User login
Gestational hypertension-diabetes combo signals CVD risk
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
FROM JAMA NETWORK OPEN
Buprenorphine linked with lower risk for neonatal harms than methadone
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Women need not wait to conceive after miscarriage, abortion
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who conceived within 6 months of having a miscarriage or an induced abortion did not appear to be at an increased risk of a problematic pregnancy, a new study of more than 70,000 live births in Norway has found.
The findings, published online in PLOS Medicine, should help women and clinicians navigate conflicting guidance over how soon it is safe to conceive again after a pregnancy loss, said Gizachew Tessema, PhD, senior research fellow at Curtin University, Perth, Australia, and the lead author of the research.
“Especially after a miscarriage, women want to conceive again,” Dr. Tessema told this news organization. “Why should they wait if there’s no increased risk?”
On the international front, the World Health Organization advises patients not to attempt to become pregnant until a minimum of 6 months after an abortion or miscarriage. Those 2007 recommendations spurred Dr. Tessema and his colleagues to take a deeper dive into risk factors associated with pregnancies following a shorter interval.
Two-thirds of women in the study conceived again within 6 months of having a miscarriage. Only a quarter of women who had an induced abortion were pregnant again within that same timeframe.
Using Norway’s national health registries, the researchers examined the outcomes of 49,058 births following a miscarriage and 23,707 births after an induced abortion between 2008 and 2016. The birth registry includes information on livebirths, stillbirths, miscarriages, and induced abortions, with detailed descriptions provided around how a miscarriage or abortion is identified. The study included only miscarriages reported through the health care system.
Expanding on other studies that have shown no adverse outcomes with those pregnancy intervals, Dr. Tessema and colleagues found that women who became pregnant shortly after a miscarriage or abortion were not at a higher risk for delivering preterm, having newborns that were small for gestational age (SGA) or large for gestational age (LGA), or developing preeclampsia or gestational diabetes.
Dr. Tessema and his colleagues found a slightly smaller percentage of women who conceived within 3 months, compared with those who became pregnant within 6-11 months after a miscarriage (8.6% to 10.1%). Women who conceived within 3 months of an induced abortion had a slightly, but statistically nonsignificant (P = .07), increased risk for SGA, compared with those who conceived between 6 and 11 months (11.5% to 10%).
No greater risk was shown for the other adverse outcomes – preterm births, LGA, preeclampsia, and GDM – for women who became pregnant within 6 months of an abortion or miscarriage.
The results should reassure women who want to get pregnant again soon after abortions or miscarriage, according to Scott Sullivan, MD, the director of high-risk ob.gyn. at Inova Health, Fairfax, Va.
Often, patients hear conflicting advice from doctors, friends, or medical associations about the best time to try for a baby following a miscarriage or abortion, in part because there are differences in various guidelines. Adding to the confusion is a lack of robust research and data on pregnancy loss, especially in the United States, he said.
“The entire topic of pregnancy loss is underappreciated by the public at large – how painful this is for people, how common it is,” Dr. Sullivan said in an interview. “We need research and resources on it. It’s not even tracked routinely in the United States like it is in other countries.”
Dr. Sullivan said he typically tells patients they can try to get pregnant again right away, following recommendations from the American College of Obstetricians and Gynecologists, which say that patients can conceive as quickly as 2 weeks after an early pregnancy loss.
But he cautions that not all patients are mentally ready to make another attempt that soon, especially if they are still grieving their pregnancy loss.
“Even if you’re physically ready, a lot of people are not emotionally ready, because there’s a grieving process,” Dr. Sullivan said. “That’s very different for people.”
The WHO’s guidelines for developed countries
The WHO developed its guidelines based on research from lower income countries, including one study across Latin America that concluded pregnancy outcomes were worse for women who waited less than 6 months to conceive following an abortion or miscarriage.
Dr. Tessema noted his research is limited because it focused on Norway, a high-income country where women have guaranteed access to health care. Outcomes may be worse in developing countries where incomes are lower and health care inequality is greater, he said.
“The issue is when this international guideline was developed, most of the evidence is from low- and middle-income countries,” Dr. Tessema said. “No studies were conducted from high income cities. We said: ‘This is a different context.’ These recommendations may not be appropriate for this setting.”
The study was supported with funding by the Research Council of Norway through its Centres of Excellence funding program, the National Health and Medical Research Council, the Raine Medical Research Foundation, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme. None of the authors report relevant financial relationships.
A version of this article first appeared on Medscape.com.
Commentary: Shoulder dystocia and vaginal breech deliveries, December 2022
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
The safety of vaginal breech delivery has been controversial since the Term Breech Trial in 2000 suggested increased neonatal mortality and short-term morbidity associated with vaginal breech delivery. The stance against breech delivery has softened since that time. Fruscalzo and colleagues provide yet more evidence supporting the safety of vaginal breech deliveries with their single-center, retrospective study, which included 804 singleton pregnant women who underwent vaginal breech vs emergency cesarean section vs elective cesarean section in Coesfeld, Germany. They found no significant differences between the vaginal breech–delivery group vs the other two groups in regard to umbilical artery pH < 7, low Apgar scores, or neonatal intensive care unit admissions. The only significant difference noted was umbilical artery pH < 7.1. This suggests that in experienced hands (each of the candidates was referred to a senior obstetrician for consultation), vaginal breech delivery can be safe, including for nulliparous women (67% were nulliparous), showing that even the short-term morbidity associated with vaginal breech delivery approaches that of planned cesarean section.
Two other articles raise caution regarding SD and increased risk for fetal death and PPH. Linde and colleagues used data from The Medical Birth Registry of Norway and Statistics Norway to examine recurrence risk for PPH associated with various causes. PPH associated with SD led the way: The recurrence risk adjusted odds ratio (aOR) was 6.8 for SD vs 5.9 for retained products of conception, 4.0 for uterine atony, 3.9 for obstetric trauma, and 2.2 for PPH of undefined cause. This study suggests that the risks for SD recurrence should be focused not just on SD, but also on PPH. Another concern regarding shoulder dystocia is raised by Davidesko and colleagues in their analysis of risk factors for intrapartum fetal death. Using a generalized estimation equation model to help identify independent risk factors for intrapartum fetal death, they examined 344,536 deliveries from 1991 to 2016 at Soroka University Medical Center in Israel and noted that SD again led the way: aOR was 23.8 for SD vs 19.0 for uterine rupture, 11.9 for preterm birth, 6.2 for placental abruption, and 3.6 for fetal malpresentation. This high risk for intrapartum fetal death associated with SD suggests a need for even more robust SD drills to help deal with this dreaded and often unpredictable obstetric emergency.
Surgical management of early pregnancy loss
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
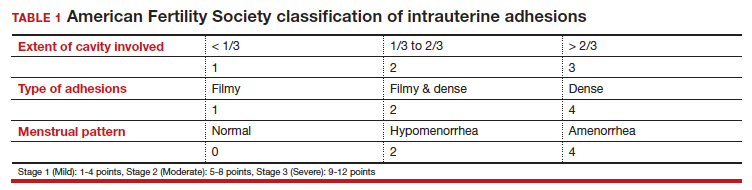
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).
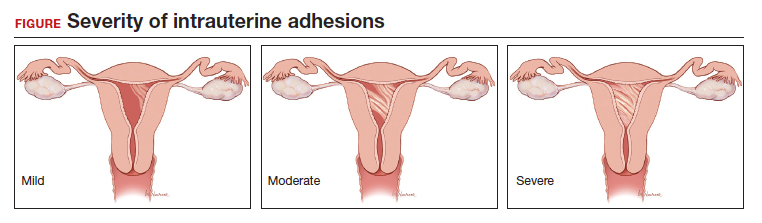
Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
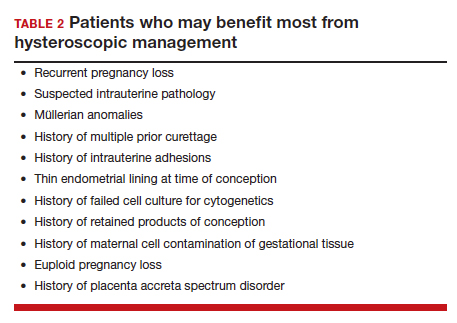
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.
CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
Twins born from embryos frozen 30 years ago
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
At-home births rose during the pandemic, CDC reports
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
Long-term behavioral follow-up of children exposed to mood stabilizers and antidepressants: A look forward
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Her child was stillborn at 39 weeks. She blames a system that doesn’t always listen to mothers
The day before doctors had scheduled Amanda Duffy to give birth, the baby jolted her awake with a kick.
A few hours later, on that bright Sunday in November 2014, she leaned back on a park bench to watch her 19-month-old son Rogen enjoy his final day of being an only child. In that moment of calm, she realized that the kick that morning was the last time she had felt the baby move.
She told herself not to worry. She had heard that babies can slow down toward the end of a pregnancy and remembered reading that sugary snacks and cold fluids can stimulate a baby’s movement. When she got back to the family’s home in suburban Minneapolis, she drank a large glass of ice water and grabbed a few Tootsie Rolls off the kitchen counter.
But something about seeing her husband, Chris, lace up his shoes to leave for a run prompted her to blurt out, “I haven’t felt the baby kick.”
Chris called Amanda’s doctor, and they headed to the hospital to be checked. Once there, a nurse maneuvered a fetal monitor around Amanda’s belly. When she had trouble locating a heartbeat, she remarked that the baby must be tucked in tight. The doctor walked into the room, turned the screen away from Amanda and Chris and began searching. She was sorry, Amanda remembers her telling them, but she could not find a heartbeat.
Amanda let out a guttural scream. She said the doctor quickly performed an internal exam, which detected faint heart activity, then rushed Amanda into an emergency cesarean section.
She woke up to the sound of doctors talking to Chris. She listened but couldn’t bring herself to face the news. Her doctor told her she needed to open her eyes.
Amanda, then 31, couldn’t fathom that her daughter had died. She said her doctors had never discussed stillbirth with her. It was not mentioned in any of the pregnancy materials she had read. She didn’t even know that stillbirth was a possibility.
But every year more than 20,000 pregnancies in the United States end in stillbirth, the death of an expected child at 20 weeks or more. That number has exceeded infant mortality every year for the last 10 years. It’s 15 times the number of babies who, according to the Centers for Disease Control and Prevention, died of Sudden Infant Death Syndrome, or SIDS, in 2020.
