User login
Telemedicine: Three fraud and abuse triggers
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
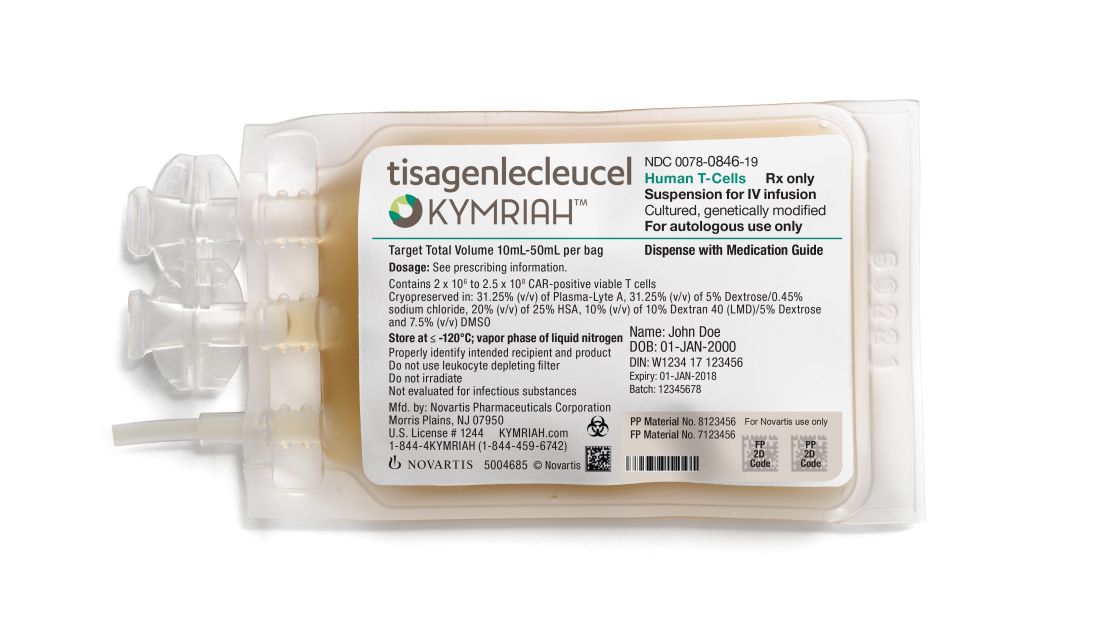
CMS finalizes CAR T-cell therapy inpatient payments
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
5 HIPAA myths in the digital age
The nexus of new technology and privacy rules springing from the Health Insurance Portability and Accountability Act of 1996 (HIPAA) leads to a lot of stress and trepidation for health care professionals. Lucia Savage, chief privacy and regulatory officer for Omada Health, and Matthew Fisher, a health law attorney based in Worcester, Mass., who specializes in compliance issues, dispel common HIPAA myths and offer advice on how to protect yourself and your practice.
Truth: Physicians are not responsible for email security flaws from patient servers, said Ms. Savage, who served as chief privacy officer for the Office of the National Coordinator for Health IT under President Obama. HIPAA requires only that health providers send emails from a secure system that protects a doctor’s message from their end, she said.
“There’s this myth out there that you cannot send an electronic message to a patient’s email box if that email is unsecured, and that’s not true,” Ms. Savage said at a recent American Bar Association meeting. “The obligation is to secure what you send, not to secure what an unregulated, private person receives.”
Just remember to warn patients that they’re responsible for the safe storage of an email message once it arrives.
Truth: An email with protected health information (PHI) accidentally sent to the wrong health provider is not likely to get doctors in trouble with the Office for Civil Rights. In the last 12 years, there have been 184,000 HIPAA-related complaints to OCR and only 55 resulted in financial settlements, according to research Ms. Savage conducted through the Department of Health & Human Services website. Of the 55 settlements, none were associated with PHI accidentally sent from one health provider to another, she said in an interview.
“[The OCR] tends to seek fines for really eye-poppingly bad behavior,” Ms. Savage said, not small-scale accidents. For example, OCR fined one hospital for including the name of a patient in a press release without patient permission. Another health professional was fined for repeated failures to encrypt their computer system.
If a document with PHI does end up in the wrong inbox, Ms. Savage advises calling the receiver and asking that they immediately delete the email.
Truth: Breaches alone are not the reason most fines are levied, nor do breach notifications mean an instant penalty, Mr. Fisher said in an interview. Fines by OCR are more often tied to further noncompliance found when the agency begins investigating the entity after the breach report.
“Most breach reports will result in OCR conducting a follow-up investigation, usually with paper-based requests,” he said. “If responses to those requests reveal widespread or consistent noncompliance, then OCR may latch on and dig in order to impose a fine.”
For example, a breach could be the result of a lost USB drive or laptop, but OCR’s investigation might ultimately find that the practice failed to conduct an adequate risk analysis. Because a risk analysis is a fundamental component of HIPAA compliance, the inadequate risk analysis becomes the basis for a fine, Mr. Fisher said.
The best way to avoid an OCR fine is to ensure that proper HIPAA protocols are in place to assess security risks, prevent breaches, and mitigate breaches should they occur. “Part of good compliance is constant review and revision of policies as well,” Mr. Fisher said. “It is not sufficient to put the policies into place and then never revisit those policies. Circumstances change all of the time and policies need to keep up.”
Truth: Health professionals are obligated to provide copies of health information to patients and that includes electronic copies if practices have such technology. The electronic copy requirement was adopted in 2009 as part of the Health Information Technology for Economic and Clinical Health (HITECH) Act.
Despite the electronic amendment’s existence for nearly 10 years, Ms. Savage said she frequently hears from patients about the difficulty of obtaining health information and the extended time and high cost that come with requests.
“[Providing health information to patients] is an obligation,” Ms. Savage stressed. “A 21st century physician might want to be thinking about how to build on that obligation to really engage their patients in a partnership of care. If you give the patient the data, they can actually become a more valuable [participant] with you and engage in self-management.”
More information on HITECH and giving patients access to protected health information can be found here.
Truth: HIPAA is flexible and can adapt to newer technology more easily than many people think, Mr. Fisher says.
“[There is the perception] that HIPAA is archaic and does not fit with modern technology,” he said. “There are a lot of misplaced fears that digital tools cannot satisfy security requirements or will place data where they should not go.”
In actuality, many health care applications enable doctors to satisfy HIPAA requirements, while using updated technology. Secure email to send patients messages is one example, he said, as well as secure text messaging between providers.
At the same time, new technology can often assist health care privacy and advance security, Mr. Fisher noted. Technology solutions frequently automate routine tasks, such as auditing. Tools like machine learning and artificial intelligence can enhance security and catch up with attacker intelligence, he added.
