User login
As designer drugs multiply, toxicologists spring into action
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
EXPERT ANALYSIS FROM APA
Case Studies in Toxicology: An Unlikely Cause of Paralysis
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Case Studies in Toxicology: Angioedema Post-tPA: Hemorrhage Is Not the Only Risk Factor
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
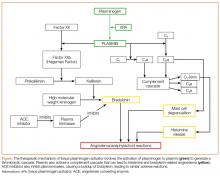
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Kratom: An Emerging Drug of Abuse
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
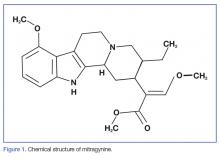
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
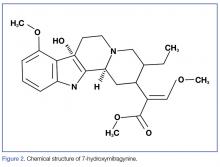
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Tavakoli HR, et al. Kratom: a new product in an expanding substance abuse market. Fed Prac. 2016;33[11]:132-136. http://www.fedprac.com).
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opioids, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most emergency physicians (EPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of this type of Web site and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite the purported raison d’être of these Web sites, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer et al4 of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patients’ digital habits, the researchers demonstrated that the majority of subjects used these Web sites and, as a result, either increased their drug use or learned about (and tried) new substances.
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opioid withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and has become increasingly popular in the United States and in the United Kingdom.2,5,6 In the United States, this poses a problem for EPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the US Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined Web sites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about $13 US currency).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a kratom product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least nine deaths.7
This article presents a case of kratom abuse. It describes a brief history of the substance, its pharmacological characteristics, the clinical presentation of kratom abuse, and the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the United States, a basic working knowledge of the drug is quickly becoming a must for EPs.
Case Presentation
At his employer’s request, a 33-year-old man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disk resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he became concerned that he was abusing his opioid medication, and it was discontinued. The patient was transferred to the local ED and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline, consistent with opioid withdrawal. Initial point-of-care (POC) admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s opioid withdrawal resolved, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia.2,3 The leaves of these trees contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-like effect, which at mega doses produces an intense euphoric state and has led to a steady growth in abuse worldwide. Although the government of Thailand banned the planting of Mitragyna speciosa as early as 1943, its continued proliferation in Southeast Asia and throughout the world has not ceased.2,3,6
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the two major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including mu-, delta-, and kappa-opioid receptors, leading to its opioid-like effects, including analgesia and euphoria. Also similar to common opioids, withdrawal symptomatology can present after only 5 days of daily use. There is limited evidence that mitragynine can activate postsynaptic alpha-2 adrenergic receptors, which may act synergistically with the mu-agonist with regard to its analgesic effect.2,5
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for EPs to be aware of the presentation of patients with kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from Web sites such as Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5-15 g of raw crushed leaves), it is believed that the mu-opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed with supportive and symptomatic care and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of a patient with an acute kratom overdose (typically requiring ingestion of >15 g of crushed leaves) begins with addressing airway support, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including POC glucose testing, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, as indicated).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong mu agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid withdrawal.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, alpha-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to EPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurological and mental status examinations.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and psychosis.3-7 Additionally, a study by Kittirattanapaiboon et al12 correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥14,000 individuals included in the study sample) with statistically significant higher suicide risk.
Detection
Because kratom is a relatively new compound in the United States, medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and two metabolites of mitragynine in urine.7 Le et al13 were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours. Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at the Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the case study patient’s urine because a urine test was not done until hospital day 5.
Case Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid-use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Conclusion
Kratom is an emerging drug of abuse in the Western world. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the United States and multiple other Western countries, kratom is easily accessible. Emergency physicians need to be aware of kratom, and during their evaluations, question appropriate patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
Drops, Ointments, Gels, and Patches: The Dangers of Topical Medications
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
Who Overdoses on Opioids at a VA Emergency Department?
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Clement C, Stock C. Who overdoses at a VA emergency department? Fed Prac. 2016;33[11]:14-19. http://www.fedprac.com).
Overdose deaths remain epidemic throughout the United States. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation, with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the United States occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary disease, cardiovascular disease (CVD), and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than doses prescribed to nonveterans.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is approved by the US Food and Drug Administration for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the ED may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the Veterans Affairs (VA) setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose-prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA Institutional Review Boards, and conducted at the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next of kin or other contact at the same address as the veteran; diagnoses based on International Classification of Diseases, 9th Revision (ICD-9) codes, including sleep apnea, obesity, cardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]), cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine-drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than three patient-care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of at or greater than 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 codes 304.1*), alcohol (ICD-9 codes 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes et al18: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed. Prescription opioids and BZDs were converted to daily morphineequivalent dose (MED) and daily lorazepam equivalent dose (LED), using established methods.19,20
Veterans were stratified into four groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications were not included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including four veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next of kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was less than or equal to 200 mg in 71.6% and less than or equal to 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; standard deviation [SD], 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least one diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included CVD, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in greater than 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for less than 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were, on average, older than the overdose fatalities in the United States. Opioid-overdose deaths in all US states and in Utah alone occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the Veterans Health Administration (VHA); however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed eight veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the United States is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half of the veterans in this study had a contact or next of kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid-overdose death include sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least one medical or psychiatric diagnosis.26 Less than half of the 18,000 VA primary care patients in five VA centers had any psychiatric condition, and less than 65% had CVD, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell et al16 found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample. Bohnert et al9 found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund et al10 found that less than 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD. Bohnert et al9 found an HR of 21.95 for overdose death among those with opioid-use disorders. The sample in this study had a much higher incidence of nonopioid SUDD compared with that of the study by Edlund et al,10 but none of the veterans in this study had a documented history of opioid-use disorder. The absence of opioid-use disorders in this sample is unexpected and points to a need for providers to screen for opioid-use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders.
Gomes et al18 found that greater than 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality. The VA/Department of Defense Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe et al29 found that 28% of overdose victims were prescribed less than 50 mg MED. In this study, the average dose available to veterans was greater than 100 mg MED; however, one-third of all study veterans had less than 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200-mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to opioids, regardless of dose.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ- opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the Centers for Disease Control and Prevention.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) of the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of an opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Providing prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program, when discussing the risk of overdose with veterans.
Most veterans had at least one UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next of kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in at or less than 30% of the sample.35 Urine-drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose-prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine-drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND), promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug-overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose-prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average US victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose-prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Clinicians should screen patients for opioid-use disorder whenever opioids are prescribed and continue to screen them throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med . 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med . 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care . 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry . 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain . 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract . 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines. Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert]. Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med . 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med . 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med . 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.
20. Washington State Agency Medical Directors’ Group. Opioid dose calculator. http://www.agen cymeddirectors.wa.gov/Calculator/DoseCalculator.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med . 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA . 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care . 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care . 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care . 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med . 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med . 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend . 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician . 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med . 1996;3(7):660-667.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Clement C, Stock C. Who overdoses at a VA emergency department? Fed Prac. 2016;33[11]:14-19. http://www.fedprac.com).
