User login
Hormonal contraception and lactation: Reset your practices based on the evidence
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.
Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8
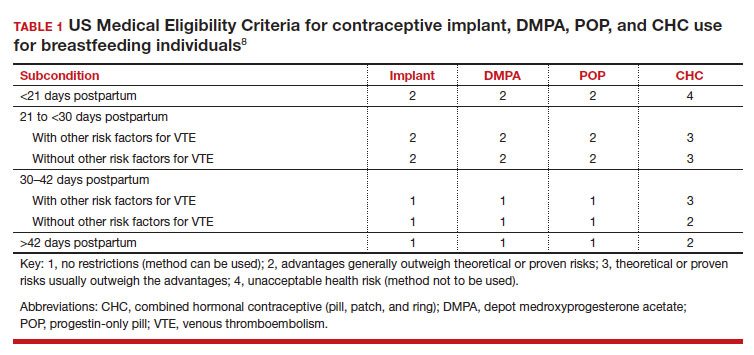
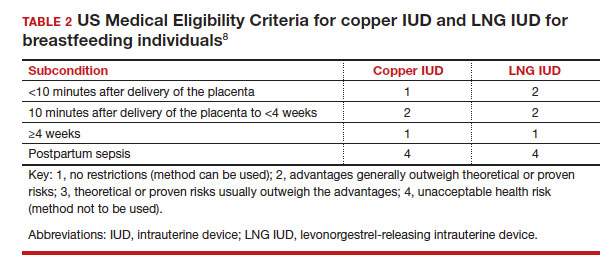
Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
Is it time to reconsider Rh testing and Rh D immune globulin treatment for miscarriage and abortion care in early pregnancy?
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