“Technology should be viewed as a means of enhancing and expanding capabilities,” he said. “Using the auditing example, an individual really cannot adequately review all records or access points, but a program may be able to do so and begin to identify small trends that represent a security concern. From this perspective, the technology, as indicated, is about enhancing what can be done.”
The nexus of new technology and privacy rules springing from the Health Insurance Portability and Accountability Act of 1996 (HIPAA) leads to a lot of stress and trepidation for health care professionals. Lucia Savage, chief privacy and regulatory officer for Omada Health, and Matthew Fisher, a health law attorney based in Worcester, Mass., who specializes in compliance issues, dispel common HIPAA myths and offer advice on how to protect yourself and your practice.
Truth: Physicians are not responsible for email security flaws from patient servers, said Ms. Savage, who served as chief privacy officer for the Office of the National Coordinator for Health IT under President Obama. HIPAA requires only that health providers send emails from a secure system that protects a doctor’s message from their end, she said.
“There’s this myth out there that you cannot send an electronic message to a patient’s email box if that email is unsecured, and that’s not true,” Ms. Savage said at a recent American Bar Association meeting. “The obligation is to secure what you send, not to secure what an unregulated, private person receives.”
Just remember to warn patients that they’re responsible for the safe storage of an email message once it arrives.
Truth: An email with protected health information (PHI) accidentally sent to the wrong health provider is not likely to get doctors in trouble with the Office for Civil Rights. In the last 12 years, there have been 184,000 HIPAA-related complaints to OCR and only 55 resulted in financial settlements, according to research Ms. Savage conducted through the Department of Health & Human Services website. Of the 55 settlements, none were associated with PHI accidentally sent from one health provider to another, she said in an interview.
“[The OCR] tends to seek fines for really eye-poppingly bad behavior,” Ms. Savage said, not small-scale accidents. For example, OCR fined one hospital for including the name of a patient in a press release without patient permission. Another health professional was fined for repeated failures to encrypt their computer system.
If a document with PHI does end up in the wrong inbox, Ms. Savage advises calling the receiver and asking that they immediately delete the email.
Truth: Breaches alone are not the reason most fines are levied, nor do breach notifications mean an instant penalty, Mr. Fisher said in an interview. Fines by OCR are more often tied to further noncompliance found when the agency begins investigating the entity after the breach report.
“Most breach reports will result in OCR conducting a follow-up investigation, usually with paper-based requests,” he said. “If responses to those requests reveal widespread or consistent noncompliance, then OCR may latch on and dig in order to impose a fine.”
For example, a breach could be the result of a lost USB drive or laptop, but OCR’s investigation might ultimately find that the practice failed to conduct an adequate risk analysis. Because a risk analysis is a fundamental component of HIPAA compliance, the inadequate risk analysis becomes the basis for a fine, Mr. Fisher said.
The best way to avoid an OCR fine is to ensure that proper HIPAA protocols are in place to assess security risks, prevent breaches, and mitigate breaches should they occur. “Part of good compliance is constant review and revision of policies as well,” Mr. Fisher said. “It is not sufficient to put the policies into place and then never revisit those policies. Circumstances change all of the time and policies need to keep up.”
Truth: Health professionals are obligated to provide copies of health information to patients and that includes electronic copies if practices have such technology. The electronic copy requirement was adopted in 2009 as part of the Health Information Technology for Economic and Clinical Health (HITECH) Act.
Despite the electronic amendment’s existence for nearly 10 years, Ms. Savage said she frequently hears from patients about the difficulty of obtaining health information and the extended time and high cost that come with requests.
“[Providing health information to patients] is an obligation,” Ms. Savage stressed. “A 21st century physician might want to be thinking about how to build on that obligation to really engage their patients in a partnership of care. If you give the patient the data, they can actually become a more valuable [participant] with you and engage in self-management.”
More information on HITECH and giving patients access to protected health information can be found here.
Truth: HIPAA is flexible and can adapt to newer technology more easily than many people think, Mr. Fisher says.
“[There is the perception] that HIPAA is archaic and does not fit with modern technology,” he said. “There are a lot of misplaced fears that digital tools cannot satisfy security requirements or will place data where they should not go.”
In actuality, many health care applications enable doctors to satisfy HIPAA requirements, while using updated technology. Secure email to send patients messages is one example, he said, as well as secure text messaging between providers.
At the same time, new technology can often assist health care privacy and advance security, Mr. Fisher noted. Technology solutions frequently automate routine tasks, such as auditing. Tools like machine learning and artificial intelligence can enhance security and catch up with attacker intelligence, he added.
“Technology should be viewed as a means of enhancing and expanding capabilities,” he said. “Using the auditing example, an individual really cannot adequately review all records or access points, but a program may be able to do so and begin to identify small trends that represent a security concern. From this perspective, the technology, as indicated, is about enhancing what can be done.”
The nexus of new technology and privacy rules springing from the Health Insurance Portability and Accountability Act of 1996 (HIPAA) leads to a lot of stress and trepidation for health care professionals. Lucia Savage, chief privacy and regulatory officer for Omada Health, and Matthew Fisher, a health law attorney based in Worcester, Mass., who specializes in compliance issues, dispel common HIPAA myths and offer advice on how to protect yourself and your practice.
Truth: Physicians are not responsible for email security flaws from patient servers, said Ms. Savage, who served as chief privacy officer for the Office of the National Coordinator for Health IT under President Obama. HIPAA requires only that health providers send emails from a secure system that protects a doctor’s message from their end, she said.
“There’s this myth out there that you cannot send an electronic message to a patient’s email box if that email is unsecured, and that’s not true,” Ms. Savage said at a recent American Bar Association meeting. “The obligation is to secure what you send, not to secure what an unregulated, private person receives.”
Just remember to warn patients that they’re responsible for the safe storage of an email message once it arrives.
Truth: An email with protected health information (PHI) accidentally sent to the wrong health provider is not likely to get doctors in trouble with the Office for Civil Rights. In the last 12 years, there have been 184,000 HIPAA-related complaints to OCR and only 55 resulted in financial settlements, according to research Ms. Savage conducted through the Department of Health & Human Services website. Of the 55 settlements, none were associated with PHI accidentally sent from one health provider to another, she said in an interview.
“[The OCR] tends to seek fines for really eye-poppingly bad behavior,” Ms. Savage said, not small-scale accidents. For example, OCR fined one hospital for including the name of a patient in a press release without patient permission. Another health professional was fined for repeated failures to encrypt their computer system.