Overdose deaths remain epidemic throughout the United States. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation, with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the United States occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary disease, cardiovascular disease (CVD), and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than doses prescribed to nonveterans.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is approved by the US Food and Drug Administration for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the ED may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the Veterans Affairs (VA) setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose-prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA Institutional Review Boards, and conducted at the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next of kin or other contact at the same address as the veteran; diagnoses based on International Classification of Diseases, 9th Revision (ICD-9) codes, including sleep apnea, obesity, cardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]), cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine-drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than three patient-care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of at or greater than 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 codes 304.1*), alcohol (ICD-9 codes 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes et al18: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed. Prescription opioids and BZDs were converted to daily morphineequivalent dose (MED) and daily lorazepam equivalent dose (LED), using established methods.19,20
Veterans were stratified into four groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications were not included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including four veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next of kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was less than or equal to 200 mg in 71.6% and less than or equal to 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; standard deviation [SD], 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least one diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included CVD, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in greater than 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for less than 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were, on average, older than the overdose fatalities in the United States. Opioid-overdose deaths in all US states and in Utah alone occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the Veterans Health Administration (VHA); however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed eight veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the United States is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half of the veterans in this study had a contact or next of kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid-overdose death include sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least one medical or psychiatric diagnosis.26 Less than half of the 18,000 VA primary care patients in five VA centers had any psychiatric condition, and less than 65% had CVD, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell et al16 found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample. Bohnert et al9 found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund et al10 found that less than 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD. Bohnert et al9 found an HR of 21.95 for overdose death among those with opioid-use disorders. The sample in this study had a much higher incidence of nonopioid SUDD compared with that of the study by Edlund et al,10 but none of the veterans in this study had a documented history of opioid-use disorder. The absence of opioid-use disorders in this sample is unexpected and points to a need for providers to screen for opioid-use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders.
Gomes et al18 found that greater than 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality. The VA/Department of Defense Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe et al29 found that 28% of overdose victims were prescribed less than 50 mg MED. In this study, the average dose available to veterans was greater than 100 mg MED; however, one-third of all study veterans had less than 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200-mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to opioids, regardless of dose.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ- opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the Centers for Disease Control and Prevention.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) of the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of an opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Providing prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program, when discussing the risk of overdose with veterans.
Most veterans had at least one UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next of kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in at or less than 30% of the sample.35 Urine-drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose-prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine-drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND), promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug-overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose-prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average US victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose-prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Clinicians should screen patients for opioid-use disorder whenever opioids are prescribed and continue to screen them throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
Editor’s Note: This article has been adapted from an article originally published in Federal Practitioner (Clement C, Stock C. Who overdoses at a VA emergency department? Fed Prac. 2016;33[11]:14-19. http://www.fedprac.com).
Overdose deaths remain epidemic throughout the United States. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation, with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the United States occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary disease, cardiovascular disease (CVD), and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than doses prescribed to nonveterans.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is approved by the US Food and Drug Administration for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the ED may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the Veterans Affairs (VA) setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose-prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA Institutional Review Boards, and conducted at the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next of kin or other contact at the same address as the veteran; diagnoses based on International Classification of Diseases, 9th Revision (ICD-9) codes, including sleep apnea, obesity, cardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]), cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine-drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than three patient-care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of at or greater than 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 codes 304.1*), alcohol (ICD-9 codes 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes et al18: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed. Prescription opioids and BZDs were converted to daily morphineequivalent dose (MED) and daily lorazepam equivalent dose (LED), using established methods.19,20
Veterans were stratified into four groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications were not included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including four veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next of kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was less than or equal to 200 mg in 71.6% and less than or equal to 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; standard deviation [SD], 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least one diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included CVD, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in greater than 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for less than 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were, on average, older than the overdose fatalities in the United States. Opioid-overdose deaths in all US states and in Utah alone occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the Veterans Health Administration (VHA); however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed eight veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the United States is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half of the veterans in this study had a contact or next of kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid-overdose death include sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least one medical or psychiatric diagnosis.26 Less than half of the 18,000 VA primary care patients in five VA centers had any psychiatric condition, and less than 65% had CVD, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell et al16 found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample. Bohnert et al9 found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund et al10 found that less than 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD. Bohnert et al9 found an HR of 21.95 for overdose death among those with opioid-use disorders. The sample in this study had a much higher incidence of nonopioid SUDD compared with that of the study by Edlund et al,10 but none of the veterans in this study had a documented history of opioid-use disorder. The absence of opioid-use disorders in this sample is unexpected and points to a need for providers to screen for opioid-use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary disease or CVD, and/or a history of psychiatric disorders.
Gomes et al18 found that greater than 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality. The VA/Department of Defense Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe et al29 found that 28% of overdose victims were prescribed less than 50 mg MED. In this study, the average dose available to veterans was greater than 100 mg MED; however, one-third of all study veterans had less than 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200-mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to opioids, regardless of dose.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ- opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the Centers for Disease Control and Prevention.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) of the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of an opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Providing prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program, when discussing the risk of overdose with veterans.
Most veterans had at least one UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next of kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in at or less than 30% of the sample.35 Urine-drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose-prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine-drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND), promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug-overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose-prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average US victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose-prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Clinicians should screen patients for opioid-use disorder whenever opioids are prescribed and continue to screen them throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med . 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med . 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care . 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry . 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain . 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract . 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines. Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert]. Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med . 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med . 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med . 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.
20. Washington State Agency Medical Directors’ Group. Opioid dose calculator. http://www.agen cymeddirectors.wa.gov/Calculator/DoseCalculator.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med . 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA . 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care . 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care . 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care . 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med . 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med . 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend . 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician . 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med . 1996;3(7):660-667.
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med . 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med . 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care . 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry . 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain . 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract . 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines. Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert]. Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med . 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med . 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med . 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.
20. Washington State Agency Medical Directors’ Group. Opioid dose calculator. http://www.agen cymeddirectors.wa.gov/Calculator/DoseCalculator.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med . 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA . 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care . 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care . 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care . 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med . 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med . 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend . 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician . 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med . 1996;3(7):660-667.
Case Studies in Toxicology: Drink the Water, but Don’t Eat the Paint
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
First EDition: Emergency Physicians’ Rates of Opioid Prescribing, more
BY JEFF BAUER
A large retrospective analysis found a wide variation in opioid prescribing among emergency physicians (EPs) working within the same ED. The study also found that Medicare patients treated by EPs who wrote the most prescriptions for opioids were more likely to use opioids for 6 months after their ED visit than were those treated by EPs who wrote fewer opioid prescriptions.