The deaths are not inevitable. One study found that nearly one in four U.S. stillbirths may be preventable. For pregnancies that last 37 weeks or more, that research shows, the figure jumps to nearly half. Thousands more babies could potentially be delivered safely every year.
But federal agencies have not prioritized critical stillbirth-focused studies that could lead to fewer deaths. Nearly two decades ago, both the CDC and the National Institutes of Health launched key stillbirth tracking and research studies, but the agencies ended those projects within about a decade. The CDC never analyzed some of the data that was collected.
Unlike with SIDS, a leading cause of infant death, federal officials have failed to launch a national campaign to reduce the risk of stillbirth or adequately raise awareness about it. Placental exams and autopsies, which can sometimes explain why stillbirths happened, are underutilized, in part because parents are not counseled on their benefits.
Federal agencies, state health departments, hospitals and doctors have also done a poor job of educating expectant parents about stillbirth or diligently counseling on fetal movement, despite research showing that patients who have had a stillbirth are more likely to have experienced abnormal fetal movements, including decreased activity. Neither the CDC nor the NIH have consistently promoted guidance telling those who are pregnant to be aware of their babies’ movement in the womb as a way to possibly reduce their risk of stillbirth.
The American College of Obstetricians and Gynecologists, the nation’s leading obstetrics organization, has been slow to update its own guidance to doctors on managing a stillbirth. In 2009, ACOG issued a set of guidelines that included a single paragraph regarding fetal movement. Those guidelines weren’t significantly updated for another 11 years.
Perhaps it’s no surprise that federal goals for reducing stillbirths keep moving in the wrong direction. In 2005, the U.S. stillbirth rate was 6.2 per 1,000 live births. The U.S. Department of Health and Human Services, in an effort to eliminate health disparities and establish a target that was “better than the best racial or ethnic group rate,” set a goal of reducing it to 4.1 for 2010. When that wasn’t met, federal officials changed their approach and set what they called more “science-based” and “realistic” goals, raising the 2020 target to 5.6. The U.S. still fell short. The 2030 goal of 5.7 was so attainable that it was met before the decade started. The 2020 rate, the most current according to the CDC, is 5.74.
By comparison, other wealthy countries have implemented national action plans to prevent stillbirth through awareness, research and care. Among other approaches, those countries have focused on increasing education around stillbirth and the importance of a baby’s movements, reducing rates of smoking and identifying fetuses that grow too slowly in the womb.
The efforts have paid off. The Netherlands, for instance, has reduced its rate of stillbirths at 28 weeks or later by more than half, from 5.2 in 2000 to 2.3 in 2019, according to a study published last year in The Lancet.
Dr. Bob Silver, chair of the obstetrics/gynecology department at University of Utah Health and a leading stillbirth expert, coauthored the study that estimated nearly one in four stillbirths are potentially preventable, a figure he referred to as conservative. He called on federal agencies to declare stillbirth reduction a priority the same way they have done for premature birth and maternal mortality.
“I’d like to see us say we really want to reduce the rate of stillbirth and raise awareness and try to do all of the reasonable things that may contribute to reducing stillbirths that other countries have done,” Silver said.
The lack of comprehensive attention and action has contributed to a stillbirth crisis, shrouded in an acceptance that some babies just die. Compounding the tragedy is a stigma and guilt so crushing that the first words some mothers utter when their lifeless babies are placed in their arms are “I’m sorry.”
In the hospital room, Amanda Duffy finally opened her eyes. She named her daughter Reese Christine, the name she had picked out for her before they found out she had died. She was 8 pounds, 3 ounces and 20 1/2 inches long and was born with her umbilical cord wrapped tightly around her neck twice. The baby was still warm when the nurse placed her in Amanda’s arms. Amanda was struck by how lovely her daughter was. Rosy skin. Chris’ red hair. Rogen’s chubby cheeks.
As Amanda held Reese, Chris hunched over the toilet, vomiting. Later that night, as he lay next to Amanda on the hospital bed, he held his daughter. He hadn’t initially wanted to see her. He worried she would be disfigured or, worse, that she would be beautiful and he would fall apart when he couldn’t take her home.
The nurses taught Amanda and Chris how to grieve and love simultaneously. One nurse told Amanda how cute Reese was and asked if she could hold her. Another placed ice packs in Reese’s swaddle to preserve her body so Amanda could keep holding her. Amanda asked the nurses to tuck cotton balls soaked in an orange scent into Reese’s blanket so the smell would trigger the memory of her daughter. And just as if Reese had been born alive, the nurses took pictures and made prints of her hands and feet.
“I felt such a deep, abiding love for her,” Amanda said. “And I was so proud to be her mom.”
On the way home from the hospital, Amanda broke down at the sight of Reese’s empty car seat. The next few weeks passed in a sleep-filled fog punctuated by intense periods of crying. The smell of oranges wrecked her. Her breast milk coming in was agonizing, physically and emotionally. She wore sports bras stuffed with ice packs to ease the pain and dry up her milk supply. While Rogen was at day care, she sobbed in his bed.
In the months that followed, Amanda and Chris searched for answers and wondered whether their medical team had missed warning signs. Late at night, Amanda turned to Google to find information about stillbirths. She mailed her medical records to a doctor who studies stillbirths, who she said told her that Reese’s death could have been prevented. They briefly discussed legal action against her doctors, but she said a lawyer told her it would be difficult to sue.
Amanda and Chris pinpointed her last two months of pregnancy as the time things started to go wrong. She had been diagnosed with polyhydramnios, meaning there was excess amniotic fluid in the womb. Her doctor had scheduled additional weekly testing.
One of those ultrasounds revealed problems with the blood flow in the umbilical cord. Reese’s cord also appeared to be wrapped around her neck, Amanda said later, but was told that was less of a concern, since it occurs in about 20% of normal deliveries. At another appointment, Amanda’s medical records show, Reese failed the portion of a test that measures fetal breathing movements.
At 37 weeks, Amanda told one of the midwives the baby’s movements felt different, but, she said, the midwife told her that it was common for movements to feel weaker with polyhydramnios. At that point, Amanda felt her baby was safer outside than inside and, her medical records show, she asked to schedule a C-section.
Despite voicing concerns about a change in the baby’s movement and asking to deliver earlier, Amanda said she and her husband were told by her midwife she couldn’t deliver for another two weeks. The doctor “continues to advise 39wks,” her medical records show. Waiting until 39 weeks is usually based on a guideline that deliveries should not happen before then unless a medical condition specifically warrants it, because early delivery can lead to complications.
Amanda would have to wait until 39 weeks and one day because, she said, her doctors didn’t typically do elective deliveries on weekends. Amanda was disappointed but said she trusted her team of doctors and midwives.
“I’m not a pushy person,” she said. “My husband is not a pushy person. That was out of our comfort zone to be, like, ‘What are we waiting for?’ But really what we wanted them to say was ‘We should deliver you.’”
Amanda’s final appointment was a maximum 30-minute-long ultrasound that combined a number of assessments to check amniotic fluid, fetal muscle tone, breathing and body movement. After 29 minutes of inactivity, Amanda said, the baby moved a hand. In the parking lot, Amanda called her mother, crying in relief. Four more days, she told her.
Less than 24 hours before the scheduled C-section, Reese was stillborn.
Four months after her death, Amanda, then a career advisor at the University of Minnesota, and Chris, a public relations specialist, wrote a letter to the University of Minnesota Medical Center, where Amanda had given birth to her dead daughter. They said they had “no ill feelings” toward anyone, but “it pains us to know that her death could’ve been prevented if we would have been sent to labor and delivery following that ultrasound.”
They noted that though they were told that Reese had passed the final ultrasound where she took 29 minutes to move, they had since come to believe that she had failed because, according to national standards, at least three movements were required. They also blamed a strict adherence to the 39-week guideline. And they encouraged the hospital staff to read more on umbilical cord accidents and acute polyhydramnios, which they later learned carries an increased stillbirth risk.
The positive feelings they had from speaking up were replaced by dismay when the hospital responded with a three-paragraph letter, signed by seven doctors and eight nurses. They said they had reexamined each medical decision in her case and concluded they had made “the best decisions medically possible.” They expressed their sympathy and said it was “so very heartwarming that you are trying to turn your tragic loss into something that will benefit others.”
Amanda felt dismissed by the medical team all over again. She didn’t expect them to admit fault, but she said she hoped that they would at least learn from Reese’s death to do things differently in the future. She was angry, and hurt, and knew that she would need to find a new doctor.
A spokesperson for the University of Minnesota Medical School told ProPublica she could not comment on individual patient cases and did not respond to questions about general protocols. “We share the physicians’ condolences,” she wrote, adding that the doctors and the university “are dedicated to delivering high quality, accessible and inclusive health care.”
For many expectant parents, it’s hard to muster the courage to call a doctor about something they’re not even sure is a problem.
“Moms self-censor a lot. No one wants to be that mom that all the doctors are rolling their eyes at because she’s freaking out over nothing,” said Samantha Banerjee, executive director of PUSH for Empowered Pregnancy, a nonprofit based in New York state that works to prevent stillbirths. Banerjee’s daughter, Alana, was stillborn two days before her due date.
In addition to raising awareness that stillbirths can happen even in low-risk pregnancies, PUSH teaches pregnant people how to advocate for themselves. The volunteers advise them to put their requests in writing and not to spend time drinking juice or lying on their side if they are worried about their baby’s lack of movement. In the majority of cases, a call or visit to the hospital reassures them.
But, the group tells parents, if their baby is in distress, calling their doctor can save their life.
Debbie Haine Vijayvergiya is fighting another narrative: that stillbirths are a rare fluke that “just happen.” When her daughter Autumn Joy was born without a heartbeat in 2011, Haine Vijayvergiya said, her doctor told her having a stillborn baby was as rare as being struck by lightning.
She believed him, but then she looked up the odds of a lightning strike and found they are less than one in a million – and most people survive. In 2020, according to the CDC, there was one stillbirth for about every 175 births.
“I’ve spoken to more women than I can count that said, ‘I raised the red flag, and I was sent home. I was told to eat a piece of cake and have some orange juice and lay on my left side,’ only to wake up the next day and their baby is not alive,” said Haine Vijayvergiya, a New Jersey mother and maternal health advocate.