Progress in breast cancer screening over the past 50 years: A remarkable story, but still work to do
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3
While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.
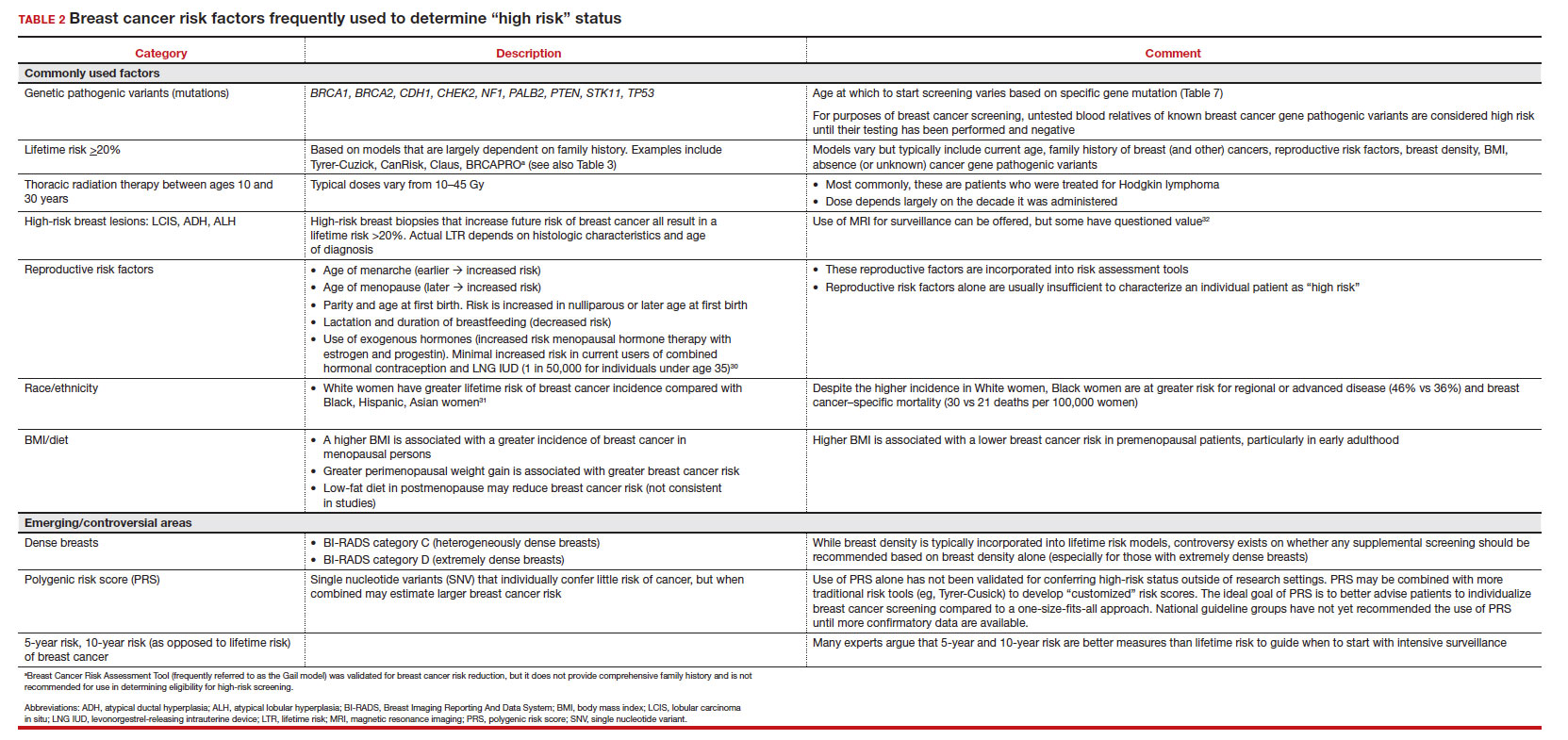
Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7
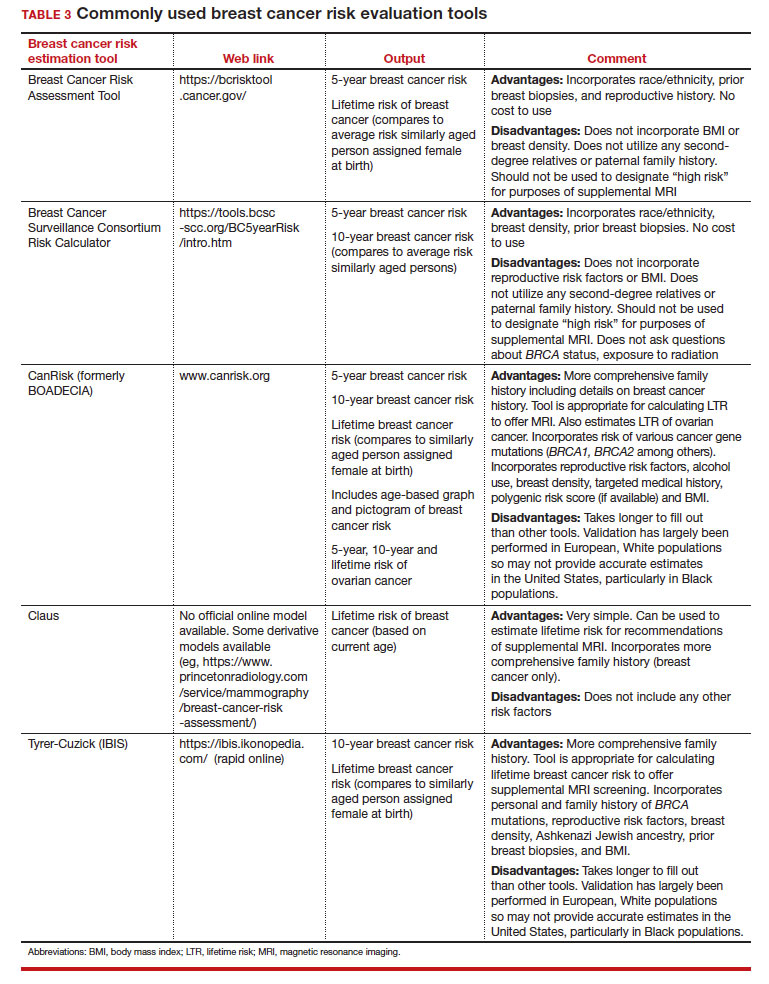
Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10
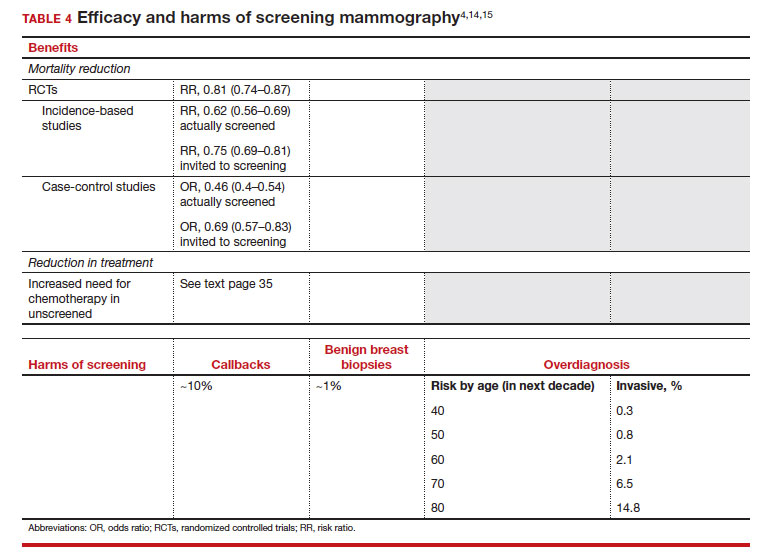
With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).
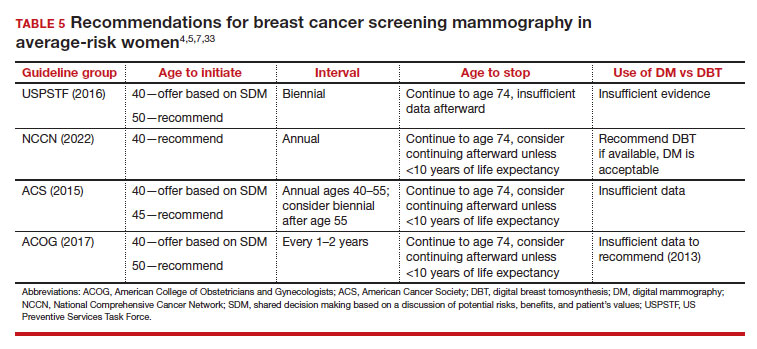
Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.