If a document with PHI does end up in the wrong inbox, Ms. Savage advises calling the receiver and asking that they immediately delete the email.
Truth: Breaches alone are not the reason most fines are levied, nor do breach notifications mean an instant penalty, Mr. Fisher said in an interview. Fines by OCR are more often tied to further noncompliance found when the agency begins investigating the entity after the breach report.
“Most breach reports will result in OCR conducting a follow-up investigation, usually with paper-based requests,” he said. “If responses to those requests reveal widespread or consistent noncompliance, then OCR may latch on and dig in order to impose a fine.”
For example, a breach could be the result of a lost USB drive or laptop, but OCR’s investigation might ultimately find that the practice failed to conduct an adequate risk analysis. Because a risk analysis is a fundamental component of HIPAA compliance, the inadequate risk analysis becomes the basis for a fine, Mr. Fisher said.
The best way to avoid an OCR fine is to ensure that proper HIPAA protocols are in place to assess security risks, prevent breaches, and mitigate breaches should they occur. “Part of good compliance is constant review and revision of policies as well,” Mr. Fisher said. “It is not sufficient to put the policies into place and then never revisit those policies. Circumstances change all of the time and policies need to keep up.”
Truth: Health professionals are obligated to provide copies of health information to patients and that includes electronic copies if practices have such technology. The electronic copy requirement was adopted in 2009 as part of the Health Information Technology for Economic and Clinical Health (HITECH) Act.
Despite the electronic amendment’s existence for nearly 10 years, Ms. Savage said she frequently hears from patients about the difficulty of obtaining health information and the extended time and high cost that come with requests.
“[Providing health information to patients] is an obligation,” Ms. Savage stressed. “A 21st century physician might want to be thinking about how to build on that obligation to really engage their patients in a partnership of care. If you give the patient the data, they can actually become a more valuable [participant] with you and engage in self-management.”
More information on HITECH and giving patients access to protected health information can be found here.
Truth: HIPAA is flexible and can adapt to newer technology more easily than many people think, Mr. Fisher says.
“[There is the perception] that HIPAA is archaic and does not fit with modern technology,” he said. “There are a lot of misplaced fears that digital tools cannot satisfy security requirements or will place data where they should not go.”
In actuality, many health care applications enable doctors to satisfy HIPAA requirements, while using updated technology. Secure email to send patients messages is one example, he said, as well as secure text messaging between providers.
At the same time, new technology can often assist health care privacy and advance security, Mr. Fisher noted. Technology solutions frequently automate routine tasks, such as auditing. Tools like machine learning and artificial intelligence can enhance security and catch up with attacker intelligence, he added.
“Technology should be viewed as a means of enhancing and expanding capabilities,” he said. “Using the auditing example, an individual really cannot adequately review all records or access points, but a program may be able to do so and begin to identify small trends that represent a security concern. From this perspective, the technology, as indicated, is about enhancing what can be done.”
Malpractice reforms reduce invasive cardiac testing
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
FROM JAMA CARDIOLOGY
Key clinical point: Physicians in states with medical malpractice damages caps perform fewer angiographies.
Major finding: In the damages-cap states, doctors ordered 24% fewer angiographies as a first diagnostic test compared with no-cap physicians.
Study details: A study of 36,647 doctors in nine states that have noneconomic damages caps and 39,154 doctors in 20 no-cap states.
Disclosures: No disclosures were reported.
Source: Farmer et al. JAMA Cardiol. 2018 June 6 doi: 10.1001/jamacardio.2018.1360.
Medical associations want withdrawal of Title X changes
Leading medical societies are calling on the Trump administration to withdraw its proposed changes to the federal Title X family planning program, calling the modifications a threat to essential health care for women.
In late May, the Department of Health & Human Services proposed broad changes to Title X, including no longer allowing staff at Title X clinics to counsel, refer, or provide information to women about abortions and mandating that Title X clinics that offer abortions maintain a separate facility for abortion services. The proposed changes aim to “refocus” the Title X program and ensure that all Title X services align with its family planning mission, according to the proposed rule published June 1.
In a July 31 letter to HHS, the American Medical Association requested that HHS withdraw the proposal, citing concerns from the medical community.
“We are very concerned that the proposed changes, if implemented, would undermine patients’ access to high-quality medical care and information, dangerously interfere with the patient-physician relationship and conflict with physicians’ ethical obligations, exclude qualified providers, and jeopardize public health,” James L. Madara, MD, chief executive officer and vice president of the AMA, wrote in a letter. “We urge HHS to withdraw this [proposal].”
The American College of Obstetricians and Gynecologists, the American College of Physicians, the American Academy of Pediatrics, the American Psychiatric Association, and 13 other health care associations also have called on the HHS to rescind its proposed rule. According to a statement from these associations, the proposal endangers women’s lives by restricting access to medically accurate information and preventive health care.
Title X is a long-standing federal program that provides funding for women’s health care and comprehensive family planning services, primarily to low-income and uninsured patients. Federal law prohibits the use of Title X funds to pay for abortions.
Under the proposed regulations, the Trump administration would define “family planning” as the voluntary process of identifying goals and developing a plan for the number and spacing of children and the means by which those goals may be achieved. This includes planning methods and services “to limit or enhance the likelihood of conception, including contraceptive methods and natural family planning or other fertility awareness-based methods,” according to the proposal. HHS specifies that family planning does not include postconception care, obstetric or prenatal care, or abortion as a method of family planning. HHS has proposed that, if a woman comes to a Title X–funded clinic and is pregnant, she be referred externally for pregnancy services. However, the proposed rule would no longer allow Title X programs to provide abortion counseling and/or referral.
According to HHS, requiring separate facilities for abortion-related care would ensure that Title X funds are used for the purposes expressly mandated by Congress – to offer family planning methods and services – and that any infrastructure built with Title X funds would not be used for impermissible purposes.
More than 100,000 comments have been submitted on the proposed rule since June. Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support for the proposed rule.
“The American people have repeatedly expressed their predominant policy preferences by supporting Congressional enactments designed to distinguish and separate abortion from family planning,” SBA List President Marjorie Dannenfelser wrote in a comment. “Abortion is not health care, nor is abortion family planning. The Clinton administration and subsequent presidential administrations have erroneously allowed the blatant distribution of Title X funding to abortion centers and abortion-referral facilities for years and in direct violation of the original purpose of Title X funding.”
A group of 14 state governors, meanwhile, has threatened legal action if the Trump administration moves forward with finalizing its rule. In a May 31 letter, the 14 Democratic governors urged HHS to halt its changes to the Title X program and said they would explore all options, including legal avenues, to protect patients’ access to care. More recently, Democratic governors in Washington, Oregon, Hawaii, and New York have said they will refuse all Title X funding if the Trump administration does not rescind its proposed changes to the program.
