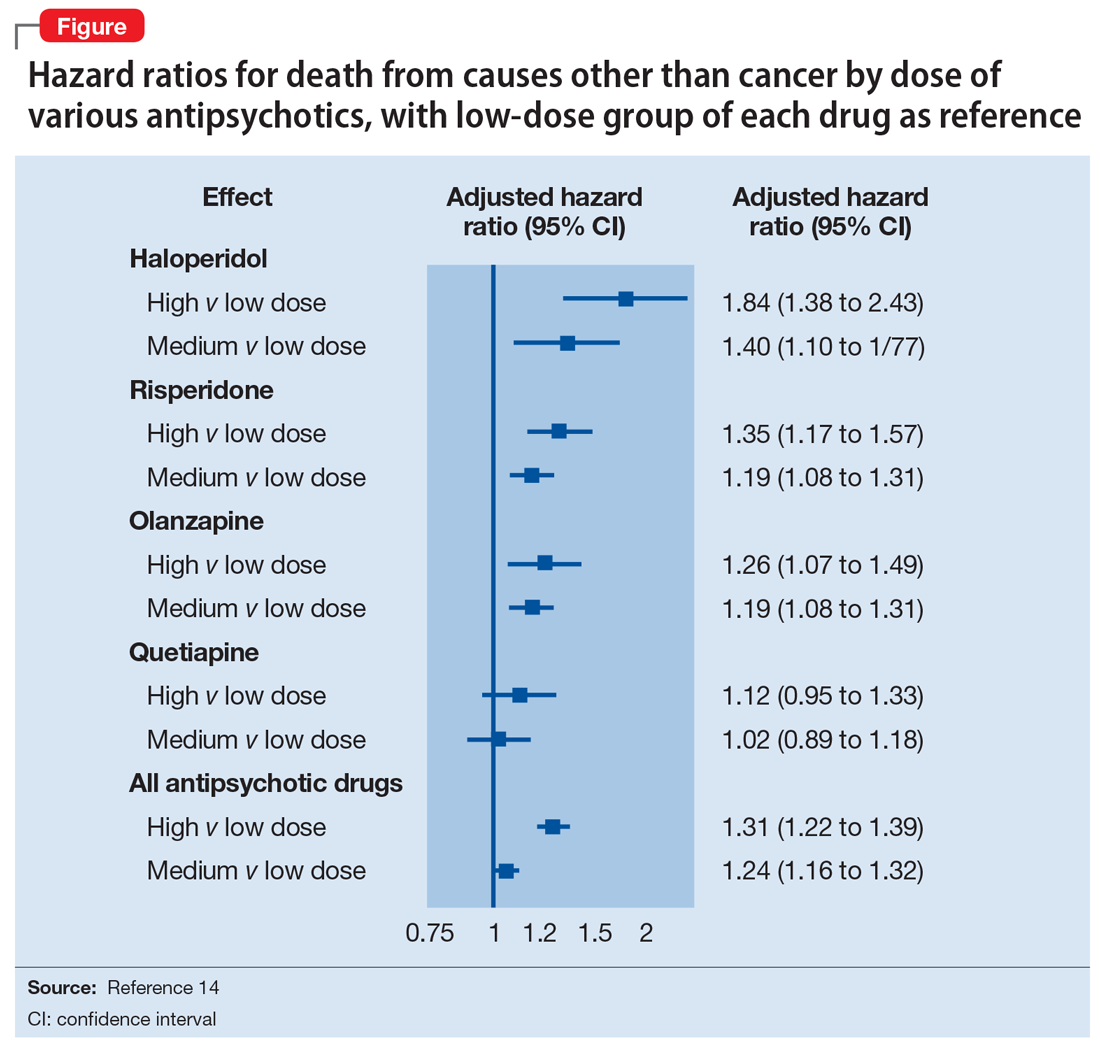
Gradual structural changes occur in the dopaminergic system with age and increase the propensity for antipsychotic adverse effects. The number of dopaminergic neurons and D2 receptors decreases approximately 10% per decade. In order to avoid the development of adverse effects related to extrapyramidal symptoms, approximately 20% of receptors need to be free. FGAs tend to block approximately 90% of D2 receptors, whereas SGAs block less than 70% to 80% and dissociate more rapidly from D2 receptors.13 FGAs should therefore be avoided, as they have been associated with numerous adverse effects, including parkinsonism, tardive dyskinesia, akathisia, sedation, peripheral and central anticholinergic effects, postural hypotension, cardiac conduction defects, and falls. As noted above, they have been linked to a greater risk of mortality (Figure14 ).
When the decision to use an antipsychotic agent is made for a person with dementia, SGAs appear to be a better choice. There appear to be modest differences within the class of SGAs in terms of effectiveness, tolerability, and adverse effect profile. Although the association between the dose of an antipsychotic and the risk of mortality or stroke remains undefined, other common adverse effects, such as sedation, extrapyramidal symptoms, and risk of falls, can be reduced by starting at the lowest dose possible and titrating slowly.
Dosing considerations
Dose increments should be modest and, in a nonemergent setting, may be adjusted at weekly intervals depending on response. Prior to starting a treatment trial, it is advisable to estimate what will constitute a worthwhile clinical response, the duration of treatment, and the maximum dose. Avoid high doses or prolonged use of antipsychotics that have not significantly improved the target behavior.
When the decision to use a SGA is made, choosing the initial starting dose is challenging given that none of these medications has an indication for use in this population. We propose doses that have been used in completed randomized trials that reflect the best information available about the dose likely to maximize benefit and minimize risk. On the basis of those trials, reasonable starting doses would be15-22:
- quetiapine 25 to 50 mg/d
- risperidone 0.5 to 1 mg/d
- aripiprazole 2 to 10 mg/d
- olanzapine 2.5 to 5 mg/d
- ziprasidone 20 mg/d
Continued to: The highest doses tested...
The highest doses tested for each of these compounds in randomized clinical trials for this population were: risperidone 2 mg/d, olanzapine 10 mg/d, and aripiprazole 15 mg/d. A wide variety of maximum doses of quetiapine were studied in clinical trials, with a top dose of 200 mg being most common. It is worth noting that doses higher than these have been used for other indications.15-22
Quetiapine. One of the most commonly prescribed antipsychotics for the treatment of BPSD in individuals with memory disorders is quetiapine. The reasons for this preference include a low risk of extrapyramidal adverse effects, flexibility of dosing, ability to use lower dosages, and evidence of the lower risk of mortality when compared with other second-generation agents.5,15 If an antipsychotic is indicated, quetiapine should be considered as a first-line antipsychotic therapy. Quetiapine has well-established effects on mood, anxiety, and sleep, all of which can be disrupted in dementia and can act as drivers for agitation.5,15 Starting quetiapine may mitigate the need for separate agents to treat insomnia, loss of appetite, or anxiety, although it is not FDA-indicated for these comorbid conditions. Quetiapine is also less likely to exacerbate motor symptoms compared with other SGAs but has the potential to increase the risk of falls, and orthostasis, and carries a considerable anticholinergic burden.5,15
Risperidone has been shown to provide modest improvements in some people exhibiting symptoms of aggression, agitation, and psychosis.5,15 There is no evidence that risperidone is any more effective than other SGAs, but it has been tested on more geriatric patients than other SGAs. The fact that it is also available in an orally disintegrating tablet makes it a practical treatment in certain populations of patients, such as those who have difficulty swallowing. Risperidone carries the highest extrapyramidal symptom burden among the SGAs due to its potent D2 receptor binding. 5,15
Aripiprazole. There have been several studies of aripiprazole for the treatment of psychosis and agitation in Alzheimer’s dementia.15 This medication showed modest effect and was generally well tolerated. Aripiprazole appears to have less associated weight gain, which may be pertinent for some patients. It also appears to be less sedating than many of the other SGAs. However, some patients may experience activation or insomnia with this agent, particularly with doses <15 mg/d. This activating effect may be beneficial for treating comorbid depressive symptoms, although lower doses could theoretically worsen psychosis due to the activating effects.