She has fought for more than a decade to pass stillbirth legislation as her daughter’s legacy. Her current undertaking is her most ambitious. The federal Stillbirth Health Improvement and Education (SHINE) for Autumn Act, named after her daughter, would authorize $9 million a year for five years in federal funding for research, better data collection and training for fetal autopsies. But it is currently sitting in the Senate Committee on Health, Education, Labor, and Pensions.
Not all stillbirths are preventable, and medical experts agree more research is needed to determine who is most at risk and which babies can potentially be saved. Complicating matters is the wide range of risk factors, including hypertension and diabetes, smoking, obesity, being pregnant with multiples, being 35 or older and having had a previous stillbirth.
ProPublica reported this summer on how the U.S. botched the rollout of COVID-19 vaccines for pregnant people, who faced an increased risk for stillbirth if they were unvaccinated and contracted the virus, especially during the Delta wave.
Doctors often work to balance the risk of stillbirth with other dangers, particularly an increased chance of being admitted to neonatal intensive care units or even death of the baby if it is born too early. ACOG and the Society for Maternal-Fetal Medicine have issued guidance to try to slow a rise in elective deliveries before 39 weeks and the potential harm that can result. The Joint Commission, a national accrediting organization, began evaluating hospitals in 2010 based on that standard.
A 2019 study found that the risks of stillbirth slightly increased after the rule went into effect, but fewer infants died after birth. Other studies have not found an effect on stillbirths.
Last year, the obstetric groups updated their guidance to allow doctors to consider an early delivery if a woman has anxiety and a history of stillbirth, writing that a previous stillbirth “may” warrant an early delivery for patients who understand and accept the risks. For those who have previously had a stillbirth, one modeling analysis found that 38 weeks is the optimal timing of delivery, considering the increased risk of another stillbirth.
“A woman who has had a previous stillbirth at 37 weeks – one could argue that it’s cruel and unusual punishment to make her go to 39 weeks with her next pregnancy, although that is the current recommendation,” said Dr. Neil Mandsager, a maternal-fetal medicine specialist in Iowa and a medical advisor to a stillbirth prevention nonprofit.
At or after 40 weeks, the risk of stillbirth increases, especially for women 35 or older. Their risk, research shows, is doubled from 39 weeks to 40 and is more than six times as high at 42 weeks. In 2019 and 2020, a combined 1,200 stillbirths occurred between 40 and 42 weeks, according to the most recent CDC data.
Deciding when a patient should deliver entails weighing the risks to the mother and the infant against a possible stillbirth as the pregnancy continues, said Dr. Mark Turrentine, chair of ACOG’s Clinical Consensus Committee-Obstetrics, which helped create the guidance on managing a stillbirth. He said ACOG has addressed stillbirth in other documents and extensively in its 2021 guidance on fetal surveillance and testing, which is done to reduce the risk of stillbirth.
ACOG said it routinely reviewed its guidance on management of stillbirth but was unable to make significant updates “due to the lack of new, evidence-based research.” While prevention is a great concern to ACOG, Turrentine said it’s difficult to know how many stillbirths are preventable.
He said it’s standard practice for doctors to ask about fetal movement, and ACOG updated its guidance after new research became available. Doctors also need to include patients in decision-making and tailor care to them, he said, whether that›s using aspirin in patients at high risk of preeclampsia – a serious high blood pressure condition during pregnancy – or ordering additional tests.
After Reese’s death, Amanda and Chris Duffy wanted to get pregnant again. They sought out an obstetrician-gynecologist who would educate and listen to them. They set up several consultations until they found Dr. Emily Hawes-Van Pelt, who was recommended by another family who had had a stillbirth.
Hawes-Van Pelt cried with Amanda and Chris at their first meeting.
“I told her I was scared to be involved,” Hawes-Van Pelt said. “It’s such a tricky subsequent pregnancy because there’s so much worry and anxiety about the horrible, awful thing happening again.”
Amanda’s fear of delivering another dead baby led to an all-consuming anxiety, but Hawes-Van Pelt supported her when she asked for additional monitoring, testing and an early delivery.
When Hawes-Van Pelt switched practices midway through Amanda’s pregnancy, Amanda followed her. But the new hospital pushed back on the early delivery.
“We intervene early for poorly controlled diabetes,” Hawes-Van Pelt said. “We intervene early for all sorts of medical issues. Anxiety and prior stillbirth are two medical issues that we can intervene earlier for.”
Hawes-Van Pelt said she learned a lot from caring for Amanda, who made her reevaluate some of her own assumptions around stillbirths.
“I had a horrible fear of scaring women unnecessarily, and then realized that I was just not preparing women or educating them because of my own fears around it,” she said. “If you can carry a human being in your body and birth that human being and take care of it, you can hear those words.”
The hospital eventually agreed to let Hawes-Van Pelt schedule Amanda for a 37-week C-section. But after Amanda was again diagnosed with polyhydramnios, she went in for a C-section even earlier. She gave birth in 2015 to a healthy boy she and Chris named Rhett. Two years later, Amanda and Hawes-Van Pelt followed the same pregnancy plan, and she delivered a girl named Maeda Reese. Amanda chose the name because, when said quickly, it sounds like “made of Reese.”
Federal agencies, national organizations and state and city officials have mobilized in recent years to address maternal mortality, when mothers die during pregnancy, at delivery or soon after childbirth. They have focused on improving data collection, passing legislation and creating awareness campaigns that encourage medical professionals and others to listen when women say something doesn›t feel right.
In 2017, ProPublica and NPR documented the U.S. maternal mortality crisis, including alarming racial disparities.
According to CDC data, Black women face nearly three times the risk of maternal mortality. They also are more than twice – and in some states close to three times – as likely to have a stillbirth than white women, meaning not only are Black mothers dying at a disproportionate rate, so are their babies.
Janet Petersen, a state senator from Iowa, said it gives her hope to see how the country has turned its attention to maternal mortality and disparities in health care. She simply cannot understand why stillbirth isn’t being met with the same urgency.
In 2020, the CDC reported 861 mothers died either while pregnant or within six weeks of giving birth. That same year, 20,854 babies were stillborn.
Stillbirth, Petersen said, is a missing piece of the puzzle. Research shows the likelihood of severe maternal complications was more than four times higher for pregnancies that ended in stillbirths, and mothers who died within six weeks of delivery were more likely to have had a stillbirth.
“We see it over and over again that stillbirth is one of the maternal health care issues that continuously gets ignored,” said Petersen, a Democrat.
Petersen was a young legislator in 2003 when her daughter Grace was born still. Devastated, she thought of her grandmother, who lost a baby to stillbirth in 1920, just a few weeks before women got the right to vote.
“I was laying in my hospital bed thinking, ‘How could this still be happening in our country?’” Petersen recalled. “And it seemed, from the medical perspective, that, well, stillbirth happens. We can’t do anything to prevent them.”
Over the next few months, Petersen heard from other mothers who had lost their babies and wanted to spark change. As an elected official, Petersen was in a position to do that. In 2004, she introduced legislation that required the Iowa Department of Public Health to create a stillbirths work group, later securing funding through the CDC to create a stillbirth registry.
But the CDC didn’t renew the funding and never analyzed the data from the registry, though a CDC spokesperson said the Iowa Department of Public Health examined the data. Officials from the department did not respond to requests for comment.
Petersen and her fellow mothers pivoted. After hearing how researchers in Norway were able to increase awareness around fetal movement, they co-founded a nonprofit aimed at doing the same in the U.S.
The group, Healthy Birth Day, created colorful “Count the Kicks” pamphlets – and later an app – teaching pregnant people how to track a baby’s movements and establish what is normal for them. Monitoring a baby’s movements is the earliest and sometimes only indication that something may be wrong, said Emily Price, chief executive officer of Healthy Birth Day. One of the organization’s main messages is for pregnant people to speak up and clinicians to listen.
“Unfortunately, there are still doctors who brush women off or send them home when they come in with a complaint of a change in their baby’s movements,” Price said. “And babies are dying because of it.”
One Indiana county, which recorded 65 stillbirths from 2017 through 2019, reported that 74% had either some chance or a good chance of prevention, according to St. Joseph County Department of Health’s Fetal Infant Mortality Review program. For mothers who experienced decreased fetal movement in the few hours or days before the stillbirth, that estimate jumped to 90%.
Although there is not a scientific consensus that kick counting can prevent stillbirths, national groups, including ACOG, recommend that medical professionals encourage their patients to be aware of fetal movement patterns. ACOG also advises medical professionals to be attentive to a mother’s concerns about reduced movement and address them “in a systematic way.”
One complaint the CDC hears too often, an agency spokesperson said, is that pregnant people and those who gave birth recently find that their concerns are dismissed or ignored. “Listening and taking the concerns of pregnant and recently pregnant people seriously,” she said, “is a simple, yet powerful action to prevent serious health complications and even death.”
The CDC, she said, is “very interested” in expanding its research on stillbirth, which is “a crucial part of the development of any awareness or prevention campaigns.” In addition to working to improve its stillbirth data quality, the agency has funded some pilot programs at the city and state level to better track stillbirths, survey people who have had a stillbirth and research risk factors and causes. The Iowa registry, she said, led the CDC to fund different research projects in Arkansas and Massachusetts, which are ongoing.
In 2009, the CDC acknowledged that fetal mortality remained a “major, but often overlooked, public health problem.” Officials wrote that much of the public health concern had been focused on infant mortality “in part due to lesser awareness of the magnitude of fetal mortality, its causes, and prevention strategies.”
But little has changed over the past 13 years. Echoing its earlier message, the CDC this year declared that “much work remains” and that “stillbirth is not often viewed as a public health issue, so increased awareness is key.”
A spokesperson for the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which is part of the NIH, said the agency has continually funded research on stillbirths, even after one of its key studies ended. The agency, she said, also supports research on conditions that increase the risk of stillbirth.
As a scientific research institute, it does not issue clinical guidelines or recommendations, she said, though it did launch the Safe to Sleep campaign in 1994, two decades after Congress put it in charge of SIDS federal research efforts. That campaign, which educates parents and caregivers on ways to reduce the risk of SIDS, highlights recommendations issued by the American Academy of Pediatrics. She said the agency will continue to collaborate with organizations that raise awareness about stillbirth and other pregnancy complications “to amplify their messages and efforts.”
“NICHD continues to support research on the prevention, causes, frequency, and risk factors of stillbirth,” the spokesperson said in an email. “Our commitment to enhancing understanding of stillbirth and improving outcomes focuses on building the scientific knowledge base.”