“This is not an issue about life or choices. This is an issue about the rights of millions of individuals who deserve the best health care available,” Hawaii governor David Ige said in a July 30 statement. “Hawaii will not accept federal funds for these programs if the proposed rules are implemented.”
Public comment on the proposed rule closed on July 31.
Leading medical societies are calling on the Trump administration to withdraw its proposed changes to the federal Title X family planning program, calling the modifications a threat to essential health care for women.
In late May, the Department of Health & Human Services proposed broad changes to Title X, including no longer allowing staff at Title X clinics to counsel, refer, or provide information to women about abortions and mandating that Title X clinics that offer abortions maintain a separate facility for abortion services. The proposed changes aim to “refocus” the Title X program and ensure that all Title X services align with its family planning mission, according to the proposed rule published June 1.
In a July 31 letter to HHS, the American Medical Association requested that HHS withdraw the proposal, citing concerns from the medical community.
“We are very concerned that the proposed changes, if implemented, would undermine patients’ access to high-quality medical care and information, dangerously interfere with the patient-physician relationship and conflict with physicians’ ethical obligations, exclude qualified providers, and jeopardize public health,” James L. Madara, MD, chief executive officer and vice president of the AMA, wrote in a letter. “We urge HHS to withdraw this [proposal].”
The American College of Obstetricians and Gynecologists, the American College of Physicians, the American Academy of Pediatrics, the American Psychiatric Association, and 13 other health care associations also have called on the HHS to rescind its proposed rule. According to a statement from these associations, the proposal endangers women’s lives by restricting access to medically accurate information and preventive health care.
Title X is a long-standing federal program that provides funding for women’s health care and comprehensive family planning services, primarily to low-income and uninsured patients. Federal law prohibits the use of Title X funds to pay for abortions.
Under the proposed regulations, the Trump administration would define “family planning” as the voluntary process of identifying goals and developing a plan for the number and spacing of children and the means by which those goals may be achieved. This includes planning methods and services “to limit or enhance the likelihood of conception, including contraceptive methods and natural family planning or other fertility awareness-based methods,” according to the proposal. HHS specifies that family planning does not include postconception care, obstetric or prenatal care, or abortion as a method of family planning. HHS has proposed that, if a woman comes to a Title X–funded clinic and is pregnant, she be referred externally for pregnancy services. However, the proposed rule would no longer allow Title X programs to provide abortion counseling and/or referral.
According to HHS, requiring separate facilities for abortion-related care would ensure that Title X funds are used for the purposes expressly mandated by Congress – to offer family planning methods and services – and that any infrastructure built with Title X funds would not be used for impermissible purposes.
More than 100,000 comments have been submitted on the proposed rule since June. Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support for the proposed rule.
“The American people have repeatedly expressed their predominant policy preferences by supporting Congressional enactments designed to distinguish and separate abortion from family planning,” SBA List President Marjorie Dannenfelser wrote in a comment. “Abortion is not health care, nor is abortion family planning. The Clinton administration and subsequent presidential administrations have erroneously allowed the blatant distribution of Title X funding to abortion centers and abortion-referral facilities for years and in direct violation of the original purpose of Title X funding.”
A group of 14 state governors, meanwhile, has threatened legal action if the Trump administration moves forward with finalizing its rule. In a May 31 letter, the 14 Democratic governors urged HHS to halt its changes to the Title X program and said they would explore all options, including legal avenues, to protect patients’ access to care. More recently, Democratic governors in Washington, Oregon, Hawaii, and New York have said they will refuse all Title X funding if the Trump administration does not rescind its proposed changes to the program.
“This is not an issue about life or choices. This is an issue about the rights of millions of individuals who deserve the best health care available,” Hawaii governor David Ige said in a July 30 statement. “Hawaii will not accept federal funds for these programs if the proposed rules are implemented.”
Public comment on the proposed rule closed on July 31.
Leading medical societies are calling on the Trump administration to withdraw its proposed changes to the federal Title X family planning program, calling the modifications a threat to essential health care for women.
In late May, the Department of Health & Human Services proposed broad changes to Title X, including no longer allowing staff at Title X clinics to counsel, refer, or provide information to women about abortions and mandating that Title X clinics that offer abortions maintain a separate facility for abortion services. The proposed changes aim to “refocus” the Title X program and ensure that all Title X services align with its family planning mission, according to the proposed rule published June 1.
In a July 31 letter to HHS, the American Medical Association requested that HHS withdraw the proposal, citing concerns from the medical community.
“We are very concerned that the proposed changes, if implemented, would undermine patients’ access to high-quality medical care and information, dangerously interfere with the patient-physician relationship and conflict with physicians’ ethical obligations, exclude qualified providers, and jeopardize public health,” James L. Madara, MD, chief executive officer and vice president of the AMA, wrote in a letter. “We urge HHS to withdraw this [proposal].”
The American College of Obstetricians and Gynecologists, the American College of Physicians, the American Academy of Pediatrics, the American Psychiatric Association, and 13 other health care associations also have called on the HHS to rescind its proposed rule. According to a statement from these associations, the proposal endangers women’s lives by restricting access to medically accurate information and preventive health care.
Title X is a long-standing federal program that provides funding for women’s health care and comprehensive family planning services, primarily to low-income and uninsured patients. Federal law prohibits the use of Title X funds to pay for abortions.
Under the proposed regulations, the Trump administration would define “family planning” as the voluntary process of identifying goals and developing a plan for the number and spacing of children and the means by which those goals may be achieved. This includes planning methods and services “to limit or enhance the likelihood of conception, including contraceptive methods and natural family planning or other fertility awareness-based methods,” according to the proposal. HHS specifies that family planning does not include postconception care, obstetric or prenatal care, or abortion as a method of family planning. HHS has proposed that, if a woman comes to a Title X–funded clinic and is pregnant, she be referred externally for pregnancy services. However, the proposed rule would no longer allow Title X programs to provide abortion counseling and/or referral.
According to HHS, requiring separate facilities for abortion-related care would ensure that Title X funds are used for the purposes expressly mandated by Congress – to offer family planning methods and services – and that any infrastructure built with Title X funds would not be used for impermissible purposes.
More than 100,000 comments have been submitted on the proposed rule since June. Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support for the proposed rule.
“The American people have repeatedly expressed their predominant policy preferences by supporting Congressional enactments designed to distinguish and separate abortion from family planning,” SBA List President Marjorie Dannenfelser wrote in a comment. “Abortion is not health care, nor is abortion family planning. The Clinton administration and subsequent presidential administrations have erroneously allowed the blatant distribution of Title X funding to abortion centers and abortion-referral facilities for years and in direct violation of the original purpose of Title X funding.”