Aripiprazole has also been studied in Parkinson’s disease. While some patients had favorable responses with improvement in psychosis and behavioral disturbances, this medication was also associated with worsening of motor symptoms. Certain individuals also experienced a worsening of their psychosis.23 For this reason, it is unlikely to be a useful agent for patients displaying evidence of parkinsonism, Parkinson’s dementia, or dementia with Lewy bodies.
Olanzapine. Several studies have shown that low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients suffering from Alzheimer’s and vascular dementias.24 The medication is also available in an orally disintegrating form, which may be beneficial when treating individuals whose swallowing abilities are compromised. Olanzapine also has been associated with significant weight gain and metabolic syndrome.24
Continued to: Ziprasidone
Ziprasidone. There are no specific studies of ziprasidone for geriatric patients and none for patients with dementia. However, case reports have suggested both oral and injectable forms of the medication may be well tolerated and have some benefit in treating agitation in this population.25 Based on evidence from younger populations, ziprasidone is less likely to be associated with weight gain or orthostatic hypotension. Medication has been associated with QTc prolongation and should be used with caution and monitored with an ECG.
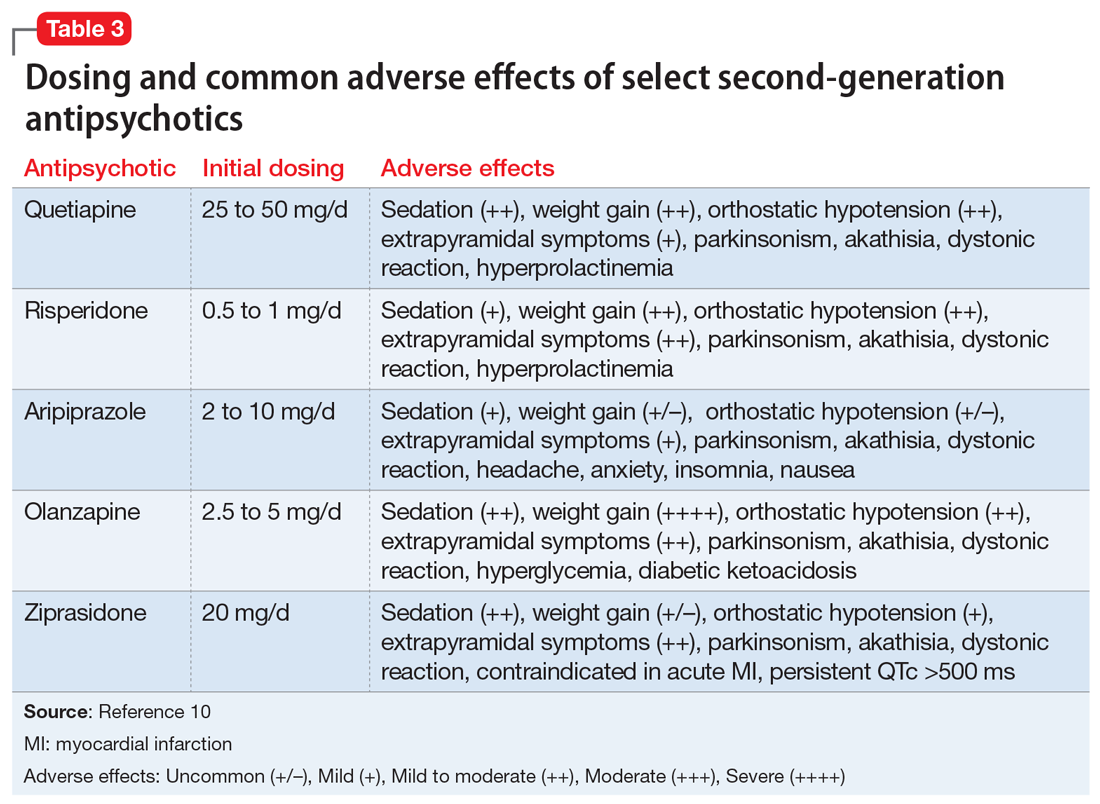
The initial dosing and potential adverse effects of quetiapine, risperidone, aripiprazole, olanzapine, and ziprasidone are highlighted in Table 3.10
Other SGAs. Newer antipsychotics have recently become available and may serve as additional tools for managing BPSD in the future. Unfortunately, there are currently no available studies regarding their efficacy in the treatment of agitation and psychosis in dementia. One notable exception is pimavaserin, a serotonin 2A receptor inverse agonist. This medication has recently been FDA-approved for the treatment of Parkinson’s disease psychosis. The medication was extensively studied in older patients. It appeared to be effective in reducing delusions and hallucinations while not impairing motor function or causing sedation or hypotension.23 Additional studies are currently ongoing for the treatment of Alzheimer’s dementia psychosis.
Monitor treatment, consider discontinuation
American Psychiatric Association guidelines on the use of antipsychotics to treat agitation or psychosis in patients with dementia currently recommend that clinicians use a quantitative measure to track symptoms and response to treatment.26 These measures may be formal, such as an overall assessment of symptom severity on a Likert scale, or as simple as monitoring the changes in the frequency of periods of agitation.
After starting an antipsychotic, a follow-up appointment should typically take place within 1 month. If the patient is at high risk for developing adverse effects, or if the symptoms are severe, a follow-up appointment for monitoring the response to treatment and potential adverse effects should occur within 1 week. At a minimum, expert consensus suggests follow-up visits should occur every 3 months.
If there is no clinical response after 4 weeks of adequate dosing of an antipsychotic, the medication should be tapered and withdrawn. Switching to an alternative agent may be appropriate.
Many patients will have only partial remission of target symptoms. Therefore, increasing the dose or switching to an alternative agent may be necessary. Concurrent use of multiple antipsychotic agents should be avoided.
Continued to: Maintenance treatment may be appropriate
Maintenance treatment may be appropriate for patients who have demonstrated a clear benefit from antipsychotic treatment without undue adverse effects, and in whom a trial dose reduction has resulted in reappearance of the target symptoms. A formal monitoring plan to assess changes in response and the significance of adverse effects should be in place. Review the target behavior, changes in function, and significance of adverse effects at least every 3 months.
