User login
Optimal psychiatric treatment: Target the brain and avoid the body
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.
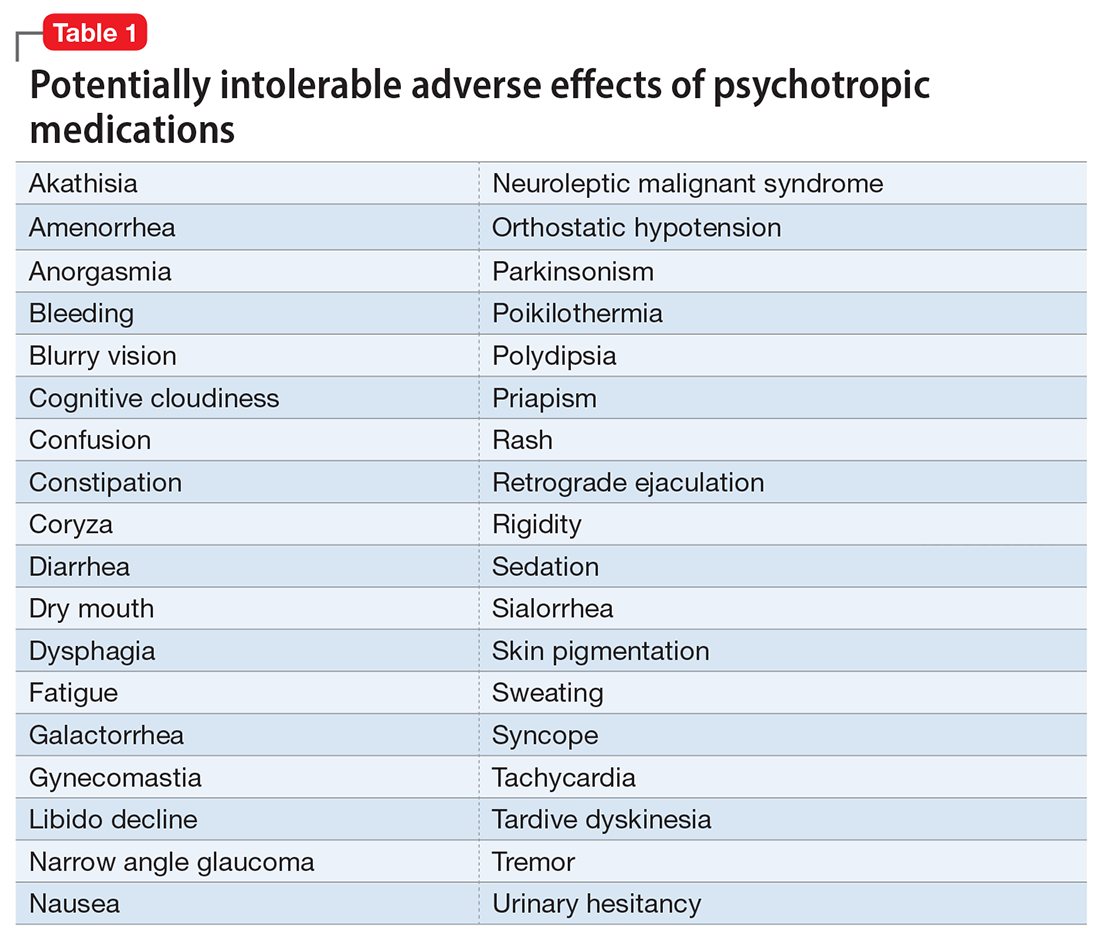
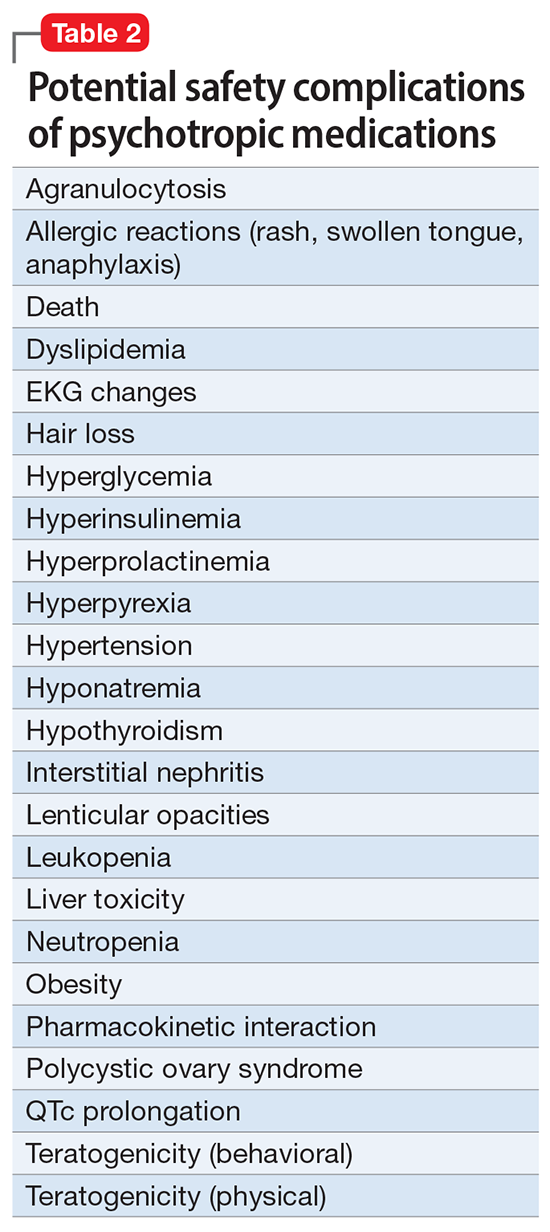
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.
Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
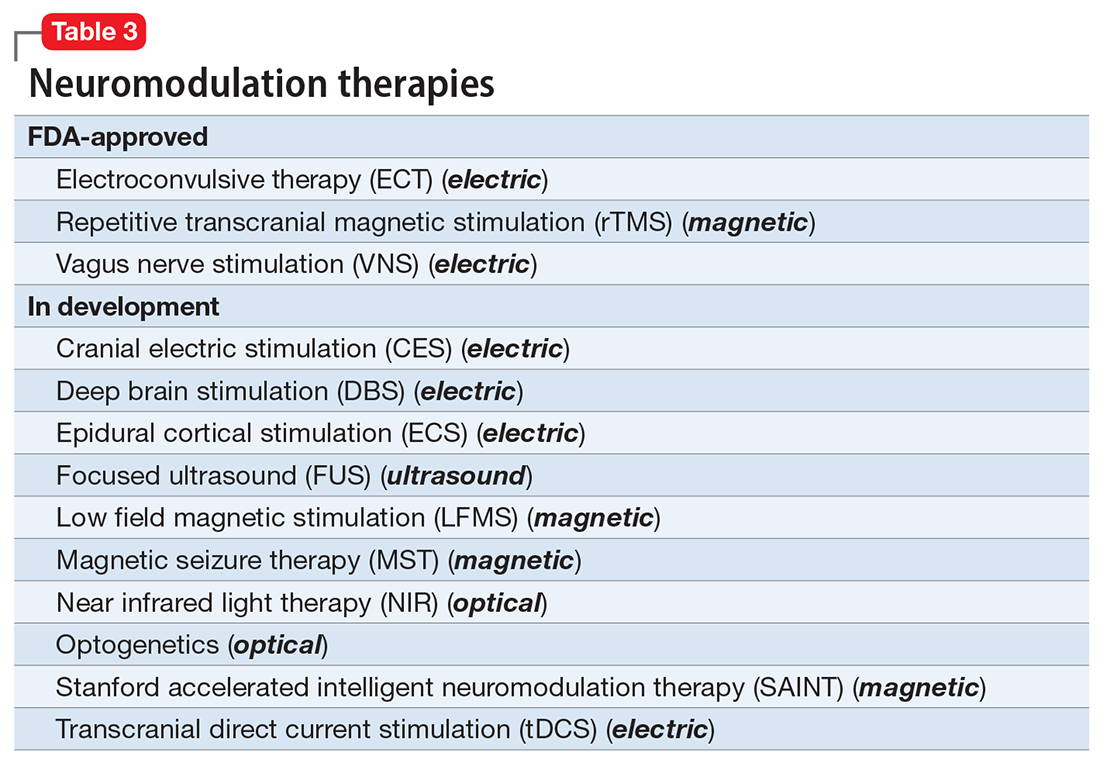
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17
A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
More on social entropy
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
Transitioning patients with opioid use disorder from methadone to buprenorphine
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
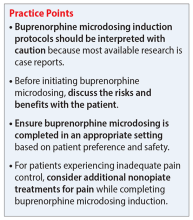
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
An overlooked cause of catatonia
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
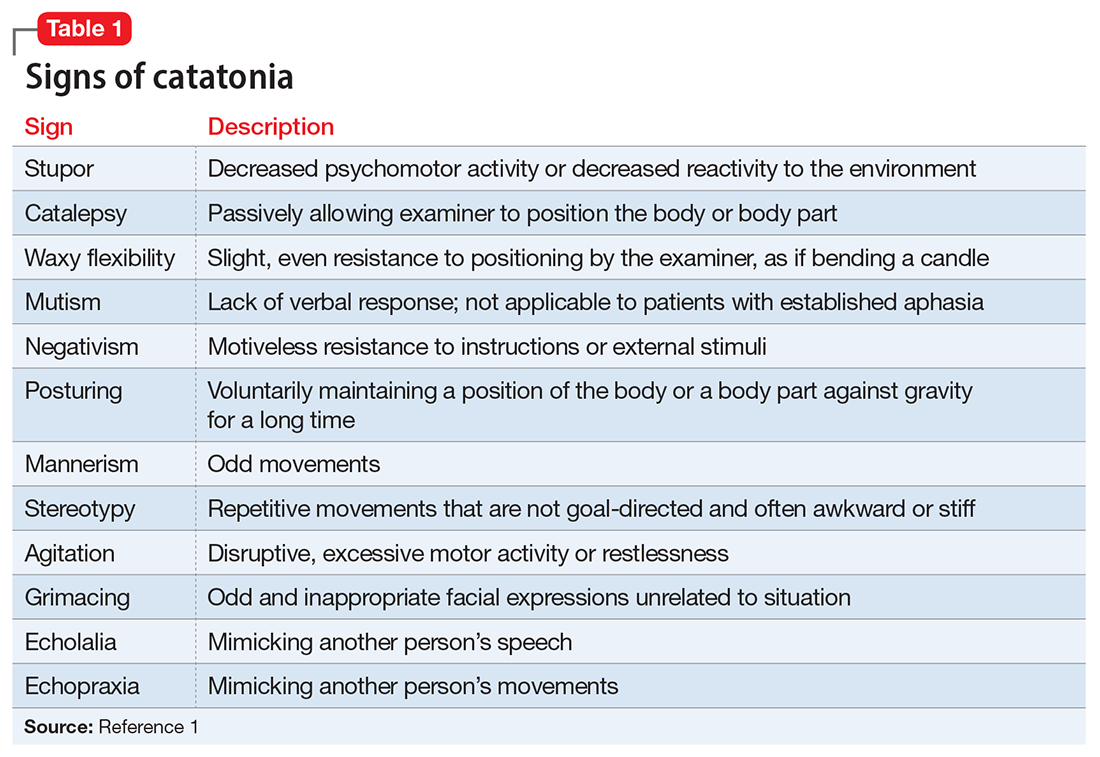
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.
EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).