A group of 14 state governors, meanwhile, has threatened legal action if the Trump administration moves forward with finalizing its rule. In a May 31 letter, the 14 Democratic governors urged HHS to halt its changes to the Title X program and said they would explore all options, including legal avenues, to protect patients’ access to care. More recently, Democratic governors in Washington, Oregon, Hawaii, and New York have said they will refuse all Title X funding if the Trump administration does not rescind its proposed changes to the program.
“This is not an issue about life or choices. This is an issue about the rights of millions of individuals who deserve the best health care available,” Hawaii governor David Ige said in a July 30 statement. “Hawaii will not accept federal funds for these programs if the proposed rules are implemented.”
Public comment on the proposed rule closed on July 31.
Physicians give Medicare QPP proposals mixed reviews
Physician associations are expressing mixed feelings about dramatic changes proposed for Year 3 of the Quality Payment Program (QPP) by the Centers for Medicare & Medicaid Services.
Under its 2019 QPP proposal, CMS plans to remove 34 process-based measures within the program considered to be low value or low priority so that providers can focus more on meaningful measures that affect health outcomes, according to the agency. CMS also calls for the streamlining of the “interoperability performance category” within the Merit-Based Incentive Payment System (MIPS) to create a more simplified scoring methodology.
In regard to alternative payment models (APMs), CMS seeks to update the Advanced APM Certified EHR Technology (CEHRT) threshold so that an Advanced APM must require at least 75% of eligible clinicians in each APM entity to use CEHRT. The agency also plans to extend the 8% revenue-based nominal amount standard for Advanced APMs through performance year 2024.
Ana Maria Lopez, MD, president for the American College of Physicians said the they were pleased that CMS proposed the removal of a number of quality measures deemed to be of low value. “ACP commends CMS for taking major steps to reduce unnecessary administrative tasks that are detracting from the patient-physician relationship,” Dr. Lopez said in a statement.
But ACP officials also noted that they were disappointed that CMS did not heed calls to streamline MIPS requirements and scoring, establish a minimum 90-day reporting period for all performance categories, or reduce the total number of quality measures.
ACP supports CMS’ promoting of interoperability by requiring CEHRT, but it urged the agency to allow at least 6 months for vendors and physicians to implement system upgrades in order to ensure a smooth transition and avoid disruptions to patient care. Additionally, CMS needs to “think about ways to mitigate costs associated with implementation, especially for small practices,” Dr. Lopez said in the statement.
In an interview, Michael L. Munger, MD, president for the American Academy of Family Physicians, said that his group is reviewing the proposed QPP regulations and plans to offer its full perspective during the comment period.
“Our initial assessment indicates CMS continues progress to simplify and modernize in documentation requirements for evaluation and management office visits,” Dr. Munger said. “Reforming the U.S. health care system is an ongoing process, and the AAFP looks forward to working with CMS to ensure continued progress.”
As part of the proposed QPP changes, CMS for the first time is proposing to allow physicians to opt-in to the MIPS program if they are prepared to meet the program’s requirements. The proposal also adds a new exemption to MIPS participation: Doctors who perform 200 or fewer services under the Medicare fee schedule. The proposal retains its previous exemption thresholds for participating in MIPS – physicians who bill Medicare $90,000 or less annually and see 200 or fewer Medicare patients are excused from the program.
CMS also proposes to add new episode-based measures to the cost performance category and to create an option to use facility-based quality and cost performance measures for certain facility-based clinicians. The QPP proposal includes a number of changes for small medical practices, which include the following:
- Continuing the small practice bonus, but including it in the quality performance category score of clinicians in small practices instead of as a standalone bonus.
- Awarding small practices three points for quality measures that don’t meet the data completeness requirements.
- Consolidating the low-volume threshold determination periods with the determination period for identifying a small practice.
David Daikh, MD, president for the American College of Rheumatology, said the ACR appreciates CMS’ emphasis on supporting the development of alternative payment models (APMs) and is encouraged by the agency’s proposal to allow more physicians to participate.
“However, we are concerned that eliminating the MIPS small practice bonus as a stand-alone bonus and instead folding it into the quality performance score would dilute the bonus and hurt small and rural providers,” Dr. Daikh said in a statement. “The ACR strongly supports maintaining the small practice bonus as a five-point stand-alone bonus that is added to the final score.”
Meanwhile, Jerry Penso, MD, president and CEO for the American Medical Group Association expressed disappointment that CMS did not lower its exclusion threshold for MIPS. Through the Bipartisan Budget Act of 2018, CMS plans to continue “the gradual implementation of certain MIPS requirements to ease administrative burden on clinicians,” according to a CMS fact sheet. This includes flexible performance thresholds until the fifth year of the QPP. The agency previously required the MIPS cost performance category to have a weight of 30% in Year 3 of the program (performance period 2019); however, the weight is now required to be no less than 10% and no more than 30% for the third, fourth and fifth years of the QPP.
Dr. Penso noted that as authorized by the Medicare Access and CHIP Reauthorization Act (MACRA), providers were given the opportunity to earn an adjustment of up to 7% on their Medicare Part B payments in 2021 based on their 2019 performance.
“However, as indicated in [the] proposal, CMS estimates the overall payment adjustment will be 2%,” Dr. Penso said in a statement. “We are concerned that CMS has again opted not to recognize the efforts of high-performing AMGA members. As we enter the program’s third year, it is time for CMS to honor congressional intent and use MIPS to create value for Medicare.”
Some specialty associations, including the American Academy of Dermatology, said they are still reviewing the proposed policies and could not comment on the changes at this time.
“The American Academy of Dermatology is still looking at the proposed rule and the implications it may have on board-certified dermatologists and their patients,” an association spokesperson said in an interview.
Comments on the proposed rule will be accepted at www.regulations.gov until Sept. 10.
Physician associations are expressing mixed feelings about dramatic changes proposed for Year 3 of the Quality Payment Program (QPP) by the Centers for Medicare & Medicaid Services.
Under its 2019 QPP proposal, CMS plans to remove 34 process-based measures within the program considered to be low value or low priority so that providers can focus more on meaningful measures that affect health outcomes, according to the agency. CMS also calls for the streamlining of the “interoperability performance category” within the Merit-Based Incentive Payment System (MIPS) to create a more simplified scoring methodology.
In regard to alternative payment models (APMs), CMS seeks to update the Advanced APM Certified EHR Technology (CEHRT) threshold so that an Advanced APM must require at least 75% of eligible clinicians in each APM entity to use CEHRT. The agency also plans to extend the 8% revenue-based nominal amount standard for Advanced APMs through performance year 2024.