How to approach discontinuation
Behavioral and psychological symptoms of dementia are frequently temporary. If the patient has been stable, gradual dose reduction and eventual discontinuation of antipsychotics should be attempted every 3 months. Studies have reported that most patients who were taken off antipsychotics for treating BPSD showed no worsening of behavioral symptoms.27
Discontinuation of antipsychotics should be done gradually by reducing the dose by 50% every 2 weeks, and then stopping after 2 weeks on the minimum dose, with monitoring for recurrence of target symptoms or emergence of new ones. The longer a medication has been prescribed, the slower the withdrawal occurs. Thus, the possibility of emerging symptoms related to drug withdrawal will lessen.
A roadmap for judicious prescribing

When underlying treatable or reversible causes of BPSD in dementia have been ruled out or nonpharmacologic treatments have failed, a trial of an antipsychotic may be indicated. The choice of agent should focus on patient-related factors and on clearly identified target behaviors. Treatment should be started at a low dose and titrated cautiously to the lowest effective dose.
Behavioral and psychological symptoms of dementia are frequently temporary. Therefore, a gradual reduction and eventual withdrawal of antipsychotic medications should be attempted every 3 months. Studies indicate that most patients are able to tolerate elimination of antipsychotic medications with no worsening of behavioral symptoms.
Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Bottom Line
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important, albeit limited, role in the treatment of behavioral disturbances in dementia. Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Related Resources
- Kales HC, Mulsant BH, Sajatovic MS. Prescribing antipsychotics in geriatric patients: Focus on dementia. Third of 3 parts. Current Psychiatry. 2017;16(12):24-30.
- Meeks TW, Jeste DV. Antipsychotics in dementia: Beyond ‘black-box’ warnings. Current Psychiatry. 2008;7(6):51-52, 55-58, 64-65.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Quetiapine • Seroquel
Ziprasidone • Geodon
1. Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther. 2013;5(4):27.
2. Tariot PN, Blazina L. The psychopathology of dementia. In: Morris JC, ed. Handbook of dementing illnesses. New York, NY: Marcel Dekker Inc.; 1993:461-475.
3. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
4. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
5. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
6. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
7. Okura T, Plassman BL, Steffens DC, et al. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473-481.
8. Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74:1315-1316.
9. Alexopoulos GS, Jeste DV, Chung H, et al. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med. 2005;Spec No:6-22.
10. Burke AD, Hall G, Yaari R, et al. Pocket reference to Alzheimer’s disease management. Philadelphia, PA: Springer Healthcare Communications; 2015:39-46
11. Burke AD, Burke WJ, Tariot PN. Drug treatments for the behavioural and psychiatric symptoms of dementia. In: Ames D, O’Brien JT, Burns A, eds. Dementia, 5th ed. Boca Raton, FL: CRC Press; 2016:231-252.
12. Royal Australian and New Zealand College of Psychiatrists. Antipsychotics in dementia: best practice guide. https://bpac.org.nz/a4d/resources/docs/bpac_A4D_best_practice_guide.pdf. Accessed September 4, 2018.
13. Nyberg L, Backman L. Cognitive aging: a view from brain imaging. In: Dixon RA, Backman L, Nilsson LG, eds. New frontiers in cognitive aging. Oxford: Oxford Univ Press; 2004:135-60.
14. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977.
15. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
16. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology.1999;53(5):946-955.
17. De Deyn PP, Jeste DV, Auby P, et al. Aripiprazole in dementia of the Alzheimer’s type. Poster presented at: 16th Annual Meeting of American Association for Geriatric Psychiatry; March 1-4, 2003; Honolulu, HI.
18. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
19. Mintzer J, Weiner M, Greenspan A, et al. Efficacy and safety of a flexible dose of risperidone versus placebo in the treatment of psychosis of Alzheimer’s disease. In: International College of Geriatric Psychopharmacology. Basel, Switzerland; 2004.
20. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918-931.
21. Sultzer DL, Davis SM, Tariot PN, et al; CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844-854.
22. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81-93.
23. Bozymski KM, Lowe DK, Pasternak KM, et al. Pimavanserin: a novel antipsychotic for Parkinson’s disease psychosis. Ann Pharmacother. 2017;51(6):479-487.
24. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252:1186.
25. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
26. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
27. Horwitz GJ, Tariot PN, Mead K, et al. Discontinuation of antipsychotics in nursing home patients with dementia. Am J Geriatr Psychiatry. 1995;3(4):290-299.
As psychiatrists treating an aging population, we frequently face the daunting challenges of managing medically complex and behaviorally unstable patients whose fragile condition tests the brightest among us. As our population enters late life, not only are physicians confronted with aging patients whose bodies have decreased renal and hepatic function, but we also face the challenges of the aging brain, severed neuronal networks, and neurotransmitter diminution. These physiological changes can alter treatment response, increase the frequency of adverse effects, and increase the likelihood of emergence of behavioral and psychological symptoms.
During the past decade, the number of people reaching age 65 has dramatically increased. As life expectancy improves, the “oldest old”—those age 85 and older—are the fastest-growing segment of the population. The prevalence of cognitive impairment, including mild cognitive impairment and dementia, in this cohort is >40%.1 Roughly 90% of patients with dementia will develop clinically significant behavioral problems at some point in the course of their illness.2
Behavioral and psychological symptoms of dementia (BPSD) have a tremendous impact on the quality of life for both patients and their caregivers. We are experts in understanding these behaviors and crafting nonpharmacologic treatment plans to manage them. Understanding the context in which behaviors emerge allows us to modify the environment, communication strategies, and other potential triggers, in turn reducing the need for pharmacologic intervention.
However, when nonpharmacologic interventions have been exhausted, what are the options? Antipsychotics have been one of the approaches used to address the challenges of behavioral disturbances and psychosis occurring in dementia. Unfortunately, there is conflicting evidence regarding the risks and benefits associated with the use of antipsychotics in this population. In this article, we provide a roadmap for the judicious use of antipsychotics for patients with dementia.
Weighing the risks and benefits of antipsychotics
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important but limited role in the treatment of behavioral disturbances in dementia. Although safety risks exist, they can be minimized through the careful selection of appropriate patients for treatment, close monitoring, and effective communication with patients and caregivers before and during treatment.
Several studies examining the efficacy of antipsychotics in the treatment of BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.3 This evidence prompted the FDA to issue a “black-box” warning in 2005 to highlight the increased risk of mortality for patients with dementia who are treated with SGAs.4 Both first-generation antipsychotics (FGAs) and SGAs have been associated with higher rates of mortality than most other psychotropic classes, except anticonvulsants. This increased mortality risk has been shown to persist for at least 6 to 12 months.5,6 FGAs appear to be associated with a greater mortality risk compared with SGAs. As a result, if antipsychotic treatment is necessary, the use of FGAs in this population is not recommended.
The potential mechanisms leading to stroke and death remain unclear. They could include orthostatic hypotension, anticholinergic adverse effects, QT prolongation, platelet aggregation effects, and venous thromboembolism. The presence of cardiovascular and vascular risk factors, electrolyte imbalances, cardiac arrhythmias, and concomitant use of medications that prolong the QTc interval may confer additional risks.
Continued to: Although the use of antipsychotics for patients with dementia...