But getting laws on the books that could raise awareness around stillbirth – even when they don’t require additional funding – has been a struggle. Petersen and Price are pushing Congress to pass legislation that would add stillbirth research and prevention to the list of activities approved for federal maternal health dollars.
Though the bill doesn’t ask for any additional funding, it has not yet passed.
In addition, the SHINE for Autumn Act breezed through the House of Representatives in December 2021. After Haine Vijayvergiya, the New Jersey mother who has championed it, secured bipartisan support from U.S. Sens. Cory Booker, D-N.J., and Marco Rubio, R-Fla., she thought the most comprehensive stillbirth legislation in U.S. history would finally become law.
Neither bill has sparked controversy.
But months after press releases announced the SHINE legislation and referred to the U.S. stillbirth rate as “unacceptable,” lawmakers and the families they represent are running out of time as this session of Congress prepares to adjourn.
“From the day that the bill was introduced into the Senate,” Haine Vijayvergiya said, “approximately 13,000 babies have been born still.”
Last month, on a brilliant fall day much like the one when Reese was stillborn, Amanda Duffy bent down to kiss her son Rogen’s head before they walked on stage.
She wore a soft blue T-shirt tucked into her jeans that read “Be courageous.” The message was as much for her as it was for the crowd on the National Mall in Washington, D.C., many of them like her, mothers who didn’t know stillbirth happened until it happened to them. Since Reese’s death, she has coached doctors and nurses on improving care for patients who have suffered pregnancy loss. Among her many suggestions, she tells them their first words when a concerned patient reaches out should be “I’m so glad you called.”
A few hundred people had gathered for The Big PUSH to End Preventable Stillbirth, billed as the first-ever march on the issue. As part of an art installation, Amanda wrote a note to Reese: “You’re pretty magical & for that I’m grateful. You’re a change maker and you are so very loved. Love, Mama.” Before she slipped the folded paper into a sea of more than 20,000 baby hats, Rogen added his own message: “Hope you are having a good time – Rogen.”
Reese would have turned 8 this month.
Before Amanda spoke, she took a deep breath and silenced her nerves. She walked onto the stage and called on Congress to pass the stillbirth legislation before it. She didn’t ask. She demanded.
“It’s time to empower pregnant people and their care providers with information that leads to prevention,” she insisted.
With the afternoon sun bearing down, Amanda and Rogen disappeared into the crowd of families marching toward the Capitol. Many carried signs. Some pushed empty strollers. Amanda was still wearing the orange-scented oil she had rubbed on her wrists that morning.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published
The day before doctors had scheduled Amanda Duffy to give birth, the baby jolted her awake with a kick.
A few hours later, on that bright Sunday in November 2014, she leaned back on a park bench to watch her 19-month-old son Rogen enjoy his final day of being an only child. In that moment of calm, she realized that the kick that morning was the last time she had felt the baby move.
She told herself not to worry. She had heard that babies can slow down toward the end of a pregnancy and remembered reading that sugary snacks and cold fluids can stimulate a baby’s movement. When she got back to the family’s home in suburban Minneapolis, she drank a large glass of ice water and grabbed a few Tootsie Rolls off the kitchen counter.
But something about seeing her husband, Chris, lace up his shoes to leave for a run prompted her to blurt out, “I haven’t felt the baby kick.”
Chris called Amanda’s doctor, and they headed to the hospital to be checked. Once there, a nurse maneuvered a fetal monitor around Amanda’s belly. When she had trouble locating a heartbeat, she remarked that the baby must be tucked in tight. The doctor walked into the room, turned the screen away from Amanda and Chris and began searching. She was sorry, Amanda remembers her telling them, but she could not find a heartbeat.
Amanda let out a guttural scream. She said the doctor quickly performed an internal exam, which detected faint heart activity, then rushed Amanda into an emergency cesarean section.
She woke up to the sound of doctors talking to Chris. She listened but couldn’t bring herself to face the news. Her doctor told her she needed to open her eyes.
Amanda, then 31, couldn’t fathom that her daughter had died. She said her doctors had never discussed stillbirth with her. It was not mentioned in any of the pregnancy materials she had read. She didn’t even know that stillbirth was a possibility.
But every year more than 20,000 pregnancies in the United States end in stillbirth, the death of an expected child at 20 weeks or more. That number has exceeded infant mortality every year for the last 10 years. It’s 15 times the number of babies who, according to the Centers for Disease Control and Prevention, died of Sudden Infant Death Syndrome, or SIDS, in 2020.
The deaths are not inevitable. One study found that nearly one in four U.S. stillbirths may be preventable. For pregnancies that last 37 weeks or more, that research shows, the figure jumps to nearly half. Thousands more babies could potentially be delivered safely every year.
But federal agencies have not prioritized critical stillbirth-focused studies that could lead to fewer deaths. Nearly two decades ago, both the CDC and the National Institutes of Health launched key stillbirth tracking and research studies, but the agencies ended those projects within about a decade. The CDC never analyzed some of the data that was collected.
Unlike with SIDS, a leading cause of infant death, federal officials have failed to launch a national campaign to reduce the risk of stillbirth or adequately raise awareness about it. Placental exams and autopsies, which can sometimes explain why stillbirths happened, are underutilized, in part because parents are not counseled on their benefits.
Federal agencies, state health departments, hospitals and doctors have also done a poor job of educating expectant parents about stillbirth or diligently counseling on fetal movement, despite research showing that patients who have had a stillbirth are more likely to have experienced abnormal fetal movements, including decreased activity. Neither the CDC nor the NIH have consistently promoted guidance telling those who are pregnant to be aware of their babies’ movement in the womb as a way to possibly reduce their risk of stillbirth.
The American College of Obstetricians and Gynecologists, the nation’s leading obstetrics organization, has been slow to update its own guidance to doctors on managing a stillbirth. In 2009, ACOG issued a set of guidelines that included a single paragraph regarding fetal movement. Those guidelines weren’t significantly updated for another 11 years.
Perhaps it’s no surprise that federal goals for reducing stillbirths keep moving in the wrong direction. In 2005, the U.S. stillbirth rate was 6.2 per 1,000 live births. The U.S. Department of Health and Human Services, in an effort to eliminate health disparities and establish a target that was “better than the best racial or ethnic group rate,” set a goal of reducing it to 4.1 for 2010. When that wasn’t met, federal officials changed their approach and set what they called more “science-based” and “realistic” goals, raising the 2020 target to 5.6. The U.S. still fell short. The 2030 goal of 5.7 was so attainable that it was met before the decade started. The 2020 rate, the most current according to the CDC, is 5.74.
By comparison, other wealthy countries have implemented national action plans to prevent stillbirth through awareness, research and care. Among other approaches, those countries have focused on increasing education around stillbirth and the importance of a baby’s movements, reducing rates of smoking and identifying fetuses that grow too slowly in the womb.
The efforts have paid off. The Netherlands, for instance, has reduced its rate of stillbirths at 28 weeks or later by more than half, from 5.2 in 2000 to 2.3 in 2019, according to a study published last year in The Lancet.
Dr. Bob Silver, chair of the obstetrics/gynecology department at University of Utah Health and a leading stillbirth expert, coauthored the study that estimated nearly one in four stillbirths are potentially preventable, a figure he referred to as conservative. He called on federal agencies to declare stillbirth reduction a priority the same way they have done for premature birth and maternal mortality.
“I’d like to see us say we really want to reduce the rate of stillbirth and raise awareness and try to do all of the reasonable things that may contribute to reducing stillbirths that other countries have done,” Silver said.
The lack of comprehensive attention and action has contributed to a stillbirth crisis, shrouded in an acceptance that some babies just die. Compounding the tragedy is a stigma and guilt so crushing that the first words some mothers utter when their lifeless babies are placed in their arms are “I’m sorry.”
In the hospital room, Amanda Duffy finally opened her eyes. She named her daughter Reese Christine, the name she had picked out for her before they found out she had died. She was 8 pounds, 3 ounces and 20 1/2 inches long and was born with her umbilical cord wrapped tightly around her neck twice. The baby was still warm when the nurse placed her in Amanda’s arms. Amanda was struck by how lovely her daughter was. Rosy skin. Chris’ red hair. Rogen’s chubby cheeks.
As Amanda held Reese, Chris hunched over the toilet, vomiting. Later that night, as he lay next to Amanda on the hospital bed, he held his daughter. He hadn’t initially wanted to see her. He worried she would be disfigured or, worse, that she would be beautiful and he would fall apart when he couldn’t take her home.
The nurses taught Amanda and Chris how to grieve and love simultaneously. One nurse told Amanda how cute Reese was and asked if she could hold her. Another placed ice packs in Reese’s swaddle to preserve her body so Amanda could keep holding her. Amanda asked the nurses to tuck cotton balls soaked in an orange scent into Reese’s blanket so the smell would trigger the memory of her daughter. And just as if Reese had been born alive, the nurses took pictures and made prints of her hands and feet.
“I felt such a deep, abiding love for her,” Amanda said. “And I was so proud to be her mom.”
On the way home from the hospital, Amanda broke down at the sight of Reese’s empty car seat. The next few weeks passed in a sleep-filled fog punctuated by intense periods of crying. The smell of oranges wrecked her. Her breast milk coming in was agonizing, physically and emotionally. She wore sports bras stuffed with ice packs to ease the pain and dry up her milk supply. While Rogen was at day care, she sobbed in his bed.
In the months that followed, Amanda and Chris searched for answers and wondered whether their medical team had missed warning signs. Late at night, Amanda turned to Google to find information about stillbirths. She mailed her medical records to a doctor who studies stillbirths, who she said told her that Reese’s death could have been prevented. They briefly discussed legal action against her doctors, but she said a lawyer told her it would be difficult to sue.
Amanda and Chris pinpointed her last two months of pregnancy as the time things started to go wrong. She had been diagnosed with polyhydramnios, meaning there was excess amniotic fluid in the womb. Her doctor had scheduled additional weekly testing.
One of those ultrasounds revealed problems with the blood flow in the umbilical cord. Reese’s cord also appeared to be wrapped around her neck, Amanda said later, but was told that was less of a concern, since it occurs in about 20% of normal deliveries. At another appointment, Amanda’s medical records show, Reese failed the portion of a test that measures fetal breathing movements.
At 37 weeks, Amanda told one of the midwives the baby’s movements felt different, but, she said, the midwife told her that it was common for movements to feel weaker with polyhydramnios. At that point, Amanda felt her baby was safer outside than inside and, her medical records show, she asked to schedule a C-section.
