User login
Oral Lichen Planus Treated With Plasma Rich in Growth Factors
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
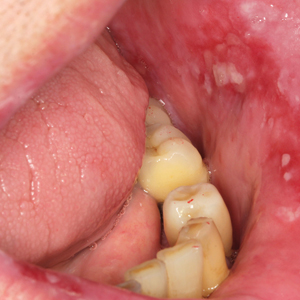
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).
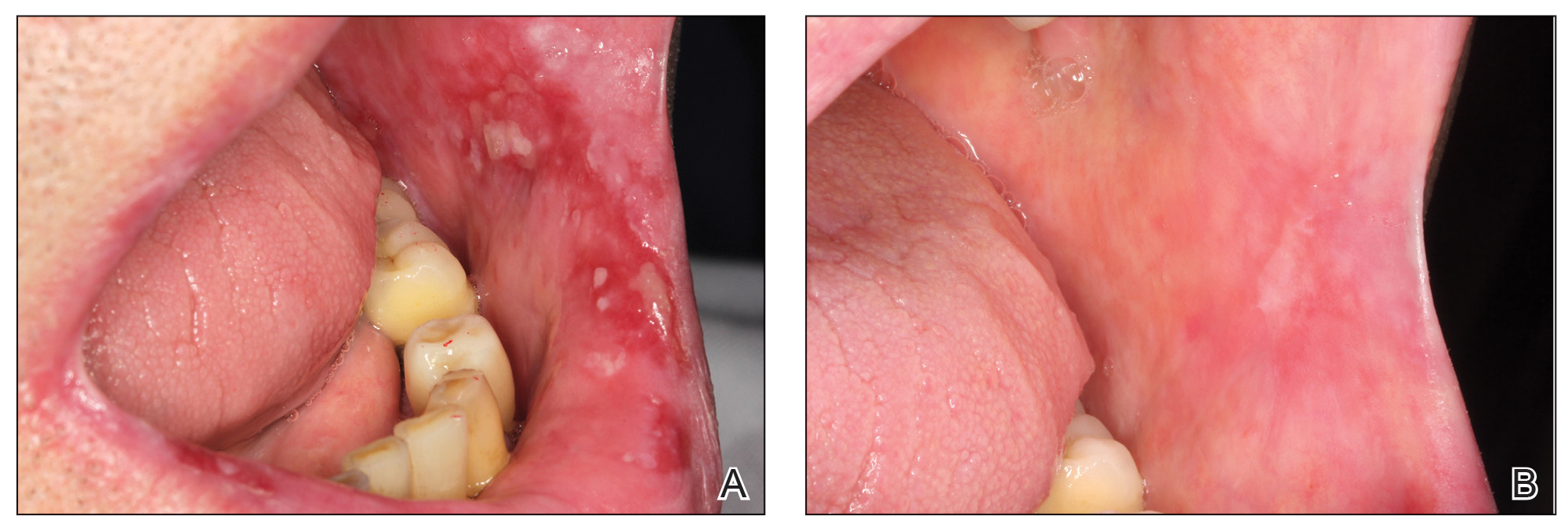
Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).
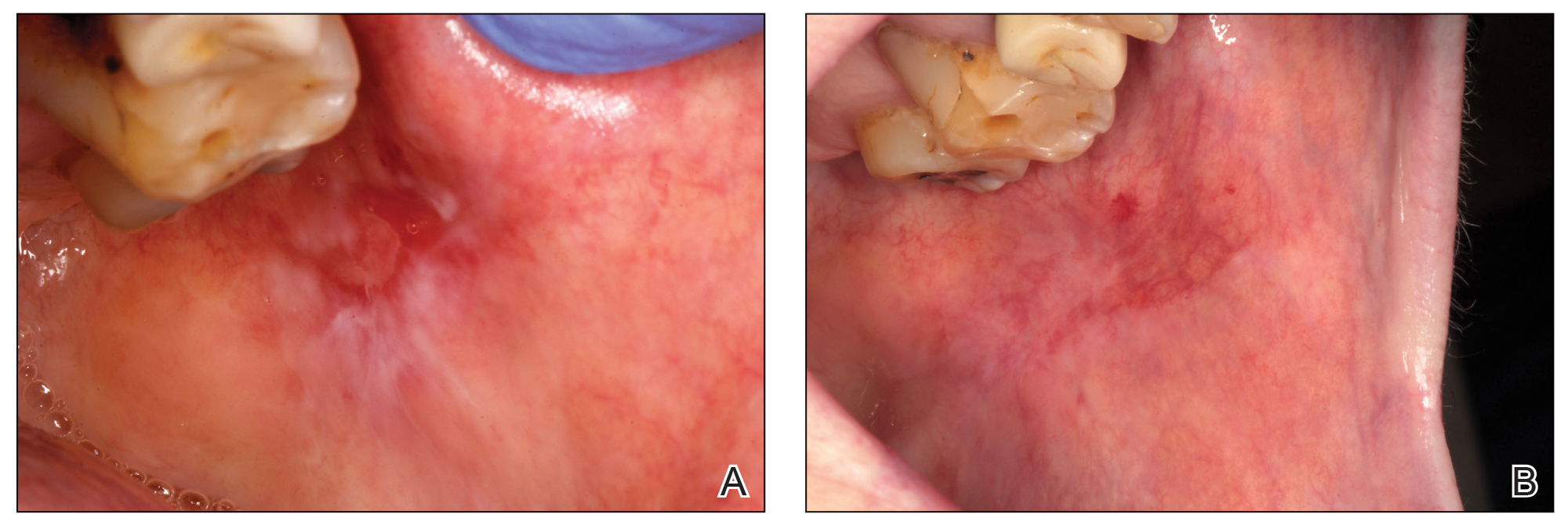
For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).

Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).

For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).

Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).

For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
Practice Points
- Treating erosive oral lichen planus lesions refractory to conventional steroid treatments can be challenging for clinicians.
- Complete re-epithelialization and total pain relief could be observed after 1 to 3 weekly perilesional infiltrations with plasma rich in growth factors.
- No relapse of the lesions in the same area or other complications could be observed during the follow-up time.
Interstitial Granulomatous Dermatitis as an Adverse Reaction to Vedolizumab
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
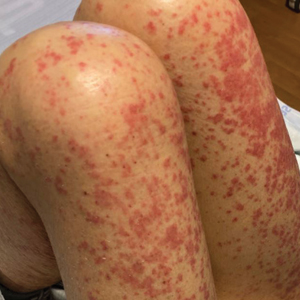
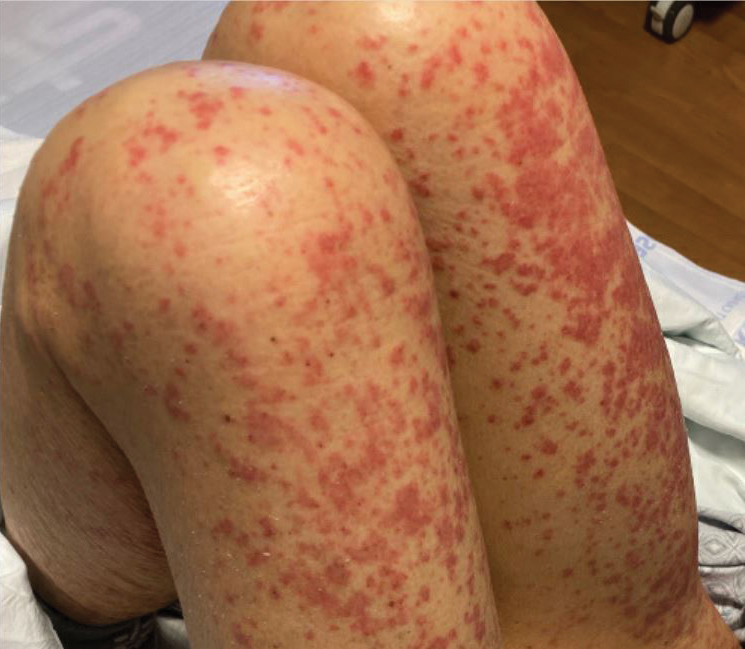
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).
A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
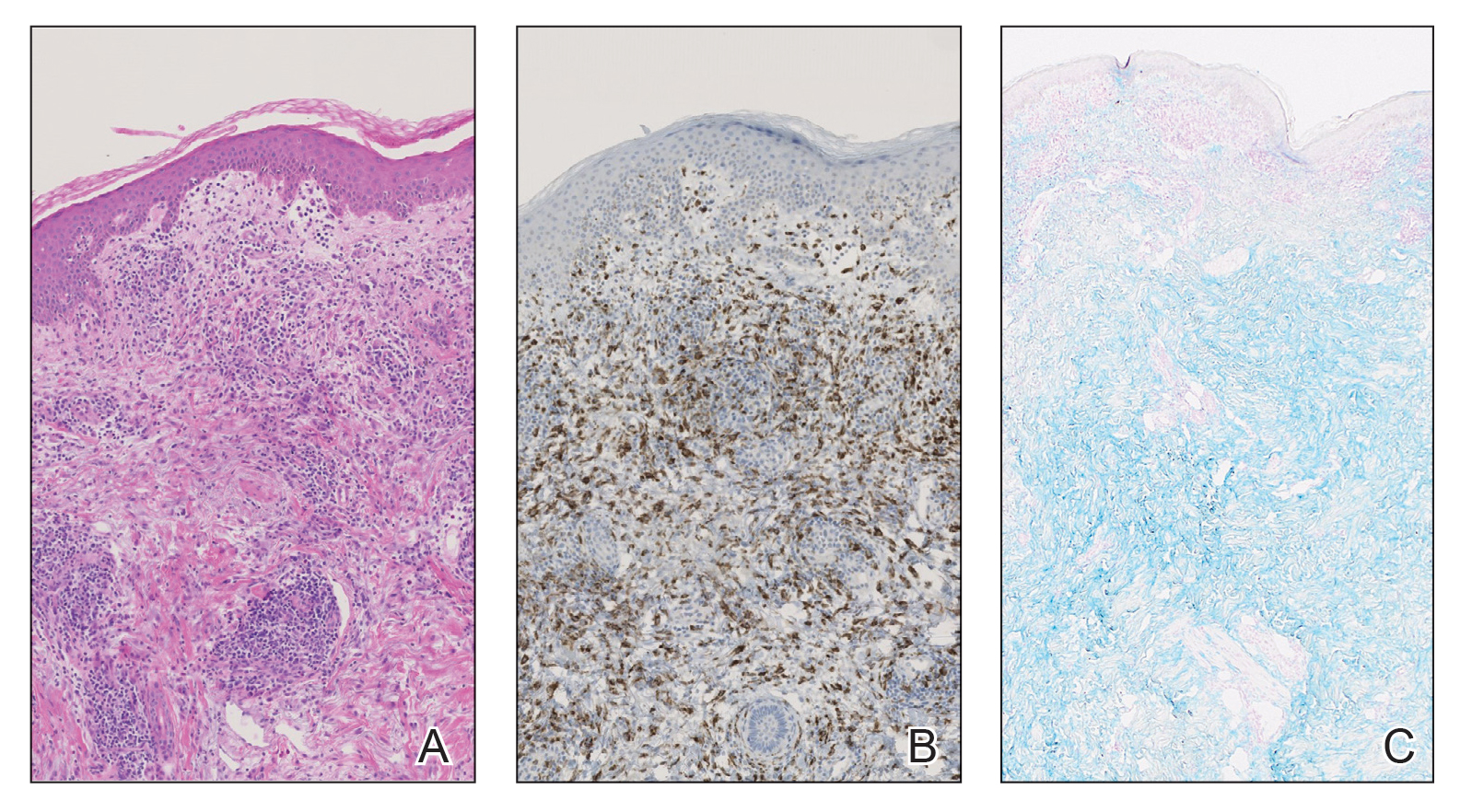
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).
Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).