Although the use of antipsychotics for patients with dementia may increase the risk of mortality, the absolute increased risk to a given individual, at least with short-term treatment, is likely small. The risk may also vary depending on the choice of SGA. Patients who were treated with quetiapine had a slightly lower risk of death than those who were treated risperidone.5 Death rates among patients prescribed aripiprazole, olanzapine, and ziprasidone were similar to the death rates of patients who were treated with risperidone. Compared with patients who were treated with risperidone, patients who were treated with the FGA haloperidol were twice as likely to die during a subsequent 6-month observation period. The largest number of deaths occurred during the first 40 days of treatment.5
While this increased risk of mortality is an important factor to discuss with patients and caregivers when deciding whether to initiate antipsychotic treatment, it is also important to put it into perspective. For example, the risk of suddenly dying from a stroke or heart attack for a person with dementia who is not taking an antipsychotic is approximately 2%. When an individual is started on one of these agents, that risk increases to approximately 4%. While the mortality risk is doubled, it remains relatively small.4 When faced with verbal or physical assaults, hostility, paranoid ideations, or other psychotic symptoms, many families feel that this relatively low risk does not outweigh the potential benefits of reducing caregiver and patient distress. If nonpharmacologic and/or other pharmacologic interventions have failed, the treatment has reached a point of no good alternatives and therapy should then focus on minimizing risk.
Informed consent is essential. A discussion of risks and benefits with the patient, family, or other decision-makers should focus on the risk of stroke, potential metabolic effects, and mortality, as well as potential worsening of cognitive decline associated with antipsychotic treatment. This should be weighed together with the evidence that suggests psychosis and agitation are associated with earlier nursing home admission and death.7,8 Families should be given ample time and opportunity to ask questions. Alternatives to immediate initiation of antipsychotics should be thoroughly reviewed.
Despite the above-noted risks, expert consensus suggests that the use of antipsychotics in the treatment of individuals with dementia can be appropriate, particularly in individuals with dangerous agitation or psychosis.9 These agents can minimize the risk of violence, reduce patient distress, improve the patient’s quality of life, and reduce caregiver burden. In clinical trials, the benefits of antipsychotics have been modest. Nevertheless, evidence has shown that these agents can reduce psychosis, agitation, aggression, hostility, and suspiciousness, which makes them a valid option when other interventions have proven insufficient.
Target specific symptoms
Despite this article’s focus on the appropriate use of antipsychotics for patients with BPSD, it is important to emphasize that the first-line approach to the management of BPSD in this population should always be a person-centered, psychosocial, multidisciplinary, nonpharmacologic approach that focuses on identifying triggers and treating potentially modifiable contributors to behavioral symptoms. Table 110 outlines common underlying causes of BPSD in dementia that should be assessed before prescribing an antipsychotic.

Continued to: Alternative psychopharmacologic treatments...
Alternative psychopharmacologic treatments based on a psychobehavioral metaphor should also be considered (Table 211). This approach matches the dominant target symptoms to the most relevant medication class.11 For example, in the case of a verbally and physically agitated patient who is also irritable, negative, socially withdrawn, and appears dysphoric, we might first undertake a trial of an antidepressant. Conversely, if the patient shows agitation in the context of increased motor activity, loud and rapid speech, and affective lability, we might consider the use of a mood stabilizer. Pharmacologic treatment should be aimed at the modification of clearly identified and documented target behaviors.

Indications to use antipsychotics for patients with dementia include:
- severe agitation and aggression associated with risk of harm
- delusions and hallucinations
- comorbid preexisting mental health conditions (eg, bipolar disorder, schizophrenia, treatment-resistant depression, etc.).
Symptoms that do not usually respond to an antipsychotic include wandering, social withdrawal, shouting, pacing, touching, cognitive defects, and incontinence.12 These symptoms may respond to interventions such as changes to the environment.
Continued to: Choosing an antipsychotic
Choosing an antipsychotic
Once you have identified that an antipsychotic is truly indicated, the choice of an agent will focus on patient-related factors. Considerations such as frailty, comorbid medical conditions including diabetes, history of falls, hepatic insufficiency, cardiac arrhythmias, and cerebrovascular risk factors, should all be analyzed prior to initiating an antipsychotic. The presence of these conditions will increase the likelihood that adverse effects may occur. It will also guide the dose trajectory and the target dose for discontinuation. Antipsychotics differ with respect to their efficacy and adverse effect profile. For practical purposes, adverse effects typically guide the selection of these agents when used for patients with dementia.
Continued to: Gradual structural changes occur...
Gradual structural changes occur in the dopaminergic system with age and increase the propensity for antipsychotic adverse effects. The number of dopaminergic neurons and D2 receptors decreases approximately 10% per decade. In order to avoid the development of adverse effects related to extrapyramidal symptoms, approximately 20% of receptors need to be free. FGAs tend to block approximately 90% of D2 receptors, whereas SGAs block less than 70% to 80% and dissociate more rapidly from D2 receptors.13 FGAs should therefore be avoided, as they have been associated with numerous adverse effects, including parkinsonism, tardive dyskinesia, akathisia, sedation, peripheral and central anticholinergic effects, postural hypotension, cardiac conduction defects, and falls. As noted above, they have been linked to a greater risk of mortality (Figure14 ).

When the decision to use an antipsychotic agent is made for a person with dementia, SGAs appear to be a better choice. There appear to be modest differences within the class of SGAs in terms of effectiveness, tolerability, and adverse effect profile. Although the association between the dose of an antipsychotic and the risk of mortality or stroke remains undefined, other common adverse effects, such as sedation, extrapyramidal symptoms, and risk of falls, can be reduced by starting at the lowest dose possible and titrating slowly.
Dosing considerations
Dose increments should be modest and, in a nonemergent setting, may be adjusted at weekly intervals depending on response. Prior to starting a treatment trial, it is advisable to estimate what will constitute a worthwhile clinical response, the duration of treatment, and the maximum dose. Avoid high doses or prolonged use of antipsychotics that have not significantly improved the target behavior.
When the decision to use a SGA is made, choosing the initial starting dose is challenging given that none of these medications has an indication for use in this population. We propose doses that have been used in completed randomized trials that reflect the best information available about the dose likely to maximize benefit and minimize risk. On the basis of those trials, reasonable starting doses would be15-22:
- quetiapine 25 to 50 mg/d
- risperidone 0.5 to 1 mg/d
- aripiprazole 2 to 10 mg/d
- olanzapine 2.5 to 5 mg/d
- ziprasidone 20 mg/d
Continued to: The highest doses tested...