Despite voicing concerns about a change in the baby’s movement and asking to deliver earlier, Amanda said she and her husband were told by her midwife she couldn’t deliver for another two weeks. The doctor “continues to advise 39wks,” her medical records show. Waiting until 39 weeks is usually based on a guideline that deliveries should not happen before then unless a medical condition specifically warrants it, because early delivery can lead to complications.
Amanda would have to wait until 39 weeks and one day because, she said, her doctors didn’t typically do elective deliveries on weekends. Amanda was disappointed but said she trusted her team of doctors and midwives.
“I’m not a pushy person,” she said. “My husband is not a pushy person. That was out of our comfort zone to be, like, ‘What are we waiting for?’ But really what we wanted them to say was ‘We should deliver you.’”
Amanda’s final appointment was a maximum 30-minute-long ultrasound that combined a number of assessments to check amniotic fluid, fetal muscle tone, breathing and body movement. After 29 minutes of inactivity, Amanda said, the baby moved a hand. In the parking lot, Amanda called her mother, crying in relief. Four more days, she told her.
Less than 24 hours before the scheduled C-section, Reese was stillborn.
Four months after her death, Amanda, then a career advisor at the University of Minnesota, and Chris, a public relations specialist, wrote a letter to the University of Minnesota Medical Center, where Amanda had given birth to her dead daughter. They said they had “no ill feelings” toward anyone, but “it pains us to know that her death could’ve been prevented if we would have been sent to labor and delivery following that ultrasound.”
They noted that though they were told that Reese had passed the final ultrasound where she took 29 minutes to move, they had since come to believe that she had failed because, according to national standards, at least three movements were required. They also blamed a strict adherence to the 39-week guideline. And they encouraged the hospital staff to read more on umbilical cord accidents and acute polyhydramnios, which they later learned carries an increased stillbirth risk.
The positive feelings they had from speaking up were replaced by dismay when the hospital responded with a three-paragraph letter, signed by seven doctors and eight nurses. They said they had reexamined each medical decision in her case and concluded they had made “the best decisions medically possible.” They expressed their sympathy and said it was “so very heartwarming that you are trying to turn your tragic loss into something that will benefit others.”
Amanda felt dismissed by the medical team all over again. She didn’t expect them to admit fault, but she said she hoped that they would at least learn from Reese’s death to do things differently in the future. She was angry, and hurt, and knew that she would need to find a new doctor.
A spokesperson for the University of Minnesota Medical School told ProPublica she could not comment on individual patient cases and did not respond to questions about general protocols. “We share the physicians’ condolences,” she wrote, adding that the doctors and the university “are dedicated to delivering high quality, accessible and inclusive health care.”
For many expectant parents, it’s hard to muster the courage to call a doctor about something they’re not even sure is a problem.
“Moms self-censor a lot. No one wants to be that mom that all the doctors are rolling their eyes at because she’s freaking out over nothing,” said Samantha Banerjee, executive director of PUSH for Empowered Pregnancy, a nonprofit based in New York state that works to prevent stillbirths. Banerjee’s daughter, Alana, was stillborn two days before her due date.
In addition to raising awareness that stillbirths can happen even in low-risk pregnancies, PUSH teaches pregnant people how to advocate for themselves. The volunteers advise them to put their requests in writing and not to spend time drinking juice or lying on their side if they are worried about their baby’s lack of movement. In the majority of cases, a call or visit to the hospital reassures them.
But, the group tells parents, if their baby is in distress, calling their doctor can save their life.
Debbie Haine Vijayvergiya is fighting another narrative: that stillbirths are a rare fluke that “just happen.” When her daughter Autumn Joy was born without a heartbeat in 2011, Haine Vijayvergiya said, her doctor told her having a stillborn baby was as rare as being struck by lightning.
She believed him, but then she looked up the odds of a lightning strike and found they are less than one in a million – and most people survive. In 2020, according to the CDC, there was one stillbirth for about every 175 births.
“I’ve spoken to more women than I can count that said, ‘I raised the red flag, and I was sent home. I was told to eat a piece of cake and have some orange juice and lay on my left side,’ only to wake up the next day and their baby is not alive,” said Haine Vijayvergiya, a New Jersey mother and maternal health advocate.
She has fought for more than a decade to pass stillbirth legislation as her daughter’s legacy. Her current undertaking is her most ambitious. The federal Stillbirth Health Improvement and Education (SHINE) for Autumn Act, named after her daughter, would authorize $9 million a year for five years in federal funding for research, better data collection and training for fetal autopsies. But it is currently sitting in the Senate Committee on Health, Education, Labor, and Pensions.
Not all stillbirths are preventable, and medical experts agree more research is needed to determine who is most at risk and which babies can potentially be saved. Complicating matters is the wide range of risk factors, including hypertension and diabetes, smoking, obesity, being pregnant with multiples, being 35 or older and having had a previous stillbirth.
ProPublica reported this summer on how the U.S. botched the rollout of COVID-19 vaccines for pregnant people, who faced an increased risk for stillbirth if they were unvaccinated and contracted the virus, especially during the Delta wave.
Doctors often work to balance the risk of stillbirth with other dangers, particularly an increased chance of being admitted to neonatal intensive care units or even death of the baby if it is born too early. ACOG and the Society for Maternal-Fetal Medicine have issued guidance to try to slow a rise in elective deliveries before 39 weeks and the potential harm that can result. The Joint Commission, a national accrediting organization, began evaluating hospitals in 2010 based on that standard.
A 2019 study found that the risks of stillbirth slightly increased after the rule went into effect, but fewer infants died after birth. Other studies have not found an effect on stillbirths.
Last year, the obstetric groups updated their guidance to allow doctors to consider an early delivery if a woman has anxiety and a history of stillbirth, writing that a previous stillbirth “may” warrant an early delivery for patients who understand and accept the risks. For those who have previously had a stillbirth, one modeling analysis found that 38 weeks is the optimal timing of delivery, considering the increased risk of another stillbirth.
“A woman who has had a previous stillbirth at 37 weeks – one could argue that it’s cruel and unusual punishment to make her go to 39 weeks with her next pregnancy, although that is the current recommendation,” said Dr. Neil Mandsager, a maternal-fetal medicine specialist in Iowa and a medical advisor to a stillbirth prevention nonprofit.
At or after 40 weeks, the risk of stillbirth increases, especially for women 35 or older. Their risk, research shows, is doubled from 39 weeks to 40 and is more than six times as high at 42 weeks. In 2019 and 2020, a combined 1,200 stillbirths occurred between 40 and 42 weeks, according to the most recent CDC data.
Deciding when a patient should deliver entails weighing the risks to the mother and the infant against a possible stillbirth as the pregnancy continues, said Dr. Mark Turrentine, chair of ACOG’s Clinical Consensus Committee-Obstetrics, which helped create the guidance on managing a stillbirth. He said ACOG has addressed stillbirth in other documents and extensively in its 2021 guidance on fetal surveillance and testing, which is done to reduce the risk of stillbirth.
ACOG said it routinely reviewed its guidance on management of stillbirth but was unable to make significant updates “due to the lack of new, evidence-based research.” While prevention is a great concern to ACOG, Turrentine said it’s difficult to know how many stillbirths are preventable.
He said it’s standard practice for doctors to ask about fetal movement, and ACOG updated its guidance after new research became available. Doctors also need to include patients in decision-making and tailor care to them, he said, whether that›s using aspirin in patients at high risk of preeclampsia – a serious high blood pressure condition during pregnancy – or ordering additional tests.
After Reese’s death, Amanda and Chris Duffy wanted to get pregnant again. They sought out an obstetrician-gynecologist who would educate and listen to them. They set up several consultations until they found Dr. Emily Hawes-Van Pelt, who was recommended by another family who had had a stillbirth.
Hawes-Van Pelt cried with Amanda and Chris at their first meeting.
“I told her I was scared to be involved,” Hawes-Van Pelt said. “It’s such a tricky subsequent pregnancy because there’s so much worry and anxiety about the horrible, awful thing happening again.”
Amanda’s fear of delivering another dead baby led to an all-consuming anxiety, but Hawes-Van Pelt supported her when she asked for additional monitoring, testing and an early delivery.
When Hawes-Van Pelt switched practices midway through Amanda’s pregnancy, Amanda followed her. But the new hospital pushed back on the early delivery.
“We intervene early for poorly controlled diabetes,” Hawes-Van Pelt said. “We intervene early for all sorts of medical issues. Anxiety and prior stillbirth are two medical issues that we can intervene earlier for.”
Hawes-Van Pelt said she learned a lot from caring for Amanda, who made her reevaluate some of her own assumptions around stillbirths.
“I had a horrible fear of scaring women unnecessarily, and then realized that I was just not preparing women or educating them because of my own fears around it,” she said. “If you can carry a human being in your body and birth that human being and take care of it, you can hear those words.”
The hospital eventually agreed to let Hawes-Van Pelt schedule Amanda for a 37-week C-section. But after Amanda was again diagnosed with polyhydramnios, she went in for a C-section even earlier. She gave birth in 2015 to a healthy boy she and Chris named Rhett. Two years later, Amanda and Hawes-Van Pelt followed the same pregnancy plan, and she delivered a girl named Maeda Reese. Amanda chose the name because, when said quickly, it sounds like “made of Reese.”
Federal agencies, national organizations and state and city officials have mobilized in recent years to address maternal mortality, when mothers die during pregnancy, at delivery or soon after childbirth. They have focused on improving data collection, passing legislation and creating awareness campaigns that encourage medical professionals and others to listen when women say something doesn›t feel right.
In 2017, ProPublica and NPR documented the U.S. maternal mortality crisis, including alarming racial disparities.
According to CDC data, Black women face nearly three times the risk of maternal mortality. They also are more than twice – and in some states close to three times – as likely to have a stillbirth than white women, meaning not only are Black mothers dying at a disproportionate rate, so are their babies.
Janet Petersen, a state senator from Iowa, said it gives her hope to see how the country has turned its attention to maternal mortality and disparities in health care. She simply cannot understand why stillbirth isn’t being met with the same urgency.
In 2020, the CDC reported 861 mothers died either while pregnant or within six weeks of giving birth. That same year, 20,854 babies were stillborn.
Stillbirth, Petersen said, is a missing piece of the puzzle. Research shows the likelihood of severe maternal complications was more than four times higher for pregnancies that ended in stillbirths, and mothers who died within six weeks of delivery were more likely to have had a stillbirth.