Researchers evaluated initial visits to an ED by approximately 378,000 Medicare beneficiaries (average age: 68 years) from 2008 through 2011. None of these patients had received a prescription for an opioid in the 6 months before the ED visit, and none of the visits resulted in a hospital admission. Prescriptions for opioids (excluding methadone) were identified by the national drug code in the Medicare Part D database. An opioid prescription was attributed to the treating EP if the patient filled the prescription within 3 days after the ED visit.
Investigators categorized the treating EPs in this study as “high-intensity” or “low-intensity” opioid prescribers by calculating the proportion of all ED visits that resulted in an opioid prescription being filled. They then grouped the EPs into quartiles of opioid prescribing within each hospital. High-intensity prescribers were those in the top quartile of opioid prescribing rates, and low-intensity prescribers were those in the bottom quartile.
The primary outcome was long-term opioid use, defined as 6 months or more of opioids supplied in the 12 months after the initial ED visit. This did not include prescriptions filled within 30 days of the initial visit.
Overall, approximately 215,700 patients were treated by low-intensity prescribers and 162,000 by high-intensity prescribers. In general, the patient characteristics and diagnoses were similar in both groups. The rate of opioid prescribing of high-intensity prescribers was approximately triple the rate of low-intensity prescribers. High-intensity prescribers provided an opioid prescription for 21.4% of ED visits, compared to 7.3% among low-intensity prescribers.
Long-term opioid use at 12 months was significantly higher among patients who had been initially treated by high-intensity prescribers compared to those who had been treated by low-intensity prescribers (1.51% vs 1.16%; unadjusted odds ratio [OR], 1.31). There was minimal change in this difference after the results were adjusted for the patients’ age, race, sex, disability status, and presence of chronic conditions (OR, 1.30). The number needed to harm was calculated as 49, meaning theoretically, for every 49 patients who received a new opioid prescription in the ED, one would become a long-term user. The authors noted, however, that “…prescriptions provided by other physicians in the months after an [ED] visit are necessary for long-term opioid use to take hold.”
Researchers pointed out several limitations to their study. Because the study was observational, it could not establish causality. Researchers were not able to directly attribute opioid prescriptions to the treating EPs, but instead used prescriptions filled within 3 days of an ED visits as a surrogate; some opioid prescriptions could have been written by another clinician, such as the patient’s primary care physician during a follow-up visit. Because the study focused on Medicare patients, the results may not be applicable to younger patients. Based on their analysis, researchers could not determine whether an opioid prescription was appropriate, and therefore they could not quantify the extent of opioid overprescribing.
For more on EPs and opioid prescribing, see “The New Opioid Epidemic and the Law of Unintended Consequences” by Emergency Medicine Editor in Chief Neal Flomenbaum, MD (Emergency Medicine. 2017;49[2]:52) and “The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs” by Rama B. Rao, MD and Emergency Medicine Associate Editor, Toxicology Lewis S. Nelson, MD (Emergency Medicine. 2017;49[2]:64-70).
Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524.
Lower Admission Rates, Other Factors Tied to High Rate of Death Soon After ED Discharge Among Older Adults
BY JEFF BAUER
Each year, approximately 10,000 older adult patients die within 7 days of discharge from an ED in the United States, despite having no obvious life-threatening illness, according to a large retrospective study. Emergency departments with lower rates of inpatient admission from the ED, lower patient volumes, and lower charges had significantly higher rates of death after discharge.
Researchers evaluated Medicare claims data related to slightly more than 10 million ED visits from 2007 to 2012. Because the goal was to study generally healthy patients, the following patients were excluded: individuals who were age 90 years and older; were receiving palliative or hospice care; or had received a life-limiting diagnosis, such as a myocardial infarction (MI) or a malignancy, either in the ED or in the year prior to the ED visit. The primary outcome was death within 7 days after discharge from an ED. The cause of death was determined by linking claims to death certificates; this information was available only for a subset of patients who visited an ED in 2007 or 2008.
Overall, during the 6-year study, 0.12% of discharged patients died within 7 days of discharge; this translates to more than 10,000 early deaths per year nationally. The leading causes of death were atherosclerotic heart disease (13.6%), MI (10.3%), and chronic obstructive pulmonary disease (9.6%).
Emergency departments ranked in the lowest fifth for admission rates admitted 15% of patients, compared to 56% of patients at EDs with the highest admission rates. The early death rate of patients treated at EDs with the lowest rates of inpatient admissions from the ED was 3.4 times higher than the death rate seen in EDs with the highest inpatient admission rates (0.27% vs 0.08%, respectively). This was true despite the fact that EDs with low-admission rates treated healthier patients, as evidenced by the overall 7-day mortality rate of all patients treated in the ED, whether they were admitted or discharged. Emergency departments that saw higher volumes of patients and had higher charges for visits had significantly fewer deaths.
Obermeyer Z, Cohn B, Wilson M, Jena AB, Cutler DM. Early death after discharge from emergency departments: analysis of national US insurance claims data. BMJ. 2017;356:j239. doi:10.1136/bmj.j239.
Tertiary Center Repeat Computed Tomography Scans Find Additional Injuries
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Imaging obtained at nontertiary trauma centers (NTCs) probably does not tell the whole story of a trauma patient’s injuries, according to a new retrospective study.
Repeat scans done at a Level 1 trauma center identified new injuries in 76% of patients who were transferred, Morgan Bonds, MD, reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma. About half of these previously unobserved injuries were considered clinically significant, said Dr Bonds, a surgical resident at the University of Oklahoma, Oklahoma City.
Her study examined imaging and clinical assessment of 203 trauma patients who were initially worked up at an NTC, and then transferred to the Level 1 University of Oklahoma tertiary trauma center (TTC). The facility’s primary radiologist reviewed all of the initial computed tomography (CT) scans while blinded to the NTC interpretation. The initial scans and interpretations were then compared with those done at the TTC.
The team split imaging and interpretation disconnects into four categories:
- Type A errors: A missed injury on the NTC scan. “This represents the expertise and experience of our primary radiologist,” Dr Bonds said.
- Type B errors: Missed injuries on scans where NTC radiologists saw other injuries that the TTC radiologist did not confirm. “This represents the experience of our radiologist and also the inexperience and overreaction of the NTC radiologists.”
- Type C errors: New injuries seen on additional TTC imaging of the same body area. “This represents the quality of the image.”
- Type D errors: New injuries found upon any new imaging, whether of a previously scanned or newly scanned body area. “This represents quality of work-up—the decision of the trauma team to more fully investigate the patient’s injuries, as well as the quality of the CT tech performing the scan.”
During the study period, 203 patients presented at the TTC with prior scans conducted at an NTC.
The mean age of the patients was 43 years; most (67%) were men. The mean Injury Severity Score was 16; 97% had experienced blunt trauma. Shock was present in 3% and a traumatic brain injury in 8%. Repeat scans were most common for neck and cervical spine injuries (54%) and thoracic/lumbar spine injuries (53%), and least common for chest injuries (32%).