The highest doses tested for each of these compounds in randomized clinical trials for this population were: risperidone 2 mg/d, olanzapine 10 mg/d, and aripiprazole 15 mg/d. A wide variety of maximum doses of quetiapine were studied in clinical trials, with a top dose of 200 mg being most common. It is worth noting that doses higher than these have been used for other indications.15-22
Quetiapine. One of the most commonly prescribed antipsychotics for the treatment of BPSD in individuals with memory disorders is quetiapine. The reasons for this preference include a low risk of extrapyramidal adverse effects, flexibility of dosing, ability to use lower dosages, and evidence of the lower risk of mortality when compared with other second-generation agents.5,15 If an antipsychotic is indicated, quetiapine should be considered as a first-line antipsychotic therapy. Quetiapine has well-established effects on mood, anxiety, and sleep, all of which can be disrupted in dementia and can act as drivers for agitation.5,15 Starting quetiapine may mitigate the need for separate agents to treat insomnia, loss of appetite, or anxiety, although it is not FDA-indicated for these comorbid conditions. Quetiapine is also less likely to exacerbate motor symptoms compared with other SGAs but has the potential to increase the risk of falls, and orthostasis, and carries a considerable anticholinergic burden.5,15
Risperidone has been shown to provide modest improvements in some people exhibiting symptoms of aggression, agitation, and psychosis.5,15 There is no evidence that risperidone is any more effective than other SGAs, but it has been tested on more geriatric patients than other SGAs. The fact that it is also available in an orally disintegrating tablet makes it a practical treatment in certain populations of patients, such as those who have difficulty swallowing. Risperidone carries the highest extrapyramidal symptom burden among the SGAs due to its potent D2 receptor binding. 5,15
Aripiprazole. There have been several studies of aripiprazole for the treatment of psychosis and agitation in Alzheimer’s dementia.15 This medication showed modest effect and was generally well tolerated. Aripiprazole appears to have less associated weight gain, which may be pertinent for some patients. It also appears to be less sedating than many of the other SGAs. However, some patients may experience activation or insomnia with this agent, particularly with doses <15 mg/d. This activating effect may be beneficial for treating comorbid depressive symptoms, although lower doses could theoretically worsen psychosis due to the activating effects.
Aripiprazole has also been studied in Parkinson’s disease. While some patients had favorable responses with improvement in psychosis and behavioral disturbances, this medication was also associated with worsening of motor symptoms. Certain individuals also experienced a worsening of their psychosis.23 For this reason, it is unlikely to be a useful agent for patients displaying evidence of parkinsonism, Parkinson’s dementia, or dementia with Lewy bodies.
Olanzapine. Several studies have shown that low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients suffering from Alzheimer’s and vascular dementias.24 The medication is also available in an orally disintegrating form, which may be beneficial when treating individuals whose swallowing abilities are compromised. Olanzapine also has been associated with significant weight gain and metabolic syndrome.24
Continued to: Ziprasidone
Ziprasidone. There are no specific studies of ziprasidone for geriatric patients and none for patients with dementia. However, case reports have suggested both oral and injectable forms of the medication may be well tolerated and have some benefit in treating agitation in this population.25 Based on evidence from younger populations, ziprasidone is less likely to be associated with weight gain or orthostatic hypotension. Medication has been associated with QTc prolongation and should be used with caution and monitored with an ECG.
The initial dosing and potential adverse effects of quetiapine, risperidone, aripiprazole, olanzapine, and ziprasidone are highlighted in Table 3.10

Other SGAs. Newer antipsychotics have recently become available and may serve as additional tools for managing BPSD in the future. Unfortunately, there are currently no available studies regarding their efficacy in the treatment of agitation and psychosis in dementia. One notable exception is pimavaserin, a serotonin 2A receptor inverse agonist. This medication has recently been FDA-approved for the treatment of Parkinson’s disease psychosis. The medication was extensively studied in older patients. It appeared to be effective in reducing delusions and hallucinations while not impairing motor function or causing sedation or hypotension.23 Additional studies are currently ongoing for the treatment of Alzheimer’s dementia psychosis.
Monitor treatment, consider discontinuation
American Psychiatric Association guidelines on the use of antipsychotics to treat agitation or psychosis in patients with dementia currently recommend that clinicians use a quantitative measure to track symptoms and response to treatment.26 These measures may be formal, such as an overall assessment of symptom severity on a Likert scale, or as simple as monitoring the changes in the frequency of periods of agitation.
After starting an antipsychotic, a follow-up appointment should typically take place within 1 month. If the patient is at high risk for developing adverse effects, or if the symptoms are severe, a follow-up appointment for monitoring the response to treatment and potential adverse effects should occur within 1 week. At a minimum, expert consensus suggests follow-up visits should occur every 3 months.
If there is no clinical response after 4 weeks of adequate dosing of an antipsychotic, the medication should be tapered and withdrawn. Switching to an alternative agent may be appropriate.
Many patients will have only partial remission of target symptoms. Therefore, increasing the dose or switching to an alternative agent may be necessary. Concurrent use of multiple antipsychotic agents should be avoided.
Continued to: Maintenance treatment may be appropriate
Maintenance treatment may be appropriate for patients who have demonstrated a clear benefit from antipsychotic treatment without undue adverse effects, and in whom a trial dose reduction has resulted in reappearance of the target symptoms. A formal monitoring plan to assess changes in response and the significance of adverse effects should be in place. Review the target behavior, changes in function, and significance of adverse effects at least every 3 months.
How to approach discontinuation
Behavioral and psychological symptoms of dementia are frequently temporary. If the patient has been stable, gradual dose reduction and eventual discontinuation of antipsychotics should be attempted every 3 months. Studies have reported that most patients who were taken off antipsychotics for treating BPSD showed no worsening of behavioral symptoms.27
Discontinuation of antipsychotics should be done gradually by reducing the dose by 50% every 2 weeks, and then stopping after 2 weeks on the minimum dose, with monitoring for recurrence of target symptoms or emergence of new ones. The longer a medication has been prescribed, the slower the withdrawal occurs. Thus, the possibility of emerging symptoms related to drug withdrawal will lessen.
A roadmap for judicious prescribing

When underlying treatable or reversible causes of BPSD in dementia have been ruled out or nonpharmacologic treatments have failed, a trial of an antipsychotic may be indicated. The choice of agent should focus on patient-related factors and on clearly identified target behaviors. Treatment should be started at a low dose and titrated cautiously to the lowest effective dose.
Behavioral and psychological symptoms of dementia are frequently temporary. Therefore, a gradual reduction and eventual withdrawal of antipsychotic medications should be attempted every 3 months. Studies indicate that most patients are able to tolerate elimination of antipsychotic medications with no worsening of behavioral symptoms.
Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Bottom Line
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important, albeit limited, role in the treatment of behavioral disturbances in dementia. Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Related Resources
- Kales HC, Mulsant BH, Sajatovic MS. Prescribing antipsychotics in geriatric patients: Focus on dementia. Third of 3 parts. Current Psychiatry. 2017;16(12):24-30.
- Meeks TW, Jeste DV. Antipsychotics in dementia: Beyond ‘black-box’ warnings. Current Psychiatry. 2008;7(6):51-52, 55-58, 64-65.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Quetiapine • Seroquel
Ziprasidone • Geodon
As psychiatrists treating an aging population, we frequently face the daunting challenges of managing medically complex and behaviorally unstable patients whose fragile condition tests the brightest among us. As our population enters late life, not only are physicians confronted with aging patients whose bodies have decreased renal and hepatic function, but we also face the challenges of the aging brain, severed neuronal networks, and neurotransmitter diminution. These physiological changes can alter treatment response, increase the frequency of adverse effects, and increase the likelihood of emergence of behavioral and psychological symptoms.