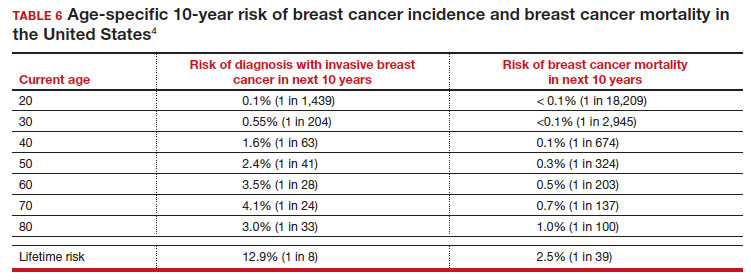
For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.
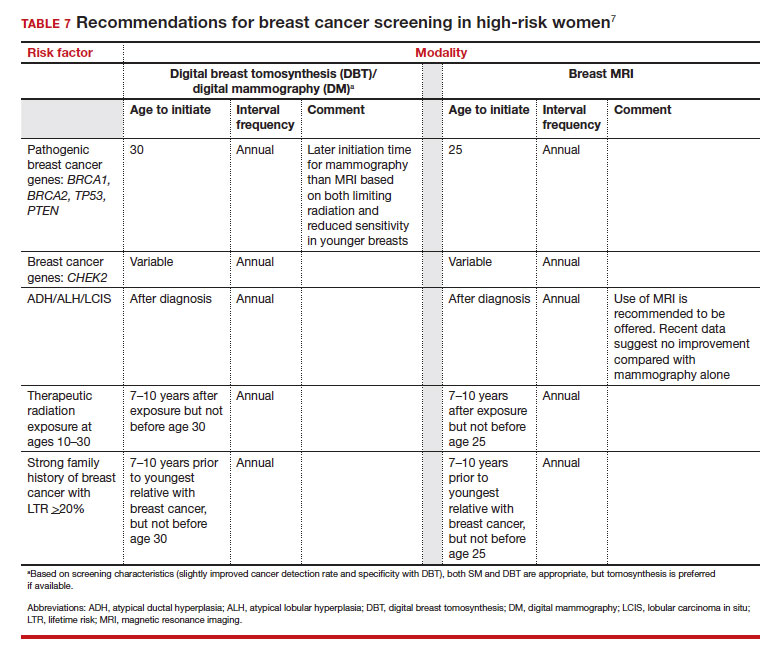
Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).
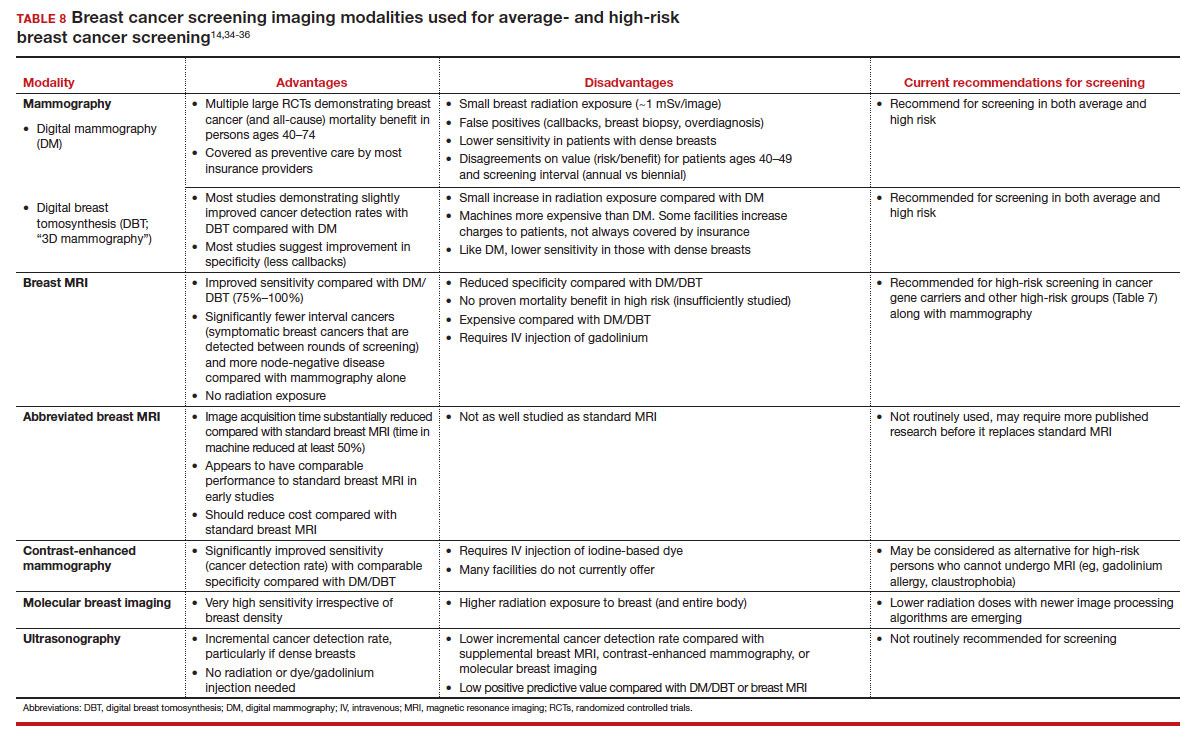
Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Teaching the Teacher: Novel Faculty Development for VA Hospitalists
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
Screen high-risk individuals for NAFLD, urges guidance
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM HEPATOLOGY
Asymptomatic Soft Tumor on the Forearm
The Diagnosis: Aneurysmal Dermatofibroma
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.
Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
The Diagnosis: Aneurysmal Dermatofibroma
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.

Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
The Diagnosis: Aneurysmal Dermatofibroma
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.

Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
A 43-year-old Black man with no notable medical history presented to our clinic with a progressively enlarging tumor on the right forearm of 12 months’ duration. Despite its progressive growth, the tumor was asymptomatic. Physical examination of the right forearm revealed a 3.7×3.0-cm, well-circumscribed, exophytic tumor with a mildly erythematous hue, scaly surface, and rubbery consistency. There was no surrounding erythema, edema, localized lymphadenopathy, or concurrent lymphedema.
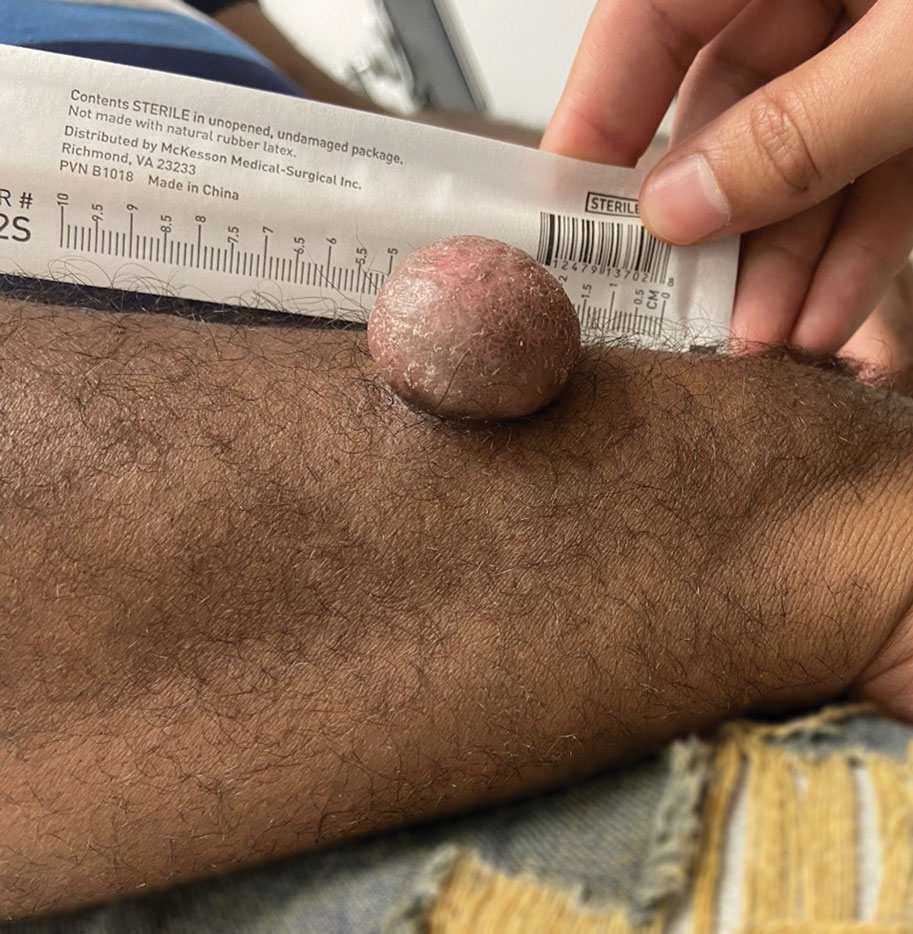
Longitudinal arm lesion
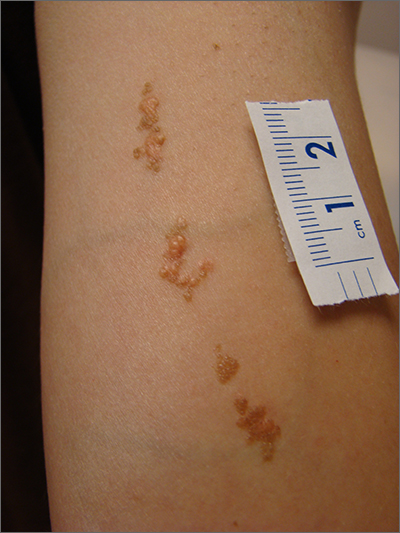
This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001

This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001
A Review of the Glycemia Reduction Approaches in Diabetes (GRADE) Study: Comparing the Effectiveness of Type 2 Diabetes Medications
Type 2 diabetes (T2D) is a chronic, progressive disease marked by ongoing decline in insulin sensitivity and beta-cell function over time. Clinical trials have shown that lowering A1C to ∼7.0% (53 mmol/mol), especially after an early diagnosis, can markedly reduce the long-term complications of T2D. Metformin has become the generally recommended first therapeutic agent in treating T2D due to the drug’s long-term experience, effectiveness, and avoidance of hypoglycemia or weight gain. However, it is clear that additional agents are necessary to regain glucose control when metformin eventually fails due to the progressive nature of the disease.