An inadequate NTC work-up as judged by the TTC attending was the most common reason for obtaining new images (76%). Poor image quality was the next most common reason (31%).
Among the 203 patients, 99 (49%) had a type A error. Of these injuries missed on the initial scan, 90% were considered to be clinically significant.
Type B errors occurred in 15% of patients. Type C errors (new injuries in different body area) occurred in 54% of patients and, of these, 76% were considered clinically significant. Type D errors (new injuries seen in any imaging of any area) occurred in 73% of patients.
“This study confirms that images are often repeated or completed after having images done at NTCs,” Dr Bonds said. “Relying on NTC image interpretation can lead to undertreating our patients. One potential solution to this issue could be image sharing between NTCs and TTCs. This might reduce both the rate of missed injuries and the need for repeat scans.”
Cutaneous Eruption Reported in Pregnant Woman With Locally Acquired Zika Virus
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Zika presented in a young, pregnant Florida woman as erythematous follicular macules and papules on the trunk and arms, scattered tender pink papules on the palms, and a few petechiae on the hard palate, according to a report in the New England Journal of Medicine.
The report advises how Zika virus may present during pregnancy. “Medical providers on the front line should be aware of the constellation of symptoms in patients reporting travel to endemic areas, including areas in Southern Florida, where other non-travel-associated cases have been confirmed,” wrote investigators led by Lucy Chen, MD, of the University of Miami.
The 23-year-old woman presented on July 7, 2016 at 23 weeks and 3 days’ gestation with a 3-day history of fever, widespread pruritic rash, and sore throat, which were followed by myalgias and joint pain 2 days later. The cutaneous eruption was noted on physical examination; neither conjunctivitis nor lymphadenopathy was present. The patient and her partner said they had not traveled outside the United States for 2 years.
Zika virus RNA was detected in the woman’s urine and serum specimens with the use of reverse-transcriptase polymerase chain reaction and persisted for 2 weeks in urine samples and for 6 weeks in serum samples. On histopathology, skin lesions revealed a mild perivascular lymphocytic infiltration in the upper dermis, admixed with some neutrophils. Liver and renal functions were normal.
Fetal ultrasonography performed on the day of presentation showed an estimated fetal weight of 644 g (53rd percentile), an estimated head circumference of 221 mm (63rd percentile), and normal intracranial anatomy. Fevers and rash subsided after 3 days of supportive care. Screening for measles, varicella, rubella, syphilis, Epstein-Barr virus, influenza, hepatitis B, hepatitis C, mumps, and dengue was negative.
An initial immunoglobulin M test on July 7 was negative; seroconversion occurred 1 week after presentation and remained positive through delivery.
A full-term infant weighing 2,990 g was delivered vaginally. Neonatal ultrasonography and magnetic resonance imaging of the head showed a normal head size and intracranial anatomy, with no calcifications. Placental tissue was negative for Zika virus, and neonatal laboratory testing revealed no evidence of infection.
The case was confirmed by the Miami-Dade County Department of Health as the first non-travel-associated Zika infection in the United States.
Chen L, Hafeez F, Curry CL, Elgart G. Cutaneous eruption in a U.S. woman with locally acquired Zika virus infection. N Engl J Med. 2017;376(4):400-401. doi:10.1056/NEJMc1610614.
Lab Values Poor Surrogate for Detecting Pediatric Rocky Mountain Spotted Fever in Children
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Three fatalities observed in a retrospective analysis of six cases of Rocky Mountain spotted fever (RMSF) in children were associated with either a delayed diagnosis pending laboratory findings or delayed anti-rickettsia treatment, researchers said.
“The fact that all fatal cases died before the convalescent period emphasizes that diagnosis should be based on clinical findings instead of RMSF serologic and histologic testing,” wrote the authors of a study published online in Pediatric Dermatology.
Rechelle Tull of the department of dermatology, Wake Forest University, Winston-Salem, NC, and her colleagues conducted a retrospective review of 3,912 inpatient dermatology consultations over a period of 10 years at a tertiary care center, and identified six patients aged 22 months to 2 years (mean, 5.1 years) diagnosed with RMSF. The patients were evaluated in the months of April, May, and June, and three of the six patients infected with the vector-borne obligate intracellular bacterium, Rickettsia rickettsii, had died within 4 days of hospitalization, according to the authors.
Two of the fatal cases involved delayed anti-rickettsial therapy after the patients were misdiagnosed with group A Streptococcus. None of the six children were initially evaluated for R rickettsii; they averaged three encounters with their clinician before being admitted for acute inpatient care, where they received intravenous doxycycline after nearly a week of symptoms.
“All fatal cases were complicated by neurologic manifestations, including seizures, obtundation, and uncal herniation,” a finding that is consistent with the literature, the authors said.
Although the high-fatality rate might be the result of the small study size, Ms Tull and her coinvestigators concluded that the disease should be considered in all differential diagnoses for children who present with a fever and rash during the summer months in endemic areas, particularly since pediatric cases of the disease are associated with poorer outcomes than adult cases.
Given that RMSF often remains subclinical in its early stages, and typically presents with nonspecific symptoms of fever, rash, headache, and abdominal pain when it does emerge, physicians might be tempted to defer treatment until after serological and histological results are in, as is the standard method. Concerns over doxycycline’s tendency to stain teeth and cause enamel hypoplasia are also common. However, empirical administration could mean the difference between life and death, since treatment within the first 5 days following infection is associated with better outcomes—an algorithm complicated by the fact that symptoms caused by R rickettsii have been known to take as long as 21 days to appear.
In the study, Ms Tull and her colleagues found that the average time between exposure to the tick and the onset of symptoms was 6.6 days (range, 1-21 days).
Currently, there are no diagnostic tests “that reliably diagnose RMSF during the first 7 days of illness,” and most patients “do not develop detectable antibodies until the second week of illness,” the investigators reported. Even then, sensitivity of indirect fluorescent antibody serum testing after the second week of illness is only between 86% and 94%, they noted. Further, the sensitivity of immunohistochemical (IHC) tissue staining has been reported at 70%, and false-negative IHC results are common in acute disease when antibody response is harder to detect.
Ms Tull and her colleagues found that five of the six patients in their study had negative IHC testing; two of the six had positive serum antibody titers. For this reason, they concluded that RMSF diagnosis should be based on “clinical history, examination, and laboratory abnormalities” rather than laboratory testing, and urged that “prompt treatment should be instituted empirically.”
Tull R, Ahn C, Daniel A, Yosipovitch G, Strowd LC. Retrospective study of Rocky Mountain spotted fever in children. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13053. [Epub ahead of print]
BY JEFF BAUER
A large retrospective analysis found a wide variation in opioid prescribing among emergency physicians (EPs) working within the same ED. The study also found that Medicare patients treated by EPs who wrote the most prescriptions for opioids were more likely to use opioids for 6 months after their ED visit than were those treated by EPs who wrote fewer opioid prescriptions.