“We see it over and over again that stillbirth is one of the maternal health care issues that continuously gets ignored,” said Petersen, a Democrat.
Petersen was a young legislator in 2003 when her daughter Grace was born still. Devastated, she thought of her grandmother, who lost a baby to stillbirth in 1920, just a few weeks before women got the right to vote.
“I was laying in my hospital bed thinking, ‘How could this still be happening in our country?’” Petersen recalled. “And it seemed, from the medical perspective, that, well, stillbirth happens. We can’t do anything to prevent them.”
Over the next few months, Petersen heard from other mothers who had lost their babies and wanted to spark change. As an elected official, Petersen was in a position to do that. In 2004, she introduced legislation that required the Iowa Department of Public Health to create a stillbirths work group, later securing funding through the CDC to create a stillbirth registry.
But the CDC didn’t renew the funding and never analyzed the data from the registry, though a CDC spokesperson said the Iowa Department of Public Health examined the data. Officials from the department did not respond to requests for comment.
Petersen and her fellow mothers pivoted. After hearing how researchers in Norway were able to increase awareness around fetal movement, they co-founded a nonprofit aimed at doing the same in the U.S.
The group, Healthy Birth Day, created colorful “Count the Kicks” pamphlets – and later an app – teaching pregnant people how to track a baby’s movements and establish what is normal for them. Monitoring a baby’s movements is the earliest and sometimes only indication that something may be wrong, said Emily Price, chief executive officer of Healthy Birth Day. One of the organization’s main messages is for pregnant people to speak up and clinicians to listen.
“Unfortunately, there are still doctors who brush women off or send them home when they come in with a complaint of a change in their baby’s movements,” Price said. “And babies are dying because of it.”
One Indiana county, which recorded 65 stillbirths from 2017 through 2019, reported that 74% had either some chance or a good chance of prevention, according to St. Joseph County Department of Health’s Fetal Infant Mortality Review program. For mothers who experienced decreased fetal movement in the few hours or days before the stillbirth, that estimate jumped to 90%.
Although there is not a scientific consensus that kick counting can prevent stillbirths, national groups, including ACOG, recommend that medical professionals encourage their patients to be aware of fetal movement patterns. ACOG also advises medical professionals to be attentive to a mother’s concerns about reduced movement and address them “in a systematic way.”
One complaint the CDC hears too often, an agency spokesperson said, is that pregnant people and those who gave birth recently find that their concerns are dismissed or ignored. “Listening and taking the concerns of pregnant and recently pregnant people seriously,” she said, “is a simple, yet powerful action to prevent serious health complications and even death.”
The CDC, she said, is “very interested” in expanding its research on stillbirth, which is “a crucial part of the development of any awareness or prevention campaigns.” In addition to working to improve its stillbirth data quality, the agency has funded some pilot programs at the city and state level to better track stillbirths, survey people who have had a stillbirth and research risk factors and causes. The Iowa registry, she said, led the CDC to fund different research projects in Arkansas and Massachusetts, which are ongoing.
In 2009, the CDC acknowledged that fetal mortality remained a “major, but often overlooked, public health problem.” Officials wrote that much of the public health concern had been focused on infant mortality “in part due to lesser awareness of the magnitude of fetal mortality, its causes, and prevention strategies.”
But little has changed over the past 13 years. Echoing its earlier message, the CDC this year declared that “much work remains” and that “stillbirth is not often viewed as a public health issue, so increased awareness is key.”
A spokesperson for the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which is part of the NIH, said the agency has continually funded research on stillbirths, even after one of its key studies ended. The agency, she said, also supports research on conditions that increase the risk of stillbirth.
As a scientific research institute, it does not issue clinical guidelines or recommendations, she said, though it did launch the Safe to Sleep campaign in 1994, two decades after Congress put it in charge of SIDS federal research efforts. That campaign, which educates parents and caregivers on ways to reduce the risk of SIDS, highlights recommendations issued by the American Academy of Pediatrics. She said the agency will continue to collaborate with organizations that raise awareness about stillbirth and other pregnancy complications “to amplify their messages and efforts.”
“NICHD continues to support research on the prevention, causes, frequency, and risk factors of stillbirth,” the spokesperson said in an email. “Our commitment to enhancing understanding of stillbirth and improving outcomes focuses on building the scientific knowledge base.”
But getting laws on the books that could raise awareness around stillbirth – even when they don’t require additional funding – has been a struggle. Petersen and Price are pushing Congress to pass legislation that would add stillbirth research and prevention to the list of activities approved for federal maternal health dollars.
Though the bill doesn’t ask for any additional funding, it has not yet passed.
In addition, the SHINE for Autumn Act breezed through the House of Representatives in December 2021. After Haine Vijayvergiya, the New Jersey mother who has championed it, secured bipartisan support from U.S. Sens. Cory Booker, D-N.J., and Marco Rubio, R-Fla., she thought the most comprehensive stillbirth legislation in U.S. history would finally become law.
Neither bill has sparked controversy.
But months after press releases announced the SHINE legislation and referred to the U.S. stillbirth rate as “unacceptable,” lawmakers and the families they represent are running out of time as this session of Congress prepares to adjourn.
“From the day that the bill was introduced into the Senate,” Haine Vijayvergiya said, “approximately 13,000 babies have been born still.”
Last month, on a brilliant fall day much like the one when Reese was stillborn, Amanda Duffy bent down to kiss her son Rogen’s head before they walked on stage.
She wore a soft blue T-shirt tucked into her jeans that read “Be courageous.” The message was as much for her as it was for the crowd on the National Mall in Washington, D.C., many of them like her, mothers who didn’t know stillbirth happened until it happened to them. Since Reese’s death, she has coached doctors and nurses on improving care for patients who have suffered pregnancy loss. Among her many suggestions, she tells them their first words when a concerned patient reaches out should be “I’m so glad you called.”
A few hundred people had gathered for The Big PUSH to End Preventable Stillbirth, billed as the first-ever march on the issue. As part of an art installation, Amanda wrote a note to Reese: “You’re pretty magical & for that I’m grateful. You’re a change maker and you are so very loved. Love, Mama.” Before she slipped the folded paper into a sea of more than 20,000 baby hats, Rogen added his own message: “Hope you are having a good time – Rogen.”
Reese would have turned 8 this month.
Before Amanda spoke, she took a deep breath and silenced her nerves. She walked onto the stage and called on Congress to pass the stillbirth legislation before it. She didn’t ask. She demanded.
“It’s time to empower pregnant people and their care providers with information that leads to prevention,” she insisted.
With the afternoon sun bearing down, Amanda and Rogen disappeared into the crowd of families marching toward the Capitol. Many carried signs. Some pushed empty strollers. Amanda was still wearing the orange-scented oil she had rubbed on her wrists that morning.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published
The day before doctors had scheduled Amanda Duffy to give birth, the baby jolted her awake with a kick.
A few hours later, on that bright Sunday in November 2014, she leaned back on a park bench to watch her 19-month-old son Rogen enjoy his final day of being an only child. In that moment of calm, she realized that the kick that morning was the last time she had felt the baby move.
She told herself not to worry. She had heard that babies can slow down toward the end of a pregnancy and remembered reading that sugary snacks and cold fluids can stimulate a baby’s movement. When she got back to the family’s home in suburban Minneapolis, she drank a large glass of ice water and grabbed a few Tootsie Rolls off the kitchen counter.
But something about seeing her husband, Chris, lace up his shoes to leave for a run prompted her to blurt out, “I haven’t felt the baby kick.”
Chris called Amanda’s doctor, and they headed to the hospital to be checked. Once there, a nurse maneuvered a fetal monitor around Amanda’s belly. When she had trouble locating a heartbeat, she remarked that the baby must be tucked in tight. The doctor walked into the room, turned the screen away from Amanda and Chris and began searching. She was sorry, Amanda remembers her telling them, but she could not find a heartbeat.
Amanda let out a guttural scream. She said the doctor quickly performed an internal exam, which detected faint heart activity, then rushed Amanda into an emergency cesarean section.
She woke up to the sound of doctors talking to Chris. She listened but couldn’t bring herself to face the news. Her doctor told her she needed to open her eyes.
Amanda, then 31, couldn’t fathom that her daughter had died. She said her doctors had never discussed stillbirth with her. It was not mentioned in any of the pregnancy materials she had read. She didn’t even know that stillbirth was a possibility.
But every year more than 20,000 pregnancies in the United States end in stillbirth, the death of an expected child at 20 weeks or more. That number has exceeded infant mortality every year for the last 10 years. It’s 15 times the number of babies who, according to the Centers for Disease Control and Prevention, died of Sudden Infant Death Syndrome, or SIDS, in 2020.
The deaths are not inevitable. One study found that nearly one in four U.S. stillbirths may be preventable. For pregnancies that last 37 weeks or more, that research shows, the figure jumps to nearly half. Thousands more babies could potentially be delivered safely every year.
But federal agencies have not prioritized critical stillbirth-focused studies that could lead to fewer deaths. Nearly two decades ago, both the CDC and the National Institutes of Health launched key stillbirth tracking and research studies, but the agencies ended those projects within about a decade. The CDC never analyzed some of the data that was collected.
Unlike with SIDS, a leading cause of infant death, federal officials have failed to launch a national campaign to reduce the risk of stillbirth or adequately raise awareness about it. Placental exams and autopsies, which can sometimes explain why stillbirths happened, are underutilized, in part because parents are not counseled on their benefits.
Federal agencies, state health departments, hospitals and doctors have also done a poor job of educating expectant parents about stillbirth or diligently counseling on fetal movement, despite research showing that patients who have had a stillbirth are more likely to have experienced abnormal fetal movements, including decreased activity. Neither the CDC nor the NIH have consistently promoted guidance telling those who are pregnant to be aware of their babies’ movement in the womb as a way to possibly reduce their risk of stillbirth.
The American College of Obstetricians and Gynecologists, the nation’s leading obstetrics organization, has been slow to update its own guidance to doctors on managing a stillbirth. In 2009, ACOG issued a set of guidelines that included a single paragraph regarding fetal movement. Those guidelines weren’t significantly updated for another 11 years.