Insufficient data on comparative efficacy and durability of effect has led to uncertainty in recommendations for the preferred second agent. Comparative effectiveness has been reported primarily in industry-sponsored trials of relatively short duration. With this in mind, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) Study. This landmark, randomized controlled study was initiated in 2013, enrolling patients on metformin alone within 10 years of diagnosis of T2D. It involved 36 research sites in the United States with a mean follow-up of 5 years. The participants were randomized to adding a dipeptidyl peptidase 4 (DPP-4) inhibitor (sitagliptin), a sulfonylurea (glimepiride), basal insulin (glargine), or a glucagon-like peptide 1 receptor agonist (GLP-1 RA) (liraglutide), with the primary outcome being time to A1C over 7.0%.
The GRADE study was unique in several ways: its size, scope, length, and the fact that the financial support and design planning stemmed from a U34 planning grant from the NIDDK. The study population of 5047 participants was very diverse, reflecting the population affected by T2D. A mix of racial and ethnic groups were represented, including 19.8% Black participants and 18.6% Hispanic participants. It is unlikely that a similar comparative effectiveness trial of pharmacologic treatment of T2D will be performed again in the future, considering the high costs and length of time required for such a study amid the dynamic drug development environment today. In fact, the final implementation of study results is somewhat complicated by the subsequent approval of GLP-1 RAs of greater efficacy, weight loss, and convenience, as well as sodium-glucose cotransporter 2 (SGLT2) inhibitors and, most recently, a dual GLP-1/gastric inhibitory polypeptide (GIP) receptor agonist (tirzepatide). Many of these newer agents have demonstrated nonglycemic benefits, such as reduced risk of cardiovascular (CV) events or reduced progression of renal disease. The findings from the GRADE study, however, did provide important insight on the long-term management of T2D.
The GRADE study was the first to compare the efficacy of 4 US Food and Drug Administration–approved drugs for T2D in maintaining blood glucose levels for the longest amount of time in patients with T2D. It also monitored microvascular complications, CV events, and adverse drug effects.
An important message of the study that may be overlooked is that all of the studied agents’ ability to maintain an A1C under 7.0% was quite low—as 71% of all participants reached the primary outcome by 5 years; the best results for a group were 67% for glargine and 68% for liraglutide. In general, the results showed that liraglutide and insulin glargine were superior to glimepiride and sitagliptin in controlling blood sugars. They provided approximately 6 months’ more time with blood glucose levels in the desired range compared with sitagliptin, which was shown to provide the least amount of time in maintaining glucose levels. Fifty-five percent of the sitagliptin group experienced the primary outcome at 1 year. Sitagliptin was particularly ineffective for the patient subgroup with an A1C at baseline of 7.8% or higher, where 70% reached the primary outcome in 1 year. The results were uniform regarding age, race, sex, and ethnicity of the trial participants. The intention-to-treat design of the study limits the conclusions about A1C differences, as failure to maintain an A1C under 7.5% required addition of prandial insulin for the glargine group and the addition of glargine to the other 3 groups. Although subjects receiving glargine had an initial glucose-lowering effect that was less than that seen with liraglutide, the ability to keep titrating the glargine likely had an impact on the long-term benefit of that agent. When the glargine group neared or in some cases even passed the secondary outcome A1C level of 7.5%, the basal insulin was increased to lower the A1C, sometimes even when the protocol would recommend adding prandial insulin.
The study was not powered specifically for determining the relative risk of CV events. However, there was some evidence that liraglutide was associated with lower CV risk than the other 3 agents by about 30%. There was no difference in microvascular risk among the agents in this study of relatively short-term disease. Side effects were not a major problem and no different than expected. Glargine and glimepiride were associated with less weight loss, while liraglutide had a particular benefit on weight. Glimepiride is associated with significantly more frequent incidents of severe hypoglycemia, though the rates of severe hypoglycemia were quite low. Liraglutide users reported significantly higher rates of nausea and had a higher early drop-out rate, but did not show a difference in continued use by the end of the study.