A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).

Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).

A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).

Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
Practice Points
- Reactive granulomatous dermatitis, interstitial granulomatous dermatitis (IGD) type, can occur as an adverse reaction to vedolizumab despite the minimal adverse effect profile of the medication.
- Evidence of IGD type reactions to monoclonal antibodies is accumulating; this disorder can be considered in the differential diagnosis for patients who develop a new rash when treated with an agent of this therapeutic class.
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Discoid Lupus
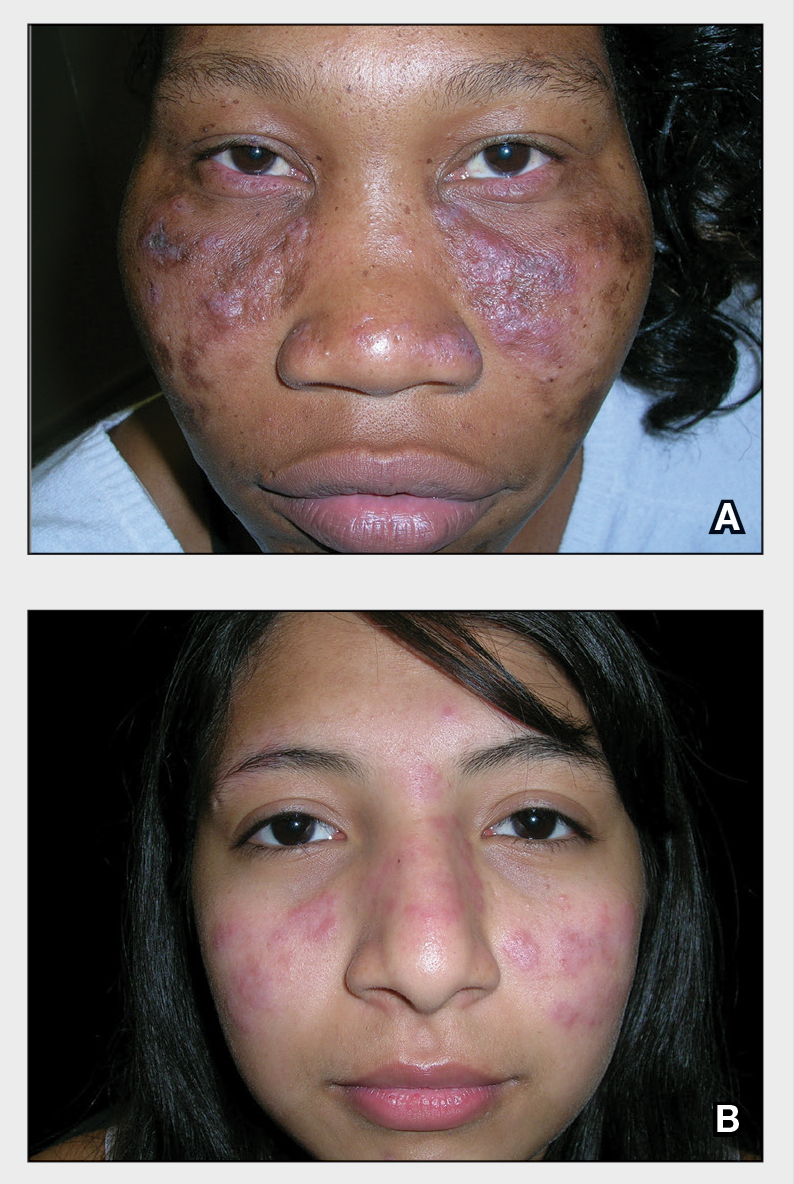
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.

THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8

THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
Unusual tongue markings
Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/

Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/
USPSTF releases updated guidance on asymptomatic A-fib
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
Mycoplasma genitalium: The Smallest Pathogen Becoming a Big Concern
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
HIV Management: Insights Into ART and Weight Gain
Antiretroviral therapy (ART) regimens provide long-lasting suppression of HIV replication and have helped people with HIV live healthier lives for decades.
Today's ART regimens are associated with fewer serious and intolerable adverse effects than those used in the past, but weight gain remains a concern in clinical practice.
In this ReCAP, Dr David Wohl, from the University of North Carolina at Chapel Hill, reports on the relationship between ART and weight gain, as well as the implications of excessive weight gain in HIV management.
He shares data from multiple studies, including the ADVANCE trial, which offer insight on how different HIV therapies affect patient weight.
Dr Wohl also discusses the steps clinicians should take if weight gain does occur in people who are on HIV therapy.
--
Professor of Medicine; Medical Director, UNC COVID-19 Vaccine Clinic, COVID-19 Monoclonal Antibody Infusion Clinic, University of North Carolina at Chapel Hill
David Wohl, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; ViiV; Janssen; Merck
Serve(d) as a speaker or a member of a speakers bureau for: Gilead
Received research grant from: Gilead; Merck; ViiV
Antiretroviral therapy (ART) regimens provide long-lasting suppression of HIV replication and have helped people with HIV live healthier lives for decades.
Today's ART regimens are associated with fewer serious and intolerable adverse effects than those used in the past, but weight gain remains a concern in clinical practice.
In this ReCAP, Dr David Wohl, from the University of North Carolina at Chapel Hill, reports on the relationship between ART and weight gain, as well as the implications of excessive weight gain in HIV management.
He shares data from multiple studies, including the ADVANCE trial, which offer insight on how different HIV therapies affect patient weight.
Dr Wohl also discusses the steps clinicians should take if weight gain does occur in people who are on HIV therapy.
--
Professor of Medicine; Medical Director, UNC COVID-19 Vaccine Clinic, COVID-19 Monoclonal Antibody Infusion Clinic, University of North Carolina at Chapel Hill
David Wohl, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; ViiV; Janssen; Merck
Serve(d) as a speaker or a member of a speakers bureau for: Gilead
Received research grant from: Gilead; Merck; ViiV
Antiretroviral therapy (ART) regimens provide long-lasting suppression of HIV replication and have helped people with HIV live healthier lives for decades.
Today's ART regimens are associated with fewer serious and intolerable adverse effects than those used in the past, but weight gain remains a concern in clinical practice.
In this ReCAP, Dr David Wohl, from the University of North Carolina at Chapel Hill, reports on the relationship between ART and weight gain, as well as the implications of excessive weight gain in HIV management.
He shares data from multiple studies, including the ADVANCE trial, which offer insight on how different HIV therapies affect patient weight.
Dr Wohl also discusses the steps clinicians should take if weight gain does occur in people who are on HIV therapy.
--
Professor of Medicine; Medical Director, UNC COVID-19 Vaccine Clinic, COVID-19 Monoclonal Antibody Infusion Clinic, University of North Carolina at Chapel Hill
David Wohl, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; ViiV; Janssen; Merck
Serve(d) as a speaker or a member of a speakers bureau for: Gilead
Received research grant from: Gilead; Merck; ViiV
Psychoses: The 5 comorbidity-defined subtypes
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.