Researchers evaluated initial visits to an ED by approximately 378,000 Medicare beneficiaries (average age: 68 years) from 2008 through 2011. None of these patients had received a prescription for an opioid in the 6 months before the ED visit, and none of the visits resulted in a hospital admission. Prescriptions for opioids (excluding methadone) were identified by the national drug code in the Medicare Part D database. An opioid prescription was attributed to the treating EP if the patient filled the prescription within 3 days after the ED visit.
Investigators categorized the treating EPs in this study as “high-intensity” or “low-intensity” opioid prescribers by calculating the proportion of all ED visits that resulted in an opioid prescription being filled. They then grouped the EPs into quartiles of opioid prescribing within each hospital. High-intensity prescribers were those in the top quartile of opioid prescribing rates, and low-intensity prescribers were those in the bottom quartile.
The primary outcome was long-term opioid use, defined as 6 months or more of opioids supplied in the 12 months after the initial ED visit. This did not include prescriptions filled within 30 days of the initial visit.
Overall, approximately 215,700 patients were treated by low-intensity prescribers and 162,000 by high-intensity prescribers. In general, the patient characteristics and diagnoses were similar in both groups. The rate of opioid prescribing of high-intensity prescribers was approximately triple the rate of low-intensity prescribers. High-intensity prescribers provided an opioid prescription for 21.4% of ED visits, compared to 7.3% among low-intensity prescribers.
Long-term opioid use at 12 months was significantly higher among patients who had been initially treated by high-intensity prescribers compared to those who had been treated by low-intensity prescribers (1.51% vs 1.16%; unadjusted odds ratio [OR], 1.31). There was minimal change in this difference after the results were adjusted for the patients’ age, race, sex, disability status, and presence of chronic conditions (OR, 1.30). The number needed to harm was calculated as 49, meaning theoretically, for every 49 patients who received a new opioid prescription in the ED, one would become a long-term user. The authors noted, however, that “…prescriptions provided by other physicians in the months after an [ED] visit are necessary for long-term opioid use to take hold.”
Researchers pointed out several limitations to their study. Because the study was observational, it could not establish causality. Researchers were not able to directly attribute opioid prescriptions to the treating EPs, but instead used prescriptions filled within 3 days of an ED visits as a surrogate; some opioid prescriptions could have been written by another clinician, such as the patient’s primary care physician during a follow-up visit. Because the study focused on Medicare patients, the results may not be applicable to younger patients. Based on their analysis, researchers could not determine whether an opioid prescription was appropriate, and therefore they could not quantify the extent of opioid overprescribing.
For more on EPs and opioid prescribing, see “The New Opioid Epidemic and the Law of Unintended Consequences” by Emergency Medicine Editor in Chief Neal Flomenbaum, MD (Emergency Medicine. 2017;49[2]:52) and “The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs” by Rama B. Rao, MD and Emergency Medicine Associate Editor, Toxicology Lewis S. Nelson, MD (Emergency Medicine. 2017;49[2]:64-70).
Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524.
Lower Admission Rates, Other Factors Tied to High Rate of Death Soon After ED Discharge Among Older Adults
BY JEFF BAUER
Each year, approximately 10,000 older adult patients die within 7 days of discharge from an ED in the United States, despite having no obvious life-threatening illness, according to a large retrospective study. Emergency departments with lower rates of inpatient admission from the ED, lower patient volumes, and lower charges had significantly higher rates of death after discharge.
Researchers evaluated Medicare claims data related to slightly more than 10 million ED visits from 2007 to 2012. Because the goal was to study generally healthy patients, the following patients were excluded: individuals who were age 90 years and older; were receiving palliative or hospice care; or had received a life-limiting diagnosis, such as a myocardial infarction (MI) or a malignancy, either in the ED or in the year prior to the ED visit. The primary outcome was death within 7 days after discharge from an ED. The cause of death was determined by linking claims to death certificates; this information was available only for a subset of patients who visited an ED in 2007 or 2008.
Overall, during the 6-year study, 0.12% of discharged patients died within 7 days of discharge; this translates to more than 10,000 early deaths per year nationally. The leading causes of death were atherosclerotic heart disease (13.6%), MI (10.3%), and chronic obstructive pulmonary disease (9.6%).
Emergency departments ranked in the lowest fifth for admission rates admitted 15% of patients, compared to 56% of patients at EDs with the highest admission rates. The early death rate of patients treated at EDs with the lowest rates of inpatient admissions from the ED was 3.4 times higher than the death rate seen in EDs with the highest inpatient admission rates (0.27% vs 0.08%, respectively). This was true despite the fact that EDs with low-admission rates treated healthier patients, as evidenced by the overall 7-day mortality rate of all patients treated in the ED, whether they were admitted or discharged. Emergency departments that saw higher volumes of patients and had higher charges for visits had significantly fewer deaths.
Obermeyer Z, Cohn B, Wilson M, Jena AB, Cutler DM. Early death after discharge from emergency departments: analysis of national US insurance claims data. BMJ. 2017;356:j239. doi:10.1136/bmj.j239.
Tertiary Center Repeat Computed Tomography Scans Find Additional Injuries
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Imaging obtained at nontertiary trauma centers (NTCs) probably does not tell the whole story of a trauma patient’s injuries, according to a new retrospective study.
Repeat scans done at a Level 1 trauma center identified new injuries in 76% of patients who were transferred, Morgan Bonds, MD, reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma. About half of these previously unobserved injuries were considered clinically significant, said Dr Bonds, a surgical resident at the University of Oklahoma, Oklahoma City.
Her study examined imaging and clinical assessment of 203 trauma patients who were initially worked up at an NTC, and then transferred to the Level 1 University of Oklahoma tertiary trauma center (TTC). The facility’s primary radiologist reviewed all of the initial computed tomography (CT) scans while blinded to the NTC interpretation. The initial scans and interpretations were then compared with those done at the TTC.
The team split imaging and interpretation disconnects into four categories:
- Type A errors: A missed injury on the NTC scan. “This represents the expertise and experience of our primary radiologist,” Dr Bonds said.
- Type B errors: Missed injuries on scans where NTC radiologists saw other injuries that the TTC radiologist did not confirm. “This represents the experience of our radiologist and also the inexperience and overreaction of the NTC radiologists.”
- Type C errors: New injuries seen on additional TTC imaging of the same body area. “This represents the quality of the image.”
- Type D errors: New injuries found upon any new imaging, whether of a previously scanned or newly scanned body area. “This represents quality of work-up—the decision of the trauma team to more fully investigate the patient’s injuries, as well as the quality of the CT tech performing the scan.”
During the study period, 203 patients presented at the TTC with prior scans conducted at an NTC.
The mean age of the patients was 43 years; most (67%) were men. The mean Injury Severity Score was 16; 97% had experienced blunt trauma. Shock was present in 3% and a traumatic brain injury in 8%. Repeat scans were most common for neck and cervical spine injuries (54%) and thoracic/lumbar spine injuries (53%), and least common for chest injuries (32%).