In summary, the GRADE trial confirmed that glucose control in T2D is a progressive problem, as the addition of all 4 classes of medication failed to keep most patients in the target glucose range. However, basal insulin and GLP-1 RAs outperformed the other 2 classes. Sitagliptin has the poorest metabolic profile. One could argue that, based on overall metabolic control and concomitant weight benefits, less need for glucose monitoring, simple titration, apparent CV benefit, and insignificant hypoglycemia, GLP-1 RAs offer the best option as an agent to add to metformin. This conclusion is fortified by the fact that the agent used to represent this class in the study appears to be less effective in reducing glucose and weight and offers less convenience than the newer, once-weekly GLP-RAs available today.
Type 2 diabetes (T2D) is a chronic, progressive disease marked by ongoing decline in insulin sensitivity and beta-cell function over time. Clinical trials have shown that lowering A1C to ∼7.0% (53 mmol/mol), especially after an early diagnosis, can markedly reduce the long-term complications of T2D. Metformin has become the generally recommended first therapeutic agent in treating T2D due to the drug’s long-term experience, effectiveness, and avoidance of hypoglycemia or weight gain. However, it is clear that additional agents are necessary to regain glucose control when metformin eventually fails due to the progressive nature of the disease.
Insufficient data on comparative efficacy and durability of effect has led to uncertainty in recommendations for the preferred second agent. Comparative effectiveness has been reported primarily in industry-sponsored trials of relatively short duration. With this in mind, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) Study. This landmark, randomized controlled study was initiated in 2013, enrolling patients on metformin alone within 10 years of diagnosis of T2D. It involved 36 research sites in the United States with a mean follow-up of 5 years. The participants were randomized to adding a dipeptidyl peptidase 4 (DPP-4) inhibitor (sitagliptin), a sulfonylurea (glimepiride), basal insulin (glargine), or a glucagon-like peptide 1 receptor agonist (GLP-1 RA) (liraglutide), with the primary outcome being time to A1C over 7.0%.
The GRADE study was unique in several ways: its size, scope, length, and the fact that the financial support and design planning stemmed from a U34 planning grant from the NIDDK. The study population of 5047 participants was very diverse, reflecting the population affected by T2D. A mix of racial and ethnic groups were represented, including 19.8% Black participants and 18.6% Hispanic participants. It is unlikely that a similar comparative effectiveness trial of pharmacologic treatment of T2D will be performed again in the future, considering the high costs and length of time required for such a study amid the dynamic drug development environment today. In fact, the final implementation of study results is somewhat complicated by the subsequent approval of GLP-1 RAs of greater efficacy, weight loss, and convenience, as well as sodium-glucose cotransporter 2 (SGLT2) inhibitors and, most recently, a dual GLP-1/gastric inhibitory polypeptide (GIP) receptor agonist (tirzepatide). Many of these newer agents have demonstrated nonglycemic benefits, such as reduced risk of cardiovascular (CV) events or reduced progression of renal disease. The findings from the GRADE study, however, did provide important insight on the long-term management of T2D.
The GRADE study was the first to compare the efficacy of 4 US Food and Drug Administration–approved drugs for T2D in maintaining blood glucose levels for the longest amount of time in patients with T2D. It also monitored microvascular complications, CV events, and adverse drug effects.
An important message of the study that may be overlooked is that all of the studied agents’ ability to maintain an A1C under 7.0% was quite low—as 71% of all participants reached the primary outcome by 5 years; the best results for a group were 67% for glargine and 68% for liraglutide. In general, the results showed that liraglutide and insulin glargine were superior to glimepiride and sitagliptin in controlling blood sugars. They provided approximately 6 months’ more time with blood glucose levels in the desired range compared with sitagliptin, which was shown to provide the least amount of time in maintaining glucose levels. Fifty-five percent of the sitagliptin group experienced the primary outcome at 1 year. Sitagliptin was particularly ineffective for the patient subgroup with an A1C at baseline of 7.8% or higher, where 70% reached the primary outcome in 1 year. The results were uniform regarding age, race, sex, and ethnicity of the trial participants. The intention-to-treat design of the study limits the conclusions about A1C differences, as failure to maintain an A1C under 7.5% required addition of prandial insulin for the glargine group and the addition of glargine to the other 3 groups. Although subjects receiving glargine had an initial glucose-lowering effect that was less than that seen with liraglutide, the ability to keep titrating the glargine likely had an impact on the long-term benefit of that agent. When the glargine group neared or in some cases even passed the secondary outcome A1C level of 7.5%, the basal insulin was increased to lower the A1C, sometimes even when the protocol would recommend adding prandial insulin.
The study was not powered specifically for determining the relative risk of CV events. However, there was some evidence that liraglutide was associated with lower CV risk than the other 3 agents by about 30%. There was no difference in microvascular risk among the agents in this study of relatively short-term disease. Side effects were not a major problem and no different than expected. Glargine and glimepiride were associated with less weight loss, while liraglutide had a particular benefit on weight. Glimepiride is associated with significantly more frequent incidents of severe hypoglycemia, though the rates of severe hypoglycemia were quite low. Liraglutide users reported significantly higher rates of nausea and had a higher early drop-out rate, but did not show a difference in continued use by the end of the study.