Ana Maria Lopez, MD, president for the American College of Physicians said the they were pleased that CMS proposed the removal of a number of quality measures deemed to be of low value. “ACP commends CMS for taking major steps to reduce unnecessary administrative tasks that are detracting from the patient-physician relationship,” Dr. Lopez said in a statement.
But ACP officials also noted that they were disappointed that CMS did not heed calls to streamline MIPS requirements and scoring, establish a minimum 90-day reporting period for all performance categories, or reduce the total number of quality measures.
ACP supports CMS’ promoting of interoperability by requiring CEHRT, but it urged the agency to allow at least 6 months for vendors and physicians to implement system upgrades in order to ensure a smooth transition and avoid disruptions to patient care. Additionally, CMS needs to “think about ways to mitigate costs associated with implementation, especially for small practices,” Dr. Lopez said in the statement.
In an interview, Michael L. Munger, MD, president for the American Academy of Family Physicians, said that his group is reviewing the proposed QPP regulations and plans to offer its full perspective during the comment period.
“Our initial assessment indicates CMS continues progress to simplify and modernize in documentation requirements for evaluation and management office visits,” Dr. Munger said. “Reforming the U.S. health care system is an ongoing process, and the AAFP looks forward to working with CMS to ensure continued progress.”
As part of the proposed QPP changes, CMS for the first time is proposing to allow physicians to opt-in to the MIPS program if they are prepared to meet the program’s requirements. The proposal also adds a new exemption to MIPS participation: Doctors who perform 200 or fewer services under the Medicare fee schedule. The proposal retains its previous exemption thresholds for participating in MIPS – physicians who bill Medicare $90,000 or less annually and see 200 or fewer Medicare patients are excused from the program.
CMS also proposes to add new episode-based measures to the cost performance category and to create an option to use facility-based quality and cost performance measures for certain facility-based clinicians. The QPP proposal includes a number of changes for small medical practices, which include the following:
- Continuing the small practice bonus, but including it in the quality performance category score of clinicians in small practices instead of as a standalone bonus.
- Awarding small practices three points for quality measures that don’t meet the data completeness requirements.
- Consolidating the low-volume threshold determination periods with the determination period for identifying a small practice.
David Daikh, MD, president for the American College of Rheumatology, said the ACR appreciates CMS’ emphasis on supporting the development of alternative payment models (APMs) and is encouraged by the agency’s proposal to allow more physicians to participate.
“However, we are concerned that eliminating the MIPS small practice bonus as a stand-alone bonus and instead folding it into the quality performance score would dilute the bonus and hurt small and rural providers,” Dr. Daikh said in a statement. “The ACR strongly supports maintaining the small practice bonus as a five-point stand-alone bonus that is added to the final score.”
Meanwhile, Jerry Penso, MD, president and CEO for the American Medical Group Association expressed disappointment that CMS did not lower its exclusion threshold for MIPS. Through the Bipartisan Budget Act of 2018, CMS plans to continue “the gradual implementation of certain MIPS requirements to ease administrative burden on clinicians,” according to a CMS fact sheet. This includes flexible performance thresholds until the fifth year of the QPP. The agency previously required the MIPS cost performance category to have a weight of 30% in Year 3 of the program (performance period 2019); however, the weight is now required to be no less than 10% and no more than 30% for the third, fourth and fifth years of the QPP.
Dr. Penso noted that as authorized by the Medicare Access and CHIP Reauthorization Act (MACRA), providers were given the opportunity to earn an adjustment of up to 7% on their Medicare Part B payments in 2021 based on their 2019 performance.
“However, as indicated in [the] proposal, CMS estimates the overall payment adjustment will be 2%,” Dr. Penso said in a statement. “We are concerned that CMS has again opted not to recognize the efforts of high-performing AMGA members. As we enter the program’s third year, it is time for CMS to honor congressional intent and use MIPS to create value for Medicare.”
Some specialty associations, including the American Academy of Dermatology, said they are still reviewing the proposed policies and could not comment on the changes at this time.
“The American Academy of Dermatology is still looking at the proposed rule and the implications it may have on board-certified dermatologists and their patients,” an association spokesperson said in an interview.
Comments on the proposed rule will be accepted at www.regulations.gov until Sept. 10.
Physician associations are expressing mixed feelings about dramatic changes proposed for Year 3 of the Quality Payment Program (QPP) by the Centers for Medicare & Medicaid Services.
Under its 2019 QPP proposal, CMS plans to remove 34 process-based measures within the program considered to be low value or low priority so that providers can focus more on meaningful measures that affect health outcomes, according to the agency. CMS also calls for the streamlining of the “interoperability performance category” within the Merit-Based Incentive Payment System (MIPS) to create a more simplified scoring methodology.
In regard to alternative payment models (APMs), CMS seeks to update the Advanced APM Certified EHR Technology (CEHRT) threshold so that an Advanced APM must require at least 75% of eligible clinicians in each APM entity to use CEHRT. The agency also plans to extend the 8% revenue-based nominal amount standard for Advanced APMs through performance year 2024.
Ana Maria Lopez, MD, president for the American College of Physicians said the they were pleased that CMS proposed the removal of a number of quality measures deemed to be of low value. “ACP commends CMS for taking major steps to reduce unnecessary administrative tasks that are detracting from the patient-physician relationship,” Dr. Lopez said in a statement.
But ACP officials also noted that they were disappointed that CMS did not heed calls to streamline MIPS requirements and scoring, establish a minimum 90-day reporting period for all performance categories, or reduce the total number of quality measures.
ACP supports CMS’ promoting of interoperability by requiring CEHRT, but it urged the agency to allow at least 6 months for vendors and physicians to implement system upgrades in order to ensure a smooth transition and avoid disruptions to patient care. Additionally, CMS needs to “think about ways to mitigate costs associated with implementation, especially for small practices,” Dr. Lopez said in the statement.
In an interview, Michael L. Munger, MD, president for the American Academy of Family Physicians, said that his group is reviewing the proposed QPP regulations and plans to offer its full perspective during the comment period.
“Our initial assessment indicates CMS continues progress to simplify and modernize in documentation requirements for evaluation and management office visits,” Dr. Munger said. “Reforming the U.S. health care system is an ongoing process, and the AAFP looks forward to working with CMS to ensure continued progress.”