Perhaps it’s no surprise that federal goals for reducing stillbirths keep moving in the wrong direction. In 2005, the U.S. stillbirth rate was 6.2 per 1,000 live births. The U.S. Department of Health and Human Services, in an effort to eliminate health disparities and establish a target that was “better than the best racial or ethnic group rate,” set a goal of reducing it to 4.1 for 2010. When that wasn’t met, federal officials changed their approach and set what they called more “science-based” and “realistic” goals, raising the 2020 target to 5.6. The U.S. still fell short. The 2030 goal of 5.7 was so attainable that it was met before the decade started. The 2020 rate, the most current according to the CDC, is 5.74.
By comparison, other wealthy countries have implemented national action plans to prevent stillbirth through awareness, research and care. Among other approaches, those countries have focused on increasing education around stillbirth and the importance of a baby’s movements, reducing rates of smoking and identifying fetuses that grow too slowly in the womb.
The efforts have paid off. The Netherlands, for instance, has reduced its rate of stillbirths at 28 weeks or later by more than half, from 5.2 in 2000 to 2.3 in 2019, according to a study published last year in The Lancet.
Dr. Bob Silver, chair of the obstetrics/gynecology department at University of Utah Health and a leading stillbirth expert, coauthored the study that estimated nearly one in four stillbirths are potentially preventable, a figure he referred to as conservative. He called on federal agencies to declare stillbirth reduction a priority the same way they have done for premature birth and maternal mortality.
“I’d like to see us say we really want to reduce the rate of stillbirth and raise awareness and try to do all of the reasonable things that may contribute to reducing stillbirths that other countries have done,” Silver said.
The lack of comprehensive attention and action has contributed to a stillbirth crisis, shrouded in an acceptance that some babies just die. Compounding the tragedy is a stigma and guilt so crushing that the first words some mothers utter when their lifeless babies are placed in their arms are “I’m sorry.”
In the hospital room, Amanda Duffy finally opened her eyes. She named her daughter Reese Christine, the name she had picked out for her before they found out she had died. She was 8 pounds, 3 ounces and 20 1/2 inches long and was born with her umbilical cord wrapped tightly around her neck twice. The baby was still warm when the nurse placed her in Amanda’s arms. Amanda was struck by how lovely her daughter was. Rosy skin. Chris’ red hair. Rogen’s chubby cheeks.
As Amanda held Reese, Chris hunched over the toilet, vomiting. Later that night, as he lay next to Amanda on the hospital bed, he held his daughter. He hadn’t initially wanted to see her. He worried she would be disfigured or, worse, that she would be beautiful and he would fall apart when he couldn’t take her home.
The nurses taught Amanda and Chris how to grieve and love simultaneously. One nurse told Amanda how cute Reese was and asked if she could hold her. Another placed ice packs in Reese’s swaddle to preserve her body so Amanda could keep holding her. Amanda asked the nurses to tuck cotton balls soaked in an orange scent into Reese’s blanket so the smell would trigger the memory of her daughter. And just as if Reese had been born alive, the nurses took pictures and made prints of her hands and feet.
“I felt such a deep, abiding love for her,” Amanda said. “And I was so proud to be her mom.”
On the way home from the hospital, Amanda broke down at the sight of Reese’s empty car seat. The next few weeks passed in a sleep-filled fog punctuated by intense periods of crying. The smell of oranges wrecked her. Her breast milk coming in was agonizing, physically and emotionally. She wore sports bras stuffed with ice packs to ease the pain and dry up her milk supply. While Rogen was at day care, she sobbed in his bed.
In the months that followed, Amanda and Chris searched for answers and wondered whether their medical team had missed warning signs. Late at night, Amanda turned to Google to find information about stillbirths. She mailed her medical records to a doctor who studies stillbirths, who she said told her that Reese’s death could have been prevented. They briefly discussed legal action against her doctors, but she said a lawyer told her it would be difficult to sue.
Amanda and Chris pinpointed her last two months of pregnancy as the time things started to go wrong. She had been diagnosed with polyhydramnios, meaning there was excess amniotic fluid in the womb. Her doctor had scheduled additional weekly testing.
One of those ultrasounds revealed problems with the blood flow in the umbilical cord. Reese’s cord also appeared to be wrapped around her neck, Amanda said later, but was told that was less of a concern, since it occurs in about 20% of normal deliveries. At another appointment, Amanda’s medical records show, Reese failed the portion of a test that measures fetal breathing movements.
At 37 weeks, Amanda told one of the midwives the baby’s movements felt different, but, she said, the midwife told her that it was common for movements to feel weaker with polyhydramnios. At that point, Amanda felt her baby was safer outside than inside and, her medical records show, she asked to schedule a C-section.
Despite voicing concerns about a change in the baby’s movement and asking to deliver earlier, Amanda said she and her husband were told by her midwife she couldn’t deliver for another two weeks. The doctor “continues to advise 39wks,” her medical records show. Waiting until 39 weeks is usually based on a guideline that deliveries should not happen before then unless a medical condition specifically warrants it, because early delivery can lead to complications.
Amanda would have to wait until 39 weeks and one day because, she said, her doctors didn’t typically do elective deliveries on weekends. Amanda was disappointed but said she trusted her team of doctors and midwives.
“I’m not a pushy person,” she said. “My husband is not a pushy person. That was out of our comfort zone to be, like, ‘What are we waiting for?’ But really what we wanted them to say was ‘We should deliver you.’”
Amanda’s final appointment was a maximum 30-minute-long ultrasound that combined a number of assessments to check amniotic fluid, fetal muscle tone, breathing and body movement. After 29 minutes of inactivity, Amanda said, the baby moved a hand. In the parking lot, Amanda called her mother, crying in relief. Four more days, she told her.
Less than 24 hours before the scheduled C-section, Reese was stillborn.
Four months after her death, Amanda, then a career advisor at the University of Minnesota, and Chris, a public relations specialist, wrote a letter to the University of Minnesota Medical Center, where Amanda had given birth to her dead daughter. They said they had “no ill feelings” toward anyone, but “it pains us to know that her death could’ve been prevented if we would have been sent to labor and delivery following that ultrasound.”
They noted that though they were told that Reese had passed the final ultrasound where she took 29 minutes to move, they had since come to believe that she had failed because, according to national standards, at least three movements were required. They also blamed a strict adherence to the 39-week guideline. And they encouraged the hospital staff to read more on umbilical cord accidents and acute polyhydramnios, which they later learned carries an increased stillbirth risk.
The positive feelings they had from speaking up were replaced by dismay when the hospital responded with a three-paragraph letter, signed by seven doctors and eight nurses. They said they had reexamined each medical decision in her case and concluded they had made “the best decisions medically possible.” They expressed their sympathy and said it was “so very heartwarming that you are trying to turn your tragic loss into something that will benefit others.”
Amanda felt dismissed by the medical team all over again. She didn’t expect them to admit fault, but she said she hoped that they would at least learn from Reese’s death to do things differently in the future. She was angry, and hurt, and knew that she would need to find a new doctor.
A spokesperson for the University of Minnesota Medical School told ProPublica she could not comment on individual patient cases and did not respond to questions about general protocols. “We share the physicians’ condolences,” she wrote, adding that the doctors and the university “are dedicated to delivering high quality, accessible and inclusive health care.”
For many expectant parents, it’s hard to muster the courage to call a doctor about something they’re not even sure is a problem.
“Moms self-censor a lot. No one wants to be that mom that all the doctors are rolling their eyes at because she’s freaking out over nothing,” said Samantha Banerjee, executive director of PUSH for Empowered Pregnancy, a nonprofit based in New York state that works to prevent stillbirths. Banerjee’s daughter, Alana, was stillborn two days before her due date.
In addition to raising awareness that stillbirths can happen even in low-risk pregnancies, PUSH teaches pregnant people how to advocate for themselves. The volunteers advise them to put their requests in writing and not to spend time drinking juice or lying on their side if they are worried about their baby’s lack of movement. In the majority of cases, a call or visit to the hospital reassures them.
But, the group tells parents, if their baby is in distress, calling their doctor can save their life.
Debbie Haine Vijayvergiya is fighting another narrative: that stillbirths are a rare fluke that “just happen.” When her daughter Autumn Joy was born without a heartbeat in 2011, Haine Vijayvergiya said, her doctor told her having a stillborn baby was as rare as being struck by lightning.
She believed him, but then she looked up the odds of a lightning strike and found they are less than one in a million – and most people survive. In 2020, according to the CDC, there was one stillbirth for about every 175 births.
“I’ve spoken to more women than I can count that said, ‘I raised the red flag, and I was sent home. I was told to eat a piece of cake and have some orange juice and lay on my left side,’ only to wake up the next day and their baby is not alive,” said Haine Vijayvergiya, a New Jersey mother and maternal health advocate.
She has fought for more than a decade to pass stillbirth legislation as her daughter’s legacy. Her current undertaking is her most ambitious. The federal Stillbirth Health Improvement and Education (SHINE) for Autumn Act, named after her daughter, would authorize $9 million a year for five years in federal funding for research, better data collection and training for fetal autopsies. But it is currently sitting in the Senate Committee on Health, Education, Labor, and Pensions.
Not all stillbirths are preventable, and medical experts agree more research is needed to determine who is most at risk and which babies can potentially be saved. Complicating matters is the wide range of risk factors, including hypertension and diabetes, smoking, obesity, being pregnant with multiples, being 35 or older and having had a previous stillbirth.
ProPublica reported this summer on how the U.S. botched the rollout of COVID-19 vaccines for pregnant people, who faced an increased risk for stillbirth if they were unvaccinated and contracted the virus, especially during the Delta wave.
Doctors often work to balance the risk of stillbirth with other dangers, particularly an increased chance of being admitted to neonatal intensive care units or even death of the baby if it is born too early. ACOG and the Society for Maternal-Fetal Medicine have issued guidance to try to slow a rise in elective deliveries before 39 weeks and the potential harm that can result. The Joint Commission, a national accrediting organization, began evaluating hospitals in 2010 based on that standard.
A 2019 study found that the risks of stillbirth slightly increased after the rule went into effect, but fewer infants died after birth. Other studies have not found an effect on stillbirths.
Last year, the obstetric groups updated their guidance to allow doctors to consider an early delivery if a woman has anxiety and a history of stillbirth, writing that a previous stillbirth “may” warrant an early delivery for patients who understand and accept the risks. For those who have previously had a stillbirth, one modeling analysis found that 38 weeks is the optimal timing of delivery, considering the increased risk of another stillbirth.