In summary, the GRADE trial confirmed that glucose control in T2D is a progressive problem, as the addition of all 4 classes of medication failed to keep most patients in the target glucose range. However, basal insulin and GLP-1 RAs outperformed the other 2 classes. Sitagliptin has the poorest metabolic profile. One could argue that, based on overall metabolic control and concomitant weight benefits, less need for glucose monitoring, simple titration, apparent CV benefit, and insignificant hypoglycemia, GLP-1 RAs offer the best option as an agent to add to metformin. This conclusion is fortified by the fact that the agent used to represent this class in the study appears to be less effective in reducing glucose and weight and offers less convenience than the newer, once-weekly GLP-RAs available today.
Type 2 diabetes (T2D) is a chronic, progressive disease marked by ongoing decline in insulin sensitivity and beta-cell function over time. Clinical trials have shown that lowering A1C to ∼7.0% (53 mmol/mol), especially after an early diagnosis, can markedly reduce the long-term complications of T2D. Metformin has become the generally recommended first therapeutic agent in treating T2D due to the drug’s long-term experience, effectiveness, and avoidance of hypoglycemia or weight gain. However, it is clear that additional agents are necessary to regain glucose control when metformin eventually fails due to the progressive nature of the disease.
Insufficient data on comparative efficacy and durability of effect has led to uncertainty in recommendations for the preferred second agent. Comparative effectiveness has been reported primarily in industry-sponsored trials of relatively short duration. With this in mind, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) Study. This landmark, randomized controlled study was initiated in 2013, enrolling patients on metformin alone within 10 years of diagnosis of T2D. It involved 36 research sites in the United States with a mean follow-up of 5 years. The participants were randomized to adding a dipeptidyl peptidase 4 (DPP-4) inhibitor (sitagliptin), a sulfonylurea (glimepiride), basal insulin (glargine), or a glucagon-like peptide 1 receptor agonist (GLP-1 RA) (liraglutide), with the primary outcome being time to A1C over 7.0%.
The GRADE study was unique in several ways: its size, scope, length, and the fact that the financial support and design planning stemmed from a U34 planning grant from the NIDDK. The study population of 5047 participants was very diverse, reflecting the population affected by T2D. A mix of racial and ethnic groups were represented, including 19.8% Black participants and 18.6% Hispanic participants. It is unlikely that a similar comparative effectiveness trial of pharmacologic treatment of T2D will be performed again in the future, considering the high costs and length of time required for such a study amid the dynamic drug development environment today. In fact, the final implementation of study results is somewhat complicated by the subsequent approval of GLP-1 RAs of greater efficacy, weight loss, and convenience, as well as sodium-glucose cotransporter 2 (SGLT2) inhibitors and, most recently, a dual GLP-1/gastric inhibitory polypeptide (GIP) receptor agonist (tirzepatide). Many of these newer agents have demonstrated nonglycemic benefits, such as reduced risk of cardiovascular (CV) events or reduced progression of renal disease. The findings from the GRADE study, however, did provide important insight on the long-term management of T2D.
The GRADE study was the first to compare the efficacy of 4 US Food and Drug Administration–approved drugs for T2D in maintaining blood glucose levels for the longest amount of time in patients with T2D. It also monitored microvascular complications, CV events, and adverse drug effects.
An important message of the study that may be overlooked is that all of the studied agents’ ability to maintain an A1C under 7.0% was quite low—as 71% of all participants reached the primary outcome by 5 years; the best results for a group were 67% for glargine and 68% for liraglutide. In general, the results showed that liraglutide and insulin glargine were superior to glimepiride and sitagliptin in controlling blood sugars. They provided approximately 6 months’ more time with blood glucose levels in the desired range compared with sitagliptin, which was shown to provide the least amount of time in maintaining glucose levels. Fifty-five percent of the sitagliptin group experienced the primary outcome at 1 year. Sitagliptin was particularly ineffective for the patient subgroup with an A1C at baseline of 7.8% or higher, where 70% reached the primary outcome in 1 year. The results were uniform regarding age, race, sex, and ethnicity of the trial participants. The intention-to-treat design of the study limits the conclusions about A1C differences, as failure to maintain an A1C under 7.5% required addition of prandial insulin for the glargine group and the addition of glargine to the other 3 groups. Although subjects receiving glargine had an initial glucose-lowering effect that was less than that seen with liraglutide, the ability to keep titrating the glargine likely had an impact on the long-term benefit of that agent. When the glargine group neared or in some cases even passed the secondary outcome A1C level of 7.5%, the basal insulin was increased to lower the A1C, sometimes even when the protocol would recommend adding prandial insulin.