An inadequate NTC work-up as judged by the TTC attending was the most common reason for obtaining new images (76%). Poor image quality was the next most common reason (31%).
Among the 203 patients, 99 (49%) had a type A error. Of these injuries missed on the initial scan, 90% were considered to be clinically significant.
Type B errors occurred in 15% of patients. Type C errors (new injuries in different body area) occurred in 54% of patients and, of these, 76% were considered clinically significant. Type D errors (new injuries seen in any imaging of any area) occurred in 73% of patients.
“This study confirms that images are often repeated or completed after having images done at NTCs,” Dr Bonds said. “Relying on NTC image interpretation can lead to undertreating our patients. One potential solution to this issue could be image sharing between NTCs and TTCs. This might reduce both the rate of missed injuries and the need for repeat scans.”
Cutaneous Eruption Reported in Pregnant Woman With Locally Acquired Zika Virus
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Zika presented in a young, pregnant Florida woman as erythematous follicular macules and papules on the trunk and arms, scattered tender pink papules on the palms, and a few petechiae on the hard palate, according to a report in the New England Journal of Medicine.
The report advises how Zika virus may present during pregnancy. “Medical providers on the front line should be aware of the constellation of symptoms in patients reporting travel to endemic areas, including areas in Southern Florida, where other non-travel-associated cases have been confirmed,” wrote investigators led by Lucy Chen, MD, of the University of Miami.
The 23-year-old woman presented on July 7, 2016 at 23 weeks and 3 days’ gestation with a 3-day history of fever, widespread pruritic rash, and sore throat, which were followed by myalgias and joint pain 2 days later. The cutaneous eruption was noted on physical examination; neither conjunctivitis nor lymphadenopathy was present. The patient and her partner said they had not traveled outside the United States for 2 years.
Zika virus RNA was detected in the woman’s urine and serum specimens with the use of reverse-transcriptase polymerase chain reaction and persisted for 2 weeks in urine samples and for 6 weeks in serum samples. On histopathology, skin lesions revealed a mild perivascular lymphocytic infiltration in the upper dermis, admixed with some neutrophils. Liver and renal functions were normal.
Fetal ultrasonography performed on the day of presentation showed an estimated fetal weight of 644 g (53rd percentile), an estimated head circumference of 221 mm (63rd percentile), and normal intracranial anatomy. Fevers and rash subsided after 3 days of supportive care. Screening for measles, varicella, rubella, syphilis, Epstein-Barr virus, influenza, hepatitis B, hepatitis C, mumps, and dengue was negative.
An initial immunoglobulin M test on July 7 was negative; seroconversion occurred 1 week after presentation and remained positive through delivery.
A full-term infant weighing 2,990 g was delivered vaginally. Neonatal ultrasonography and magnetic resonance imaging of the head showed a normal head size and intracranial anatomy, with no calcifications. Placental tissue was negative for Zika virus, and neonatal laboratory testing revealed no evidence of infection.
The case was confirmed by the Miami-Dade County Department of Health as the first non-travel-associated Zika infection in the United States.
Chen L, Hafeez F, Curry CL, Elgart G. Cutaneous eruption in a U.S. woman with locally acquired Zika virus infection. N Engl J Med. 2017;376(4):400-401. doi:10.1056/NEJMc1610614.
Lab Values Poor Surrogate for Detecting Pediatric Rocky Mountain Spotted Fever in Children
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Three fatalities observed in a retrospective analysis of six cases of Rocky Mountain spotted fever (RMSF) in children were associated with either a delayed diagnosis pending laboratory findings or delayed anti-rickettsia treatment, researchers said.
“The fact that all fatal cases died before the convalescent period emphasizes that diagnosis should be based on clinical findings instead of RMSF serologic and histologic testing,” wrote the authors of a study published online in Pediatric Dermatology.
Rechelle Tull of the department of dermatology, Wake Forest University, Winston-Salem, NC, and her colleagues conducted a retrospective review of 3,912 inpatient dermatology consultations over a period of 10 years at a tertiary care center, and identified six patients aged 22 months to 2 years (mean, 5.1 years) diagnosed with RMSF. The patients were evaluated in the months of April, May, and June, and three of the six patients infected with the vector-borne obligate intracellular bacterium, Rickettsia rickettsii, had died within 4 days of hospitalization, according to the authors.
Two of the fatal cases involved delayed anti-rickettsial therapy after the patients were misdiagnosed with group A Streptococcus. None of the six children were initially evaluated for R rickettsii; they averaged three encounters with their clinician before being admitted for acute inpatient care, where they received intravenous doxycycline after nearly a week of symptoms.
“All fatal cases were complicated by neurologic manifestations, including seizures, obtundation, and uncal herniation,” a finding that is consistent with the literature, the authors said.
Although the high-fatality rate might be the result of the small study size, Ms Tull and her coinvestigators concluded that the disease should be considered in all differential diagnoses for children who present with a fever and rash during the summer months in endemic areas, particularly since pediatric cases of the disease are associated with poorer outcomes than adult cases.
Given that RMSF often remains subclinical in its early stages, and typically presents with nonspecific symptoms of fever, rash, headache, and abdominal pain when it does emerge, physicians might be tempted to defer treatment until after serological and histological results are in, as is the standard method. Concerns over doxycycline’s tendency to stain teeth and cause enamel hypoplasia are also common. However, empirical administration could mean the difference between life and death, since treatment within the first 5 days following infection is associated with better outcomes—an algorithm complicated by the fact that symptoms caused by R rickettsii have been known to take as long as 21 days to appear.
In the study, Ms Tull and her colleagues found that the average time between exposure to the tick and the onset of symptoms was 6.6 days (range, 1-21 days).
Currently, there are no diagnostic tests “that reliably diagnose RMSF during the first 7 days of illness,” and most patients “do not develop detectable antibodies until the second week of illness,” the investigators reported. Even then, sensitivity of indirect fluorescent antibody serum testing after the second week of illness is only between 86% and 94%, they noted. Further, the sensitivity of immunohistochemical (IHC) tissue staining has been reported at 70%, and false-negative IHC results are common in acute disease when antibody response is harder to detect.
Ms Tull and her colleagues found that five of the six patients in their study had negative IHC testing; two of the six had positive serum antibody titers. For this reason, they concluded that RMSF diagnosis should be based on “clinical history, examination, and laboratory abnormalities” rather than laboratory testing, and urged that “prompt treatment should be instituted empirically.”
Tull R, Ahn C, Daniel A, Yosipovitch G, Strowd LC. Retrospective study of Rocky Mountain spotted fever in children. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13053. [Epub ahead of print]
BY JEFF BAUER
A large retrospective analysis found a wide variation in opioid prescribing among emergency physicians (EPs) working within the same ED. The study also found that Medicare patients treated by EPs who wrote the most prescriptions for opioids were more likely to use opioids for 6 months after their ED visit than were those treated by EPs who wrote fewer opioid prescriptions.