[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.
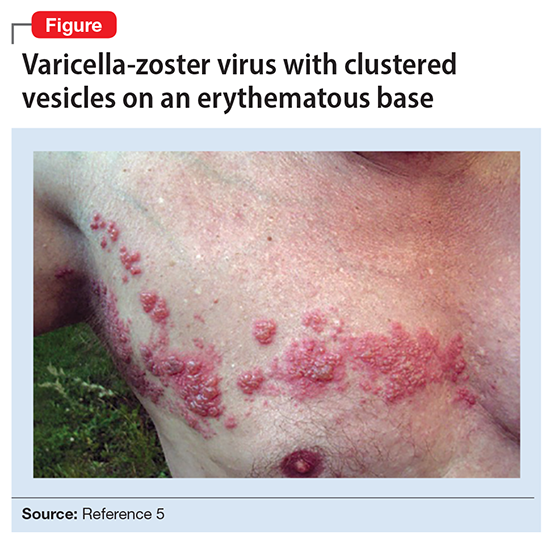
Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16
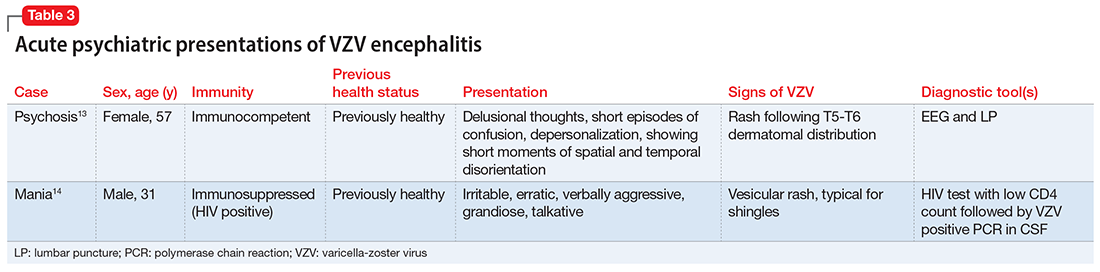
To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-
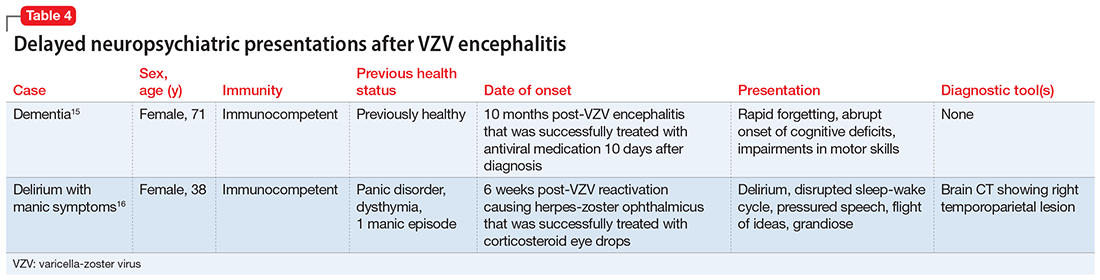
Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
Scurvy in psychiatric patients: An easy-to-miss diagnosis
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
Two years ago, I cared for Ms. L, a woman in her late 40s who had a history of generalized anxiety disorder and major depressive disorder. Unable to work and highly distressed throughout the day, Ms. L was admitted to our psychiatric unit due to her functional decompensation and symptom severity.