As part of the proposed QPP changes, CMS for the first time is proposing to allow physicians to opt-in to the MIPS program if they are prepared to meet the program’s requirements. The proposal also adds a new exemption to MIPS participation: Doctors who perform 200 or fewer services under the Medicare fee schedule. The proposal retains its previous exemption thresholds for participating in MIPS – physicians who bill Medicare $90,000 or less annually and see 200 or fewer Medicare patients are excused from the program.
CMS also proposes to add new episode-based measures to the cost performance category and to create an option to use facility-based quality and cost performance measures for certain facility-based clinicians. The QPP proposal includes a number of changes for small medical practices, which include the following:
- Continuing the small practice bonus, but including it in the quality performance category score of clinicians in small practices instead of as a standalone bonus.
- Awarding small practices three points for quality measures that don’t meet the data completeness requirements.
- Consolidating the low-volume threshold determination periods with the determination period for identifying a small practice.
David Daikh, MD, president for the American College of Rheumatology, said the ACR appreciates CMS’ emphasis on supporting the development of alternative payment models (APMs) and is encouraged by the agency’s proposal to allow more physicians to participate.
“However, we are concerned that eliminating the MIPS small practice bonus as a stand-alone bonus and instead folding it into the quality performance score would dilute the bonus and hurt small and rural providers,” Dr. Daikh said in a statement. “The ACR strongly supports maintaining the small practice bonus as a five-point stand-alone bonus that is added to the final score.”
Meanwhile, Jerry Penso, MD, president and CEO for the American Medical Group Association expressed disappointment that CMS did not lower its exclusion threshold for MIPS. Through the Bipartisan Budget Act of 2018, CMS plans to continue “the gradual implementation of certain MIPS requirements to ease administrative burden on clinicians,” according to a CMS fact sheet. This includes flexible performance thresholds until the fifth year of the QPP. The agency previously required the MIPS cost performance category to have a weight of 30% in Year 3 of the program (performance period 2019); however, the weight is now required to be no less than 10% and no more than 30% for the third, fourth and fifth years of the QPP.
Dr. Penso noted that as authorized by the Medicare Access and CHIP Reauthorization Act (MACRA), providers were given the opportunity to earn an adjustment of up to 7% on their Medicare Part B payments in 2021 based on their 2019 performance.
“However, as indicated in [the] proposal, CMS estimates the overall payment adjustment will be 2%,” Dr. Penso said in a statement. “We are concerned that CMS has again opted not to recognize the efforts of high-performing AMGA members. As we enter the program’s third year, it is time for CMS to honor congressional intent and use MIPS to create value for Medicare.”
Some specialty associations, including the American Academy of Dermatology, said they are still reviewing the proposed policies and could not comment on the changes at this time.
“The American Academy of Dermatology is still looking at the proposed rule and the implications it may have on board-certified dermatologists and their patients,” an association spokesperson said in an interview.
Comments on the proposed rule will be accepted at www.regulations.gov until Sept. 10.
CMS considers expanding telemedicine payments
Payment for more telemedicine services could be in store for physicians and other health providers if new proposals in the latest fee schedule from the Centers for Medicare & Medicaid Services are finalized.
Under the proposed physician fee schedule, announced July 12, the CMS would expand services that qualify for telemedicine payments and add reimbursement for virtual check-ins by phone or other technologies, such as Skype. Telemedicine clinicians would also be paid for time spent reviewing patient photos sent by text or e-mail under the suggested changes.
Such telehealth services would aid patients who have transportation difficulties by creating more opportunities for them to access personalized care, said CMS Administrator Seema Verma.
“CMS is committed to modernizing the Medicare program by leveraging technologies, such as audio/video applications or patient-facing health portals, that will help beneficiaries access high-quality services in a convenient manner,” Ms. Verma said in a statement.
Under the proposal, physicians could bill separately for brief, non–face-to-face patient check-ins with patients via communication technology beginning January 2019. In addition, the proposed rule carves out payments for the remote professional evaluation of patient-transmitted information conducted via prerecorded “store and forward” video or image technology. Doctors could use both services to determine whether an office visit or other service is warranted, according to the proposed rule.
The services would have limitations on when they could be separately billed. In cases where the brief communication technology–based service originated from a related evaluation and management (E/M) service provided within the previous 7 days by the same physician or other qualified health care professional, the service would be considered “bundled” into that previous E/M service and could not be billed separately. Similarly, a photo evaluation could not be separately billed if it stemmed from a related E/M service provided within the previous 7 days by the same physician, or if the evaluation results in an in-person E/M office visit with the same doctor.
Under the proposal, health providers could perform the newly covered telehealth services only with established patients, but the CMS is seeking comments as to whether in certain cases, such as dermatological or ophthalmological instances, it might be appropriate for a new patient to receive the services. Agency officials also want to know what types of communication technology are used by physicians in furnishing check-in services, including whether audio-only telephone interactions are sufficient, compared with interactions that are enhanced with video. The CMS is asking physicians whether it would be clinically appropriate to apply a frequency limitation on the use of the proposed telehealth services by the same physician.
Latoya Thomas, director of the American Telemedicine Association’s State Policy Resource Center, said the proposal is exciting because it acknowledges the pervasive growth, accessibility, and acceptance of technology advances.
“In expanding reimbursement to providers for more modality-neutral and site-neutral virtual care, such as store-and-forward and remote patient monitoring, [the rules] address longstanding barriers to broader dissemination of telehealth,” Ms. Thomas said in an interview. “By making available ‘virtual check ins’ to every Medicare beneficiary, it can improve patient engagement and reduce unnecessary trips back to their provider’s office.”
James P. Marcin, MD, a telemedicine physician and director of the Center for Health and Technology at UC Davis Children’s Hospital in Sacramento, Calif., said he was pleased with the proposed telehealth changes, but he noted that more work remains to address further telemedicine challenges.
“The needle is finally moving, albeit too slowly for some of us,” Dr. Marcin said in an interview. “There are still some areas users of telemedicine and organizations supporting the use of telemedicine want to address, including the need for verbal informed consent, the requirements for established relationships with patients, and of course, rate valuations for the remote patient monitoring and professional codes. But again, this is good news for patients.”
Public comments on the proposed rule are due by Sept. 10, 2018. Comments can be submitted to regulations.gov.
Payment for more telemedicine services could be in store for physicians and other health providers if new proposals in the latest fee schedule from the Centers for Medicare & Medicaid Services are finalized.
Under the proposed physician fee schedule, announced July 12, the CMS would expand services that qualify for telemedicine payments and add reimbursement for virtual check-ins by phone or other technologies, such as Skype. Telemedicine clinicians would also be paid for time spent reviewing patient photos sent by text or e-mail under the suggested changes.
Such telehealth services would aid patients who have transportation difficulties by creating more opportunities for them to access personalized care, said CMS Administrator Seema Verma.