Researchers evaluated initial visits to an ED by approximately 378,000 Medicare beneficiaries (average age: 68 years) from 2008 through 2011. None of these patients had received a prescription for an opioid in the 6 months before the ED visit, and none of the visits resulted in a hospital admission. Prescriptions for opioids (excluding methadone) were identified by the national drug code in the Medicare Part D database. An opioid prescription was attributed to the treating EP if the patient filled the prescription within 3 days after the ED visit.
Investigators categorized the treating EPs in this study as “high-intensity” or “low-intensity” opioid prescribers by calculating the proportion of all ED visits that resulted in an opioid prescription being filled. They then grouped the EPs into quartiles of opioid prescribing within each hospital. High-intensity prescribers were those in the top quartile of opioid prescribing rates, and low-intensity prescribers were those in the bottom quartile.
The primary outcome was long-term opioid use, defined as 6 months or more of opioids supplied in the 12 months after the initial ED visit. This did not include prescriptions filled within 30 days of the initial visit.
Overall, approximately 215,700 patients were treated by low-intensity prescribers and 162,000 by high-intensity prescribers. In general, the patient characteristics and diagnoses were similar in both groups. The rate of opioid prescribing of high-intensity prescribers was approximately triple the rate of low-intensity prescribers. High-intensity prescribers provided an opioid prescription for 21.4% of ED visits, compared to 7.3% among low-intensity prescribers.
Long-term opioid use at 12 months was significantly higher among patients who had been initially treated by high-intensity prescribers compared to those who had been treated by low-intensity prescribers (1.51% vs 1.16%; unadjusted odds ratio [OR], 1.31). There was minimal change in this difference after the results were adjusted for the patients’ age, race, sex, disability status, and presence of chronic conditions (OR, 1.30). The number needed to harm was calculated as 49, meaning theoretically, for every 49 patients who received a new opioid prescription in the ED, one would become a long-term user. The authors noted, however, that “…prescriptions provided by other physicians in the months after an [ED] visit are necessary for long-term opioid use to take hold.”
Researchers pointed out several limitations to their study. Because the study was observational, it could not establish causality. Researchers were not able to directly attribute opioid prescriptions to the treating EPs, but instead used prescriptions filled within 3 days of an ED visits as a surrogate; some opioid prescriptions could have been written by another clinician, such as the patient’s primary care physician during a follow-up visit. Because the study focused on Medicare patients, the results may not be applicable to younger patients. Based on their analysis, researchers could not determine whether an opioid prescription was appropriate, and therefore they could not quantify the extent of opioid overprescribing.
For more on EPs and opioid prescribing, see “The New Opioid Epidemic and the Law of Unintended Consequences” by Emergency Medicine Editor in Chief Neal Flomenbaum, MD (Emergency Medicine. 2017;49[2]:52) and “The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs” by Rama B. Rao, MD and Emergency Medicine Associate Editor, Toxicology Lewis S. Nelson, MD (Emergency Medicine. 2017;49[2]:64-70).
Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524.
Lower Admission Rates, Other Factors Tied to High Rate of Death Soon After ED Discharge Among Older Adults
BY JEFF BAUER
Each year, approximately 10,000 older adult patients die within 7 days of discharge from an ED in the United States, despite having no obvious life-threatening illness, according to a large retrospective study. Emergency departments with lower rates of inpatient admission from the ED, lower patient volumes, and lower charges had significantly higher rates of death after discharge.
Researchers evaluated Medicare claims data related to slightly more than 10 million ED visits from 2007 to 2012. Because the goal was to study generally healthy patients, the following patients were excluded: individuals who were age 90 years and older; were receiving palliative or hospice care; or had received a life-limiting diagnosis, such as a myocardial infarction (MI) or a malignancy, either in the ED or in the year prior to the ED visit. The primary outcome was death within 7 days after discharge from an ED. The cause of death was determined by linking claims to death certificates; this information was available only for a subset of patients who visited an ED in 2007 or 2008.
Overall, during the 6-year study, 0.12% of discharged patients died within 7 days of discharge; this translates to more than 10,000 early deaths per year nationally. The leading causes of death were atherosclerotic heart disease (13.6%), MI (10.3%), and chronic obstructive pulmonary disease (9.6%).
Emergency departments ranked in the lowest fifth for admission rates admitted 15% of patients, compared to 56% of patients at EDs with the highest admission rates. The early death rate of patients treated at EDs with the lowest rates of inpatient admissions from the ED was 3.4 times higher than the death rate seen in EDs with the highest inpatient admission rates (0.27% vs 0.08%, respectively). This was true despite the fact that EDs with low-admission rates treated healthier patients, as evidenced by the overall 7-day mortality rate of all patients treated in the ED, whether they were admitted or discharged. Emergency departments that saw higher volumes of patients and had higher charges for visits had significantly fewer deaths.
Obermeyer Z, Cohn B, Wilson M, Jena AB, Cutler DM. Early death after discharge from emergency departments: analysis of national US insurance claims data. BMJ. 2017;356:j239. doi:10.1136/bmj.j239.
Tertiary Center Repeat Computed Tomography Scans Find Additional Injuries
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Imaging obtained at nontertiary trauma centers (NTCs) probably does not tell the whole story of a trauma patient’s injuries, according to a new retrospective study.
Repeat scans done at a Level 1 trauma center identified new injuries in 76% of patients who were transferred, Morgan Bonds, MD, reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma. About half of these previously unobserved injuries were considered clinically significant, said Dr Bonds, a surgical resident at the University of Oklahoma, Oklahoma City.
Her study examined imaging and clinical assessment of 203 trauma patients who were initially worked up at an NTC, and then transferred to the Level 1 University of Oklahoma tertiary trauma center (TTC). The facility’s primary radiologist reviewed all of the initial computed tomography (CT) scans while blinded to the NTC interpretation. The initial scans and interpretations were then compared with those done at the TTC.
The team split imaging and interpretation disconnects into four categories:
- Type A errors: A missed injury on the NTC scan. “This represents the expertise and experience of our primary radiologist,” Dr Bonds said.
- Type B errors: Missed injuries on scans where NTC radiologists saw other injuries that the TTC radiologist did not confirm. “This represents the experience of our radiologist and also the inexperience and overreaction of the NTC radiologists.”
- Type C errors: New injuries seen on additional TTC imaging of the same body area. “This represents the quality of the image.”
- Type D errors: New injuries found upon any new imaging, whether of a previously scanned or newly scanned body area. “This represents quality of work-up—the decision of the trauma team to more fully investigate the patient’s injuries, as well as the quality of the CT tech performing the scan.”
During the study period, 203 patients presented at the TTC with prior scans conducted at an NTC.