During the past decade, the number of people reaching age 65 has dramatically increased. As life expectancy improves, the “oldest old”—those age 85 and older—are the fastest-growing segment of the population. The prevalence of cognitive impairment, including mild cognitive impairment and dementia, in this cohort is >40%.1 Roughly 90% of patients with dementia will develop clinically significant behavioral problems at some point in the course of their illness.2
Behavioral and psychological symptoms of dementia (BPSD) have a tremendous impact on the quality of life for both patients and their caregivers. We are experts in understanding these behaviors and crafting nonpharmacologic treatment plans to manage them. Understanding the context in which behaviors emerge allows us to modify the environment, communication strategies, and other potential triggers, in turn reducing the need for pharmacologic intervention.
However, when nonpharmacologic interventions have been exhausted, what are the options? Antipsychotics have been one of the approaches used to address the challenges of behavioral disturbances and psychosis occurring in dementia. Unfortunately, there is conflicting evidence regarding the risks and benefits associated with the use of antipsychotics in this population. In this article, we provide a roadmap for the judicious use of antipsychotics for patients with dementia.
Weighing the risks and benefits of antipsychotics
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important but limited role in the treatment of behavioral disturbances in dementia. Although safety risks exist, they can be minimized through the careful selection of appropriate patients for treatment, close monitoring, and effective communication with patients and caregivers before and during treatment.
Several studies examining the efficacy of antipsychotics in the treatment of BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.3 This evidence prompted the FDA to issue a “black-box” warning in 2005 to highlight the increased risk of mortality for patients with dementia who are treated with SGAs.4 Both first-generation antipsychotics (FGAs) and SGAs have been associated with higher rates of mortality than most other psychotropic classes, except anticonvulsants. This increased mortality risk has been shown to persist for at least 6 to 12 months.5,6 FGAs appear to be associated with a greater mortality risk compared with SGAs. As a result, if antipsychotic treatment is necessary, the use of FGAs in this population is not recommended.
The potential mechanisms leading to stroke and death remain unclear. They could include orthostatic hypotension, anticholinergic adverse effects, QT prolongation, platelet aggregation effects, and venous thromboembolism. The presence of cardiovascular and vascular risk factors, electrolyte imbalances, cardiac arrhythmias, and concomitant use of medications that prolong the QTc interval may confer additional risks.
Continued to: Although the use of antipsychotics for patients with dementia...
Although the use of antipsychotics for patients with dementia may increase the risk of mortality, the absolute increased risk to a given individual, at least with short-term treatment, is likely small. The risk may also vary depending on the choice of SGA. Patients who were treated with quetiapine had a slightly lower risk of death than those who were treated risperidone.5 Death rates among patients prescribed aripiprazole, olanzapine, and ziprasidone were similar to the death rates of patients who were treated with risperidone. Compared with patients who were treated with risperidone, patients who were treated with the FGA haloperidol were twice as likely to die during a subsequent 6-month observation period. The largest number of deaths occurred during the first 40 days of treatment.5
While this increased risk of mortality is an important factor to discuss with patients and caregivers when deciding whether to initiate antipsychotic treatment, it is also important to put it into perspective. For example, the risk of suddenly dying from a stroke or heart attack for a person with dementia who is not taking an antipsychotic is approximately 2%. When an individual is started on one of these agents, that risk increases to approximately 4%. While the mortality risk is doubled, it remains relatively small.4 When faced with verbal or physical assaults, hostility, paranoid ideations, or other psychotic symptoms, many families feel that this relatively low risk does not outweigh the potential benefits of reducing caregiver and patient distress. If nonpharmacologic and/or other pharmacologic interventions have failed, the treatment has reached a point of no good alternatives and therapy should then focus on minimizing risk.
Informed consent is essential. A discussion of risks and benefits with the patient, family, or other decision-makers should focus on the risk of stroke, potential metabolic effects, and mortality, as well as potential worsening of cognitive decline associated with antipsychotic treatment. This should be weighed together with the evidence that suggests psychosis and agitation are associated with earlier nursing home admission and death.7,8 Families should be given ample time and opportunity to ask questions. Alternatives to immediate initiation of antipsychotics should be thoroughly reviewed.
Despite the above-noted risks, expert consensus suggests that the use of antipsychotics in the treatment of individuals with dementia can be appropriate, particularly in individuals with dangerous agitation or psychosis.9 These agents can minimize the risk of violence, reduce patient distress, improve the patient’s quality of life, and reduce caregiver burden. In clinical trials, the benefits of antipsychotics have been modest. Nevertheless, evidence has shown that these agents can reduce psychosis, agitation, aggression, hostility, and suspiciousness, which makes them a valid option when other interventions have proven insufficient.
Target specific symptoms
Despite this article’s focus on the appropriate use of antipsychotics for patients with BPSD, it is important to emphasize that the first-line approach to the management of BPSD in this population should always be a person-centered, psychosocial, multidisciplinary, nonpharmacologic approach that focuses on identifying triggers and treating potentially modifiable contributors to behavioral symptoms. Table 110 outlines common underlying causes of BPSD in dementia that should be assessed before prescribing an antipsychotic.

Continued to: Alternative psychopharmacologic treatments...
Alternative psychopharmacologic treatments based on a psychobehavioral metaphor should also be considered (Table 211). This approach matches the dominant target symptoms to the most relevant medication class.11 For example, in the case of a verbally and physically agitated patient who is also irritable, negative, socially withdrawn, and appears dysphoric, we might first undertake a trial of an antidepressant. Conversely, if the patient shows agitation in the context of increased motor activity, loud and rapid speech, and affective lability, we might consider the use of a mood stabilizer. Pharmacologic treatment should be aimed at the modification of clearly identified and documented target behaviors.

Indications to use antipsychotics for patients with dementia include:
- severe agitation and aggression associated with risk of harm
- delusions and hallucinations
- comorbid preexisting mental health conditions (eg, bipolar disorder, schizophrenia, treatment-resistant depression, etc.).
Symptoms that do not usually respond to an antipsychotic include wandering, social withdrawal, shouting, pacing, touching, cognitive defects, and incontinence.12 These symptoms may respond to interventions such as changes to the environment.
Continued to: Choosing an antipsychotic
Choosing an antipsychotic
Once you have identified that an antipsychotic is truly indicated, the choice of an agent will focus on patient-related factors. Considerations such as frailty, comorbid medical conditions including diabetes, history of falls, hepatic insufficiency, cardiac arrhythmias, and cerebrovascular risk factors, should all be analyzed prior to initiating an antipsychotic. The presence of these conditions will increase the likelihood that adverse effects may occur. It will also guide the dose trajectory and the target dose for discontinuation. Antipsychotics differ with respect to their efficacy and adverse effect profile. For practical purposes, adverse effects typically guide the selection of these agents when used for patients with dementia.
Continued to: Gradual structural changes occur...
Gradual structural changes occur in the dopaminergic system with age and increase the propensity for antipsychotic adverse effects. The number of dopaminergic neurons and D2 receptors decreases approximately 10% per decade. In order to avoid the development of adverse effects related to extrapyramidal symptoms, approximately 20% of receptors need to be free. FGAs tend to block approximately 90% of D2 receptors, whereas SGAs block less than 70% to 80% and dissociate more rapidly from D2 receptors.13 FGAs should therefore be avoided, as they have been associated with numerous adverse effects, including parkinsonism, tardive dyskinesia, akathisia, sedation, peripheral and central anticholinergic effects, postural hypotension, cardiac conduction defects, and falls. As noted above, they have been linked to a greater risk of mortality (Figure14 ).