The study was not powered specifically for determining the relative risk of CV events. However, there was some evidence that liraglutide was associated with lower CV risk than the other 3 agents by about 30%. There was no difference in microvascular risk among the agents in this study of relatively short-term disease. Side effects were not a major problem and no different than expected. Glargine and glimepiride were associated with less weight loss, while liraglutide had a particular benefit on weight. Glimepiride is associated with significantly more frequent incidents of severe hypoglycemia, though the rates of severe hypoglycemia were quite low. Liraglutide users reported significantly higher rates of nausea and had a higher early drop-out rate, but did not show a difference in continued use by the end of the study.
In summary, the GRADE trial confirmed that glucose control in T2D is a progressive problem, as the addition of all 4 classes of medication failed to keep most patients in the target glucose range. However, basal insulin and GLP-1 RAs outperformed the other 2 classes. Sitagliptin has the poorest metabolic profile. One could argue that, based on overall metabolic control and concomitant weight benefits, less need for glucose monitoring, simple titration, apparent CV benefit, and insignificant hypoglycemia, GLP-1 RAs offer the best option as an agent to add to metformin. This conclusion is fortified by the fact that the agent used to represent this class in the study appears to be less effective in reducing glucose and weight and offers less convenience than the newer, once-weekly GLP-RAs available today.
Specific ACPA and anti-native protein antibodies predict risk for RA-associated ILD
Key clinical point: Six biomarkers, including fine-specificity anti-citrullinated protein antibodies (ACPA) and anti-native protein antibodies, demonstrated a significant association with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and improved prediction sensitivity.
Major finding: Six fine-specificity antibody biomarkers, including immunoglobin G, to citrullinated (adjusted odds ratio [aOR] 3.47; 95% CI 1.71-7.01) and native (aOR 2.53; 95% CI 1.47-4.34) cyclic filaggrin 48-65 were associated with an increased risk for RA-ILD. Risk scores combining antibodies with clinical features with vs without biomarkers (score 5.9 vs 2.6) demonstrated higher sensitivity (67% vs 25%) and high specificity (≥93%) for developing RA-ILD at a threshold of 50% predicted probability.
Study details: This nested case-control study within an ongoing prospective registry included adult patients with incident RA-ILD (n = 84) and matched patients with RA without ILD (n = 233).
Disclosures: This study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Several authors reported ties with various sources unrelated to this study.
Source: Kronzer VL et al. Autoantibodies against citrullinated and native proteins and prediction of rheumatoid arthritis-associated interstitial lung disease: A nested case–control study. Lancet Rheumatol. 2023;5(2):E77-E87 (Feb). Doi: 10.1016/S2665-9913(22)00380-0
Key clinical point: Six biomarkers, including fine-specificity anti-citrullinated protein antibodies (ACPA) and anti-native protein antibodies, demonstrated a significant association with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and improved prediction sensitivity.
Major finding: Six fine-specificity antibody biomarkers, including immunoglobin G, to citrullinated (adjusted odds ratio [aOR] 3.47; 95% CI 1.71-7.01) and native (aOR 2.53; 95% CI 1.47-4.34) cyclic filaggrin 48-65 were associated with an increased risk for RA-ILD. Risk scores combining antibodies with clinical features with vs without biomarkers (score 5.9 vs 2.6) demonstrated higher sensitivity (67% vs 25%) and high specificity (≥93%) for developing RA-ILD at a threshold of 50% predicted probability.
Study details: This nested case-control study within an ongoing prospective registry included adult patients with incident RA-ILD (n = 84) and matched patients with RA without ILD (n = 233).
Disclosures: This study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Several authors reported ties with various sources unrelated to this study.
Source: Kronzer VL et al. Autoantibodies against citrullinated and native proteins and prediction of rheumatoid arthritis-associated interstitial lung disease: A nested case–control study. Lancet Rheumatol. 2023;5(2):E77-E87 (Feb). Doi: 10.1016/S2665-9913(22)00380-0
Key clinical point: Six biomarkers, including fine-specificity anti-citrullinated protein antibodies (ACPA) and anti-native protein antibodies, demonstrated a significant association with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and improved prediction sensitivity.
Major finding: Six fine-specificity antibody biomarkers, including immunoglobin G, to citrullinated (adjusted odds ratio [aOR] 3.47; 95% CI 1.71-7.01) and native (aOR 2.53; 95% CI 1.47-4.34) cyclic filaggrin 48-65 were associated with an increased risk for RA-ILD. Risk scores combining antibodies with clinical features with vs without biomarkers (score 5.9 vs 2.6) demonstrated higher sensitivity (67% vs 25%) and high specificity (≥93%) for developing RA-ILD at a threshold of 50% predicted probability.
Study details: This nested case-control study within an ongoing prospective registry included adult patients with incident RA-ILD (n = 84) and matched patients with RA without ILD (n = 233).
Disclosures: This study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Several authors reported ties with various sources unrelated to this study.
Source: Kronzer VL et al. Autoantibodies against citrullinated and native proteins and prediction of rheumatoid arthritis-associated interstitial lung disease: A nested case–control study. Lancet Rheumatol. 2023;5(2):E77-E87 (Feb). Doi: 10.1016/S2665-9913(22)00380-0