Ms. L was extremely focused on physical symptoms. She had rigid rules regarding which beauty products she could and could not use (she insisted most soaps gave her a rash, though she did not have any clear documentation of this) as well as the types of food she could and could not eat due to fear of an allergic reaction (skin testing was negative for the foods she claimed were problematic, though this did not change her selective eating habits). By the time she was admitted to our unit, in addition to outpatient mental health, she was being treated by internal medicine, allergy and immunology, and dermatology, with largely equivocal objective findings.
During her psychiatric admission intake, Ms. L mentioned that due to her fear of anaphylaxis, she hadn’t eaten any fruits or vegetables for at least 2 years. As a result, I ordered testing of her vitamin C level.
Three days following admission, Ms. L requested to be discharged because she said she needed to care for her pet. She reported feeling less anxious, and because the treatment team felt she did not meet the criteria for an involuntary hold, she was discharged. A week later, the results of her vitamin C level came back, indicating a severe deficiency (<0.1 mg/dL; reference range: 0.3 to 2.7 mg/dL). I contacted her outpatient team, and vitamin C supplementation was started immediately.
Notes from Ms. L’s subsequent outpatient mental health visits indicated improvement in her somatic symptoms (less perseveration), although over the next year her scores on the Generalized Anxiety Disorder-7 and Patient Health Questionnaire-9 scales were largely unchanged (fluctuating within the range of 11 to 17 and 12 to 17, respectively). One year later, Ms. L stopped taking vitamin C supplements because she was afraid she was becoming allergic to them, though there was no objective evidence to support this belief. Her vitamin C levels were within the normal range at the time and have not been rechecked since then.
Ms. L’s obsession with “healthy eating” led to numerous red herrings for clinicians, as she was anxious about every food. Countertransference and feelings of frustration may have also led clinicians in multiple specialties to miss the diagnosis of scurvy. Vitamin C supplementation did not result in remission of Ms. L’s symptoms, which reflects the complexity and severity of her comorbid psychiatric illnesses. However, a decrease in her perseveration on somatic symptoms afforded increased opportunities to address her other psychiatric diagnoses. Ms. L eventually enrolled in an eating disorders program, which was beneficial to her.
Keep scurvy in the differential Dx
Symptoms of scurvy include malaise; lethargy; anemia; myalgia; bone pain; easy bruising; petechiae and perifollicular hemorrhages (due to capillary fragility); gum disease; mood changes; and depression.1 In later stages, the presentation can progress to edema; jaundice; hemolysis and spontaneous bleeding; neuropathy; fever; convulsions; and death.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
1. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008;54(10):1403-1406.
2. Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23(8):1281-1284.
3. Meisel K, Daggubati S, Josephson SA. Scurvy in the 21st century? Vitamin C deficiency presenting to the neurologist. Neurol Clin Pract. 2015;5(6):491-493.
Breast cancer screening in women receiving antipsychotics
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Should residents be taught how to prescribe monoamine oxidase inhibitors?
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
What my Grandma’s schizophrenia taught me
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Yellow Nodule on the Scalp
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4
The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
A 45-year-old woman was referred to dermatology by a primary care physician for evaluation of a raised skin lesion on the scalp. She was otherwise healthy. The lesion had been present for many years but recently grew in size. The patient reported that the lesion was subject to recurrent physical trauma and she wanted it removed. Physical examination revealed a 6×6-mm, domeshaped, yellow nodule on the left inferior parietal scalp. There were no similar lesions located elsewhere on the body. A shave removal was performed and sent for histopathologic evaluation.

Women need not wait to conceive after miscarriage, abortion
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.