The mean age of the patients was 43 years; most (67%) were men. The mean Injury Severity Score was 16; 97% had experienced blunt trauma. Shock was present in 3% and a traumatic brain injury in 8%. Repeat scans were most common for neck and cervical spine injuries (54%) and thoracic/lumbar spine injuries (53%), and least common for chest injuries (32%).
An inadequate NTC work-up as judged by the TTC attending was the most common reason for obtaining new images (76%). Poor image quality was the next most common reason (31%).
Among the 203 patients, 99 (49%) had a type A error. Of these injuries missed on the initial scan, 90% were considered to be clinically significant.
Type B errors occurred in 15% of patients. Type C errors (new injuries in different body area) occurred in 54% of patients and, of these, 76% were considered clinically significant. Type D errors (new injuries seen in any imaging of any area) occurred in 73% of patients.
“This study confirms that images are often repeated or completed after having images done at NTCs,” Dr Bonds said. “Relying on NTC image interpretation can lead to undertreating our patients. One potential solution to this issue could be image sharing between NTCs and TTCs. This might reduce both the rate of missed injuries and the need for repeat scans.”
Cutaneous Eruption Reported in Pregnant Woman With Locally Acquired Zika Virus
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Zika presented in a young, pregnant Florida woman as erythematous follicular macules and papules on the trunk and arms, scattered tender pink papules on the palms, and a few petechiae on the hard palate, according to a report in the New England Journal of Medicine.
The report advises how Zika virus may present during pregnancy. “Medical providers on the front line should be aware of the constellation of symptoms in patients reporting travel to endemic areas, including areas in Southern Florida, where other non-travel-associated cases have been confirmed,” wrote investigators led by Lucy Chen, MD, of the University of Miami.
The 23-year-old woman presented on July 7, 2016 at 23 weeks and 3 days’ gestation with a 3-day history of fever, widespread pruritic rash, and sore throat, which were followed by myalgias and joint pain 2 days later. The cutaneous eruption was noted on physical examination; neither conjunctivitis nor lymphadenopathy was present. The patient and her partner said they had not traveled outside the United States for 2 years.
Zika virus RNA was detected in the woman’s urine and serum specimens with the use of reverse-transcriptase polymerase chain reaction and persisted for 2 weeks in urine samples and for 6 weeks in serum samples. On histopathology, skin lesions revealed a mild perivascular lymphocytic infiltration in the upper dermis, admixed with some neutrophils. Liver and renal functions were normal.
Fetal ultrasonography performed on the day of presentation showed an estimated fetal weight of 644 g (53rd percentile), an estimated head circumference of 221 mm (63rd percentile), and normal intracranial anatomy. Fevers and rash subsided after 3 days of supportive care. Screening for measles, varicella, rubella, syphilis, Epstein-Barr virus, influenza, hepatitis B, hepatitis C, mumps, and dengue was negative.
An initial immunoglobulin M test on July 7 was negative; seroconversion occurred 1 week after presentation and remained positive through delivery.
A full-term infant weighing 2,990 g was delivered vaginally. Neonatal ultrasonography and magnetic resonance imaging of the head showed a normal head size and intracranial anatomy, with no calcifications. Placental tissue was negative for Zika virus, and neonatal laboratory testing revealed no evidence of infection.
The case was confirmed by the Miami-Dade County Department of Health as the first non-travel-associated Zika infection in the United States.
Chen L, Hafeez F, Curry CL, Elgart G. Cutaneous eruption in a U.S. woman with locally acquired Zika virus infection. N Engl J Med. 2017;376(4):400-401. doi:10.1056/NEJMc1610614.
Lab Values Poor Surrogate for Detecting Pediatric Rocky Mountain Spotted Fever in Children
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Three fatalities observed in a retrospective analysis of six cases of Rocky Mountain spotted fever (RMSF) in children were associated with either a delayed diagnosis pending laboratory findings or delayed anti-rickettsia treatment, researchers said.
“The fact that all fatal cases died before the convalescent period emphasizes that diagnosis should be based on clinical findings instead of RMSF serologic and histologic testing,” wrote the authors of a study published online in Pediatric Dermatology.
Rechelle Tull of the department of dermatology, Wake Forest University, Winston-Salem, NC, and her colleagues conducted a retrospective review of 3,912 inpatient dermatology consultations over a period of 10 years at a tertiary care center, and identified six patients aged 22 months to 2 years (mean, 5.1 years) diagnosed with RMSF. The patients were evaluated in the months of April, May, and June, and three of the six patients infected with the vector-borne obligate intracellular bacterium, Rickettsia rickettsii, had died within 4 days of hospitalization, according to the authors.
Two of the fatal cases involved delayed anti-rickettsial therapy after the patients were misdiagnosed with group A Streptococcus. None of the six children were initially evaluated for R rickettsii; they averaged three encounters with their clinician before being admitted for acute inpatient care, where they received intravenous doxycycline after nearly a week of symptoms.
“All fatal cases were complicated by neurologic manifestations, including seizures, obtundation, and uncal herniation,” a finding that is consistent with the literature, the authors said.
Although the high-fatality rate might be the result of the small study size, Ms Tull and her coinvestigators concluded that the disease should be considered in all differential diagnoses for children who present with a fever and rash during the summer months in endemic areas, particularly since pediatric cases of the disease are associated with poorer outcomes than adult cases.
Given that RMSF often remains subclinical in its early stages, and typically presents with nonspecific symptoms of fever, rash, headache, and abdominal pain when it does emerge, physicians might be tempted to defer treatment until after serological and histological results are in, as is the standard method. Concerns over doxycycline’s tendency to stain teeth and cause enamel hypoplasia are also common. However, empirical administration could mean the difference between life and death, since treatment within the first 5 days following infection is associated with better outcomes—an algorithm complicated by the fact that symptoms caused by R rickettsii have been known to take as long as 21 days to appear.
In the study, Ms Tull and her colleagues found that the average time between exposure to the tick and the onset of symptoms was 6.6 days (range, 1-21 days).
Currently, there are no diagnostic tests “that reliably diagnose RMSF during the first 7 days of illness,” and most patients “do not develop detectable antibodies until the second week of illness,” the investigators reported. Even then, sensitivity of indirect fluorescent antibody serum testing after the second week of illness is only between 86% and 94%, they noted. Further, the sensitivity of immunohistochemical (IHC) tissue staining has been reported at 70%, and false-negative IHC results are common in acute disease when antibody response is harder to detect.
Ms Tull and her colleagues found that five of the six patients in their study had negative IHC testing; two of the six had positive serum antibody titers. For this reason, they concluded that RMSF diagnosis should be based on “clinical history, examination, and laboratory abnormalities” rather than laboratory testing, and urged that “prompt treatment should be instituted empirically.”
Tull R, Ahn C, Daniel A, Yosipovitch G, Strowd LC. Retrospective study of Rocky Mountain spotted fever in children. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13053. [Epub ahead of print]
The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
The New Opioid Epidemic and the Law of Unintended Consequences
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.