When the decision to use an antipsychotic agent is made for a person with dementia, SGAs appear to be a better choice. There appear to be modest differences within the class of SGAs in terms of effectiveness, tolerability, and adverse effect profile. Although the association between the dose of an antipsychotic and the risk of mortality or stroke remains undefined, other common adverse effects, such as sedation, extrapyramidal symptoms, and risk of falls, can be reduced by starting at the lowest dose possible and titrating slowly.
Dosing considerations
Dose increments should be modest and, in a nonemergent setting, may be adjusted at weekly intervals depending on response. Prior to starting a treatment trial, it is advisable to estimate what will constitute a worthwhile clinical response, the duration of treatment, and the maximum dose. Avoid high doses or prolonged use of antipsychotics that have not significantly improved the target behavior.
When the decision to use a SGA is made, choosing the initial starting dose is challenging given that none of these medications has an indication for use in this population. We propose doses that have been used in completed randomized trials that reflect the best information available about the dose likely to maximize benefit and minimize risk. On the basis of those trials, reasonable starting doses would be15-22:
- quetiapine 25 to 50 mg/d
- risperidone 0.5 to 1 mg/d
- aripiprazole 2 to 10 mg/d
- olanzapine 2.5 to 5 mg/d
- ziprasidone 20 mg/d
Continued to: The highest doses tested...
The highest doses tested for each of these compounds in randomized clinical trials for this population were: risperidone 2 mg/d, olanzapine 10 mg/d, and aripiprazole 15 mg/d. A wide variety of maximum doses of quetiapine were studied in clinical trials, with a top dose of 200 mg being most common. It is worth noting that doses higher than these have been used for other indications.15-22
Quetiapine. One of the most commonly prescribed antipsychotics for the treatment of BPSD in individuals with memory disorders is quetiapine. The reasons for this preference include a low risk of extrapyramidal adverse effects, flexibility of dosing, ability to use lower dosages, and evidence of the lower risk of mortality when compared with other second-generation agents.5,15 If an antipsychotic is indicated, quetiapine should be considered as a first-line antipsychotic therapy. Quetiapine has well-established effects on mood, anxiety, and sleep, all of which can be disrupted in dementia and can act as drivers for agitation.5,15 Starting quetiapine may mitigate the need for separate agents to treat insomnia, loss of appetite, or anxiety, although it is not FDA-indicated for these comorbid conditions. Quetiapine is also less likely to exacerbate motor symptoms compared with other SGAs but has the potential to increase the risk of falls, and orthostasis, and carries a considerable anticholinergic burden.5,15
Risperidone has been shown to provide modest improvements in some people exhibiting symptoms of aggression, agitation, and psychosis.5,15 There is no evidence that risperidone is any more effective than other SGAs, but it has been tested on more geriatric patients than other SGAs. The fact that it is also available in an orally disintegrating tablet makes it a practical treatment in certain populations of patients, such as those who have difficulty swallowing. Risperidone carries the highest extrapyramidal symptom burden among the SGAs due to its potent D2 receptor binding. 5,15
Aripiprazole. There have been several studies of aripiprazole for the treatment of psychosis and agitation in Alzheimer’s dementia.15 This medication showed modest effect and was generally well tolerated. Aripiprazole appears to have less associated weight gain, which may be pertinent for some patients. It also appears to be less sedating than many of the other SGAs. However, some patients may experience activation or insomnia with this agent, particularly with doses <15 mg/d. This activating effect may be beneficial for treating comorbid depressive symptoms, although lower doses could theoretically worsen psychosis due to the activating effects.
Aripiprazole has also been studied in Parkinson’s disease. While some patients had favorable responses with improvement in psychosis and behavioral disturbances, this medication was also associated with worsening of motor symptoms. Certain individuals also experienced a worsening of their psychosis.23 For this reason, it is unlikely to be a useful agent for patients displaying evidence of parkinsonism, Parkinson’s dementia, or dementia with Lewy bodies.
Olanzapine. Several studies have shown that low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients suffering from Alzheimer’s and vascular dementias.24 The medication is also available in an orally disintegrating form, which may be beneficial when treating individuals whose swallowing abilities are compromised. Olanzapine also has been associated with significant weight gain and metabolic syndrome.24
Continued to: Ziprasidone
Ziprasidone. There are no specific studies of ziprasidone for geriatric patients and none for patients with dementia. However, case reports have suggested both oral and injectable forms of the medication may be well tolerated and have some benefit in treating agitation in this population.25 Based on evidence from younger populations, ziprasidone is less likely to be associated with weight gain or orthostatic hypotension. Medication has been associated with QTc prolongation and should be used with caution and monitored with an ECG.
The initial dosing and potential adverse effects of quetiapine, risperidone, aripiprazole, olanzapine, and ziprasidone are highlighted in Table 3.10

Other SGAs. Newer antipsychotics have recently become available and may serve as additional tools for managing BPSD in the future. Unfortunately, there are currently no available studies regarding their efficacy in the treatment of agitation and psychosis in dementia. One notable exception is pimavaserin, a serotonin 2A receptor inverse agonist. This medication has recently been FDA-approved for the treatment of Parkinson’s disease psychosis. The medication was extensively studied in older patients. It appeared to be effective in reducing delusions and hallucinations while not impairing motor function or causing sedation or hypotension.23 Additional studies are currently ongoing for the treatment of Alzheimer’s dementia psychosis.
Monitor treatment, consider discontinuation
American Psychiatric Association guidelines on the use of antipsychotics to treat agitation or psychosis in patients with dementia currently recommend that clinicians use a quantitative measure to track symptoms and response to treatment.26 These measures may be formal, such as an overall assessment of symptom severity on a Likert scale, or as simple as monitoring the changes in the frequency of periods of agitation.
After starting an antipsychotic, a follow-up appointment should typically take place within 1 month. If the patient is at high risk for developing adverse effects, or if the symptoms are severe, a follow-up appointment for monitoring the response to treatment and potential adverse effects should occur within 1 week. At a minimum, expert consensus suggests follow-up visits should occur every 3 months.
If there is no clinical response after 4 weeks of adequate dosing of an antipsychotic, the medication should be tapered and withdrawn. Switching to an alternative agent may be appropriate.
Many patients will have only partial remission of target symptoms. Therefore, increasing the dose or switching to an alternative agent may be necessary. Concurrent use of multiple antipsychotic agents should be avoided.
Continued to: Maintenance treatment may be appropriate
Maintenance treatment may be appropriate for patients who have demonstrated a clear benefit from antipsychotic treatment without undue adverse effects, and in whom a trial dose reduction has resulted in reappearance of the target symptoms. A formal monitoring plan to assess changes in response and the significance of adverse effects should be in place. Review the target behavior, changes in function, and significance of adverse effects at least every 3 months.