“CMS is committed to modernizing the Medicare program by leveraging technologies, such as audio/video applications or patient-facing health portals, that will help beneficiaries access high-quality services in a convenient manner,” Ms. Verma said in a statement.
Under the proposal, physicians could bill separately for brief, non–face-to-face patient check-ins with patients via communication technology beginning January 2019. In addition, the proposed rule carves out payments for the remote professional evaluation of patient-transmitted information conducted via prerecorded “store and forward” video or image technology. Doctors could use both services to determine whether an office visit or other service is warranted, according to the proposed rule.
The services would have limitations on when they could be separately billed. In cases where the brief communication technology–based service originated from a related evaluation and management (E/M) service provided within the previous 7 days by the same physician or other qualified health care professional, the service would be considered “bundled” into that previous E/M service and could not be billed separately. Similarly, a photo evaluation could not be separately billed if it stemmed from a related E/M service provided within the previous 7 days by the same physician, or if the evaluation results in an in-person E/M office visit with the same doctor.
Under the proposal, health providers could perform the newly covered telehealth services only with established patients, but the CMS is seeking comments as to whether in certain cases, such as dermatological or ophthalmological instances, it might be appropriate for a new patient to receive the services. Agency officials also want to know what types of communication technology are used by physicians in furnishing check-in services, including whether audio-only telephone interactions are sufficient, compared with interactions that are enhanced with video. The CMS is asking physicians whether it would be clinically appropriate to apply a frequency limitation on the use of the proposed telehealth services by the same physician.
Latoya Thomas, director of the American Telemedicine Association’s State Policy Resource Center, said the proposal is exciting because it acknowledges the pervasive growth, accessibility, and acceptance of technology advances.
“In expanding reimbursement to providers for more modality-neutral and site-neutral virtual care, such as store-and-forward and remote patient monitoring, [the rules] address longstanding barriers to broader dissemination of telehealth,” Ms. Thomas said in an interview. “By making available ‘virtual check ins’ to every Medicare beneficiary, it can improve patient engagement and reduce unnecessary trips back to their provider’s office.”
James P. Marcin, MD, a telemedicine physician and director of the Center for Health and Technology at UC Davis Children’s Hospital in Sacramento, Calif., said he was pleased with the proposed telehealth changes, but he noted that more work remains to address further telemedicine challenges.
“The needle is finally moving, albeit too slowly for some of us,” Dr. Marcin said in an interview. “There are still some areas users of telemedicine and organizations supporting the use of telemedicine want to address, including the need for verbal informed consent, the requirements for established relationships with patients, and of course, rate valuations for the remote patient monitoring and professional codes. But again, this is good news for patients.”
Public comments on the proposed rule are due by Sept. 10, 2018. Comments can be submitted to regulations.gov.
Payment for more telemedicine services could be in store for physicians and other health providers if new proposals in the latest fee schedule from the Centers for Medicare & Medicaid Services are finalized.
Under the proposed physician fee schedule, announced July 12, the CMS would expand services that qualify for telemedicine payments and add reimbursement for virtual check-ins by phone or other technologies, such as Skype. Telemedicine clinicians would also be paid for time spent reviewing patient photos sent by text or e-mail under the suggested changes.
Such telehealth services would aid patients who have transportation difficulties by creating more opportunities for them to access personalized care, said CMS Administrator Seema Verma.
“CMS is committed to modernizing the Medicare program by leveraging technologies, such as audio/video applications or patient-facing health portals, that will help beneficiaries access high-quality services in a convenient manner,” Ms. Verma said in a statement.
Under the proposal, physicians could bill separately for brief, non–face-to-face patient check-ins with patients via communication technology beginning January 2019. In addition, the proposed rule carves out payments for the remote professional evaluation of patient-transmitted information conducted via prerecorded “store and forward” video or image technology. Doctors could use both services to determine whether an office visit or other service is warranted, according to the proposed rule.
The services would have limitations on when they could be separately billed. In cases where the brief communication technology–based service originated from a related evaluation and management (E/M) service provided within the previous 7 days by the same physician or other qualified health care professional, the service would be considered “bundled” into that previous E/M service and could not be billed separately. Similarly, a photo evaluation could not be separately billed if it stemmed from a related E/M service provided within the previous 7 days by the same physician, or if the evaluation results in an in-person E/M office visit with the same doctor.
Under the proposal, health providers could perform the newly covered telehealth services only with established patients, but the CMS is seeking comments as to whether in certain cases, such as dermatological or ophthalmological instances, it might be appropriate for a new patient to receive the services. Agency officials also want to know what types of communication technology are used by physicians in furnishing check-in services, including whether audio-only telephone interactions are sufficient, compared with interactions that are enhanced with video. The CMS is asking physicians whether it would be clinically appropriate to apply a frequency limitation on the use of the proposed telehealth services by the same physician.
Latoya Thomas, director of the American Telemedicine Association’s State Policy Resource Center, said the proposal is exciting because it acknowledges the pervasive growth, accessibility, and acceptance of technology advances.
“In expanding reimbursement to providers for more modality-neutral and site-neutral virtual care, such as store-and-forward and remote patient monitoring, [the rules] address longstanding barriers to broader dissemination of telehealth,” Ms. Thomas said in an interview. “By making available ‘virtual check ins’ to every Medicare beneficiary, it can improve patient engagement and reduce unnecessary trips back to their provider’s office.”
James P. Marcin, MD, a telemedicine physician and director of the Center for Health and Technology at UC Davis Children’s Hospital in Sacramento, Calif., said he was pleased with the proposed telehealth changes, but he noted that more work remains to address further telemedicine challenges.
“The needle is finally moving, albeit too slowly for some of us,” Dr. Marcin said in an interview. “There are still some areas users of telemedicine and organizations supporting the use of telemedicine want to address, including the need for verbal informed consent, the requirements for established relationships with patients, and of course, rate valuations for the remote patient monitoring and professional codes. But again, this is good news for patients.”
Public comments on the proposed rule are due by Sept. 10, 2018. Comments can be submitted to regulations.gov.
No gender bias found in ABS exam
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
Key clinical point: The gender of examinees and the gender of examiners do not affect ABS exam outcomes.
Major finding: No correlation was found between surgeon gender and CE ratings received.
Study details: Investigators examined data from the 2016-2017 ABS general surgery certifying exam, which consisted of 1,341 examinees and 216 examiners.
Disclosures: The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Source: Ong et al. J Surg Res. 2018 Jun. doi: 10.1016/j.jss.2018.06.014.
Drug price transparency laws gain ground
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Supreme Court supports anti-abortion centers in free speech case
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”