How to approach discontinuation
Behavioral and psychological symptoms of dementia are frequently temporary. If the patient has been stable, gradual dose reduction and eventual discontinuation of antipsychotics should be attempted every 3 months. Studies have reported that most patients who were taken off antipsychotics for treating BPSD showed no worsening of behavioral symptoms.27
Discontinuation of antipsychotics should be done gradually by reducing the dose by 50% every 2 weeks, and then stopping after 2 weeks on the minimum dose, with monitoring for recurrence of target symptoms or emergence of new ones. The longer a medication has been prescribed, the slower the withdrawal occurs. Thus, the possibility of emerging symptoms related to drug withdrawal will lessen.
A roadmap for judicious prescribing

When underlying treatable or reversible causes of BPSD in dementia have been ruled out or nonpharmacologic treatments have failed, a trial of an antipsychotic may be indicated. The choice of agent should focus on patient-related factors and on clearly identified target behaviors. Treatment should be started at a low dose and titrated cautiously to the lowest effective dose.
Behavioral and psychological symptoms of dementia are frequently temporary. Therefore, a gradual reduction and eventual withdrawal of antipsychotic medications should be attempted every 3 months. Studies indicate that most patients are able to tolerate elimination of antipsychotic medications with no worsening of behavioral symptoms.
Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Bottom Line
Until better treatment options become available, second-generation antipsychotics (SGAs) continue to have an important, albeit limited, role in the treatment of behavioral disturbances in dementia. Despite the limitations of treatment, SGAs remain a valid consideration when other interventions have proven insufficient. However, judicious use of these agents remains the cornerstone of therapy.
Related Resources
- Kales HC, Mulsant BH, Sajatovic MS. Prescribing antipsychotics in geriatric patients: Focus on dementia. Third of 3 parts. Current Psychiatry. 2017;16(12):24-30.
- Meeks TW, Jeste DV. Antipsychotics in dementia: Beyond ‘black-box’ warnings. Current Psychiatry. 2008;7(6):51-52, 55-58, 64-65.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Quetiapine • Seroquel
Ziprasidone • Geodon
1. Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther. 2013;5(4):27.
2. Tariot PN, Blazina L. The psychopathology of dementia. In: Morris JC, ed. Handbook of dementing illnesses. New York, NY: Marcel Dekker Inc.; 1993:461-475.
3. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
4. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
5. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
6. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
7. Okura T, Plassman BL, Steffens DC, et al. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473-481.
8. Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74:1315-1316.
9. Alexopoulos GS, Jeste DV, Chung H, et al. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med. 2005;Spec No:6-22.
10. Burke AD, Hall G, Yaari R, et al. Pocket reference to Alzheimer’s disease management. Philadelphia, PA: Springer Healthcare Communications; 2015:39-46
11. Burke AD, Burke WJ, Tariot PN. Drug treatments for the behavioural and psychiatric symptoms of dementia. In: Ames D, O’Brien JT, Burns A, eds. Dementia, 5th ed. Boca Raton, FL: CRC Press; 2016:231-252.
12. Royal Australian and New Zealand College of Psychiatrists. Antipsychotics in dementia: best practice guide. https://bpac.org.nz/a4d/resources/docs/bpac_A4D_best_practice_guide.pdf. Accessed September 4, 2018.
13. Nyberg L, Backman L. Cognitive aging: a view from brain imaging. In: Dixon RA, Backman L, Nilsson LG, eds. New frontiers in cognitive aging. Oxford: Oxford Univ Press; 2004:135-60.
14. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977.
15. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
16. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology.1999;53(5):946-955.
17. De Deyn PP, Jeste DV, Auby P, et al. Aripiprazole in dementia of the Alzheimer’s type. Poster presented at: 16th Annual Meeting of American Association for Geriatric Psychiatry; March 1-4, 2003; Honolulu, HI.
18. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
19. Mintzer J, Weiner M, Greenspan A, et al. Efficacy and safety of a flexible dose of risperidone versus placebo in the treatment of psychosis of Alzheimer’s disease. In: International College of Geriatric Psychopharmacology. Basel, Switzerland; 2004.
20. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918-931.
21. Sultzer DL, Davis SM, Tariot PN, et al; CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844-854.
22. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81-93.
23. Bozymski KM, Lowe DK, Pasternak KM, et al. Pimavanserin: a novel antipsychotic for Parkinson’s disease psychosis. Ann Pharmacother. 2017;51(6):479-487.
24. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252:1186.
25. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
26. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
27. Horwitz GJ, Tariot PN, Mead K, et al. Discontinuation of antipsychotics in nursing home patients with dementia. Am J Geriatr Psychiatry. 1995;3(4):290-299.
1. Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther. 2013;5(4):27.
2. Tariot PN, Blazina L. The psychopathology of dementia. In: Morris JC, ed. Handbook of dementing illnesses. New York, NY: Marcel Dekker Inc.; 1993:461-475.
3. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
4. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
5. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
6. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
7. Okura T, Plassman BL, Steffens DC, et al. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473-481.
8. Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74:1315-1316.
9. Alexopoulos GS, Jeste DV, Chung H, et al. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med. 2005;Spec No:6-22.
10. Burke AD, Hall G, Yaari R, et al. Pocket reference to Alzheimer’s disease management. Philadelphia, PA: Springer Healthcare Communications; 2015:39-46
11. Burke AD, Burke WJ, Tariot PN. Drug treatments for the behavioural and psychiatric symptoms of dementia. In: Ames D, O’Brien JT, Burns A, eds. Dementia, 5th ed. Boca Raton, FL: CRC Press; 2016:231-252.
12. Royal Australian and New Zealand College of Psychiatrists. Antipsychotics in dementia: best practice guide. https://bpac.org.nz/a4d/resources/docs/bpac_A4D_best_practice_guide.pdf. Accessed September 4, 2018.
13. Nyberg L, Backman L. Cognitive aging: a view from brain imaging. In: Dixon RA, Backman L, Nilsson LG, eds. New frontiers in cognitive aging. Oxford: Oxford Univ Press; 2004:135-60.
14. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977.
15. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
16. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology.1999;53(5):946-955.
17. De Deyn PP, Jeste DV, Auby P, et al. Aripiprazole in dementia of the Alzheimer’s type. Poster presented at: 16th Annual Meeting of American Association for Geriatric Psychiatry; March 1-4, 2003; Honolulu, HI.
18. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
19. Mintzer J, Weiner M, Greenspan A, et al. Efficacy and safety of a flexible dose of risperidone versus placebo in the treatment of psychosis of Alzheimer’s disease. In: International College of Geriatric Psychopharmacology. Basel, Switzerland; 2004.
20. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918-931.
21. Sultzer DL, Davis SM, Tariot PN, et al; CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844-854.
22. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81-93.
23. Bozymski KM, Lowe DK, Pasternak KM, et al. Pimavanserin: a novel antipsychotic for Parkinson’s disease psychosis. Ann Pharmacother. 2017;51(6):479-487.
24. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252:1186.
25. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
26. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
27. Horwitz GJ, Tariot PN, Mead K, et al. Discontinuation of antipsychotics in nursing home patients with dementia. Am J Geriatr Psychiatry. 1995;3(4):290-299.