“A woman who has had a previous stillbirth at 37 weeks – one could argue that it’s cruel and unusual punishment to make her go to 39 weeks with her next pregnancy, although that is the current recommendation,” said Dr. Neil Mandsager, a maternal-fetal medicine specialist in Iowa and a medical advisor to a stillbirth prevention nonprofit.
At or after 40 weeks, the risk of stillbirth increases, especially for women 35 or older. Their risk, research shows, is doubled from 39 weeks to 40 and is more than six times as high at 42 weeks. In 2019 and 2020, a combined 1,200 stillbirths occurred between 40 and 42 weeks, according to the most recent CDC data.
Deciding when a patient should deliver entails weighing the risks to the mother and the infant against a possible stillbirth as the pregnancy continues, said Dr. Mark Turrentine, chair of ACOG’s Clinical Consensus Committee-Obstetrics, which helped create the guidance on managing a stillbirth. He said ACOG has addressed stillbirth in other documents and extensively in its 2021 guidance on fetal surveillance and testing, which is done to reduce the risk of stillbirth.
ACOG said it routinely reviewed its guidance on management of stillbirth but was unable to make significant updates “due to the lack of new, evidence-based research.” While prevention is a great concern to ACOG, Turrentine said it’s difficult to know how many stillbirths are preventable.
He said it’s standard practice for doctors to ask about fetal movement, and ACOG updated its guidance after new research became available. Doctors also need to include patients in decision-making and tailor care to them, he said, whether that›s using aspirin in patients at high risk of preeclampsia – a serious high blood pressure condition during pregnancy – or ordering additional tests.
After Reese’s death, Amanda and Chris Duffy wanted to get pregnant again. They sought out an obstetrician-gynecologist who would educate and listen to them. They set up several consultations until they found Dr. Emily Hawes-Van Pelt, who was recommended by another family who had had a stillbirth.
Hawes-Van Pelt cried with Amanda and Chris at their first meeting.
“I told her I was scared to be involved,” Hawes-Van Pelt said. “It’s such a tricky subsequent pregnancy because there’s so much worry and anxiety about the horrible, awful thing happening again.”
Amanda’s fear of delivering another dead baby led to an all-consuming anxiety, but Hawes-Van Pelt supported her when she asked for additional monitoring, testing and an early delivery.
When Hawes-Van Pelt switched practices midway through Amanda’s pregnancy, Amanda followed her. But the new hospital pushed back on the early delivery.
“We intervene early for poorly controlled diabetes,” Hawes-Van Pelt said. “We intervene early for all sorts of medical issues. Anxiety and prior stillbirth are two medical issues that we can intervene earlier for.”
Hawes-Van Pelt said she learned a lot from caring for Amanda, who made her reevaluate some of her own assumptions around stillbirths.
“I had a horrible fear of scaring women unnecessarily, and then realized that I was just not preparing women or educating them because of my own fears around it,” she said. “If you can carry a human being in your body and birth that human being and take care of it, you can hear those words.”
The hospital eventually agreed to let Hawes-Van Pelt schedule Amanda for a 37-week C-section. But after Amanda was again diagnosed with polyhydramnios, she went in for a C-section even earlier. She gave birth in 2015 to a healthy boy she and Chris named Rhett. Two years later, Amanda and Hawes-Van Pelt followed the same pregnancy plan, and she delivered a girl named Maeda Reese. Amanda chose the name because, when said quickly, it sounds like “made of Reese.”
Federal agencies, national organizations and state and city officials have mobilized in recent years to address maternal mortality, when mothers die during pregnancy, at delivery or soon after childbirth. They have focused on improving data collection, passing legislation and creating awareness campaigns that encourage medical professionals and others to listen when women say something doesn›t feel right.
In 2017, ProPublica and NPR documented the U.S. maternal mortality crisis, including alarming racial disparities.
According to CDC data, Black women face nearly three times the risk of maternal mortality. They also are more than twice – and in some states close to three times – as likely to have a stillbirth than white women, meaning not only are Black mothers dying at a disproportionate rate, so are their babies.
Janet Petersen, a state senator from Iowa, said it gives her hope to see how the country has turned its attention to maternal mortality and disparities in health care. She simply cannot understand why stillbirth isn’t being met with the same urgency.
In 2020, the CDC reported 861 mothers died either while pregnant or within six weeks of giving birth. That same year, 20,854 babies were stillborn.
Stillbirth, Petersen said, is a missing piece of the puzzle. Research shows the likelihood of severe maternal complications was more than four times higher for pregnancies that ended in stillbirths, and mothers who died within six weeks of delivery were more likely to have had a stillbirth.
“We see it over and over again that stillbirth is one of the maternal health care issues that continuously gets ignored,” said Petersen, a Democrat.
Petersen was a young legislator in 2003 when her daughter Grace was born still. Devastated, she thought of her grandmother, who lost a baby to stillbirth in 1920, just a few weeks before women got the right to vote.
“I was laying in my hospital bed thinking, ‘How could this still be happening in our country?’” Petersen recalled. “And it seemed, from the medical perspective, that, well, stillbirth happens. We can’t do anything to prevent them.”
Over the next few months, Petersen heard from other mothers who had lost their babies and wanted to spark change. As an elected official, Petersen was in a position to do that. In 2004, she introduced legislation that required the Iowa Department of Public Health to create a stillbirths work group, later securing funding through the CDC to create a stillbirth registry.
But the CDC didn’t renew the funding and never analyzed the data from the registry, though a CDC spokesperson said the Iowa Department of Public Health examined the data. Officials from the department did not respond to requests for comment.
Petersen and her fellow mothers pivoted. After hearing how researchers in Norway were able to increase awareness around fetal movement, they co-founded a nonprofit aimed at doing the same in the U.S.
The group, Healthy Birth Day, created colorful “Count the Kicks” pamphlets – and later an app – teaching pregnant people how to track a baby’s movements and establish what is normal for them. Monitoring a baby’s movements is the earliest and sometimes only indication that something may be wrong, said Emily Price, chief executive officer of Healthy Birth Day. One of the organization’s main messages is for pregnant people to speak up and clinicians to listen.
“Unfortunately, there are still doctors who brush women off or send them home when they come in with a complaint of a change in their baby’s movements,” Price said. “And babies are dying because of it.”
One Indiana county, which recorded 65 stillbirths from 2017 through 2019, reported that 74% had either some chance or a good chance of prevention, according to St. Joseph County Department of Health’s Fetal Infant Mortality Review program. For mothers who experienced decreased fetal movement in the few hours or days before the stillbirth, that estimate jumped to 90%.
Although there is not a scientific consensus that kick counting can prevent stillbirths, national groups, including ACOG, recommend that medical professionals encourage their patients to be aware of fetal movement patterns. ACOG also advises medical professionals to be attentive to a mother’s concerns about reduced movement and address them “in a systematic way.”
One complaint the CDC hears too often, an agency spokesperson said, is that pregnant people and those who gave birth recently find that their concerns are dismissed or ignored. “Listening and taking the concerns of pregnant and recently pregnant people seriously,” she said, “is a simple, yet powerful action to prevent serious health complications and even death.”
The CDC, she said, is “very interested” in expanding its research on stillbirth, which is “a crucial part of the development of any awareness or prevention campaigns.” In addition to working to improve its stillbirth data quality, the agency has funded some pilot programs at the city and state level to better track stillbirths, survey people who have had a stillbirth and research risk factors and causes. The Iowa registry, she said, led the CDC to fund different research projects in Arkansas and Massachusetts, which are ongoing.
In 2009, the CDC acknowledged that fetal mortality remained a “major, but often overlooked, public health problem.” Officials wrote that much of the public health concern had been focused on infant mortality “in part due to lesser awareness of the magnitude of fetal mortality, its causes, and prevention strategies.”
But little has changed over the past 13 years. Echoing its earlier message, the CDC this year declared that “much work remains” and that “stillbirth is not often viewed as a public health issue, so increased awareness is key.”
A spokesperson for the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which is part of the NIH, said the agency has continually funded research on stillbirths, even after one of its key studies ended. The agency, she said, also supports research on conditions that increase the risk of stillbirth.
As a scientific research institute, it does not issue clinical guidelines or recommendations, she said, though it did launch the Safe to Sleep campaign in 1994, two decades after Congress put it in charge of SIDS federal research efforts. That campaign, which educates parents and caregivers on ways to reduce the risk of SIDS, highlights recommendations issued by the American Academy of Pediatrics. She said the agency will continue to collaborate with organizations that raise awareness about stillbirth and other pregnancy complications “to amplify their messages and efforts.”
“NICHD continues to support research on the prevention, causes, frequency, and risk factors of stillbirth,” the spokesperson said in an email. “Our commitment to enhancing understanding of stillbirth and improving outcomes focuses on building the scientific knowledge base.”
But getting laws on the books that could raise awareness around stillbirth – even when they don’t require additional funding – has been a struggle. Petersen and Price are pushing Congress to pass legislation that would add stillbirth research and prevention to the list of activities approved for federal maternal health dollars.
Though the bill doesn’t ask for any additional funding, it has not yet passed.
In addition, the SHINE for Autumn Act breezed through the House of Representatives in December 2021. After Haine Vijayvergiya, the New Jersey mother who has championed it, secured bipartisan support from U.S. Sens. Cory Booker, D-N.J., and Marco Rubio, R-Fla., she thought the most comprehensive stillbirth legislation in U.S. history would finally become law.
Neither bill has sparked controversy.
But months after press releases announced the SHINE legislation and referred to the U.S. stillbirth rate as “unacceptable,” lawmakers and the families they represent are running out of time as this session of Congress prepares to adjourn.
“From the day that the bill was introduced into the Senate,” Haine Vijayvergiya said, “approximately 13,000 babies have been born still.”
Last month, on a brilliant fall day much like the one when Reese was stillborn, Amanda Duffy bent down to kiss her son Rogen’s head before they walked on stage.
She wore a soft blue T-shirt tucked into her jeans that read “Be courageous.” The message was as much for her as it was for the crowd on the National Mall in Washington, D.C., many of them like her, mothers who didn’t know stillbirth happened until it happened to them. Since Reese’s death, she has coached doctors and nurses on improving care for patients who have suffered pregnancy loss. Among her many suggestions, she tells them their first words when a concerned patient reaches out should be “I’m so glad you called.”
A few hundred people had gathered for The Big PUSH to End Preventable Stillbirth, billed as the first-ever march on the issue. As part of an art installation, Amanda wrote a note to Reese: “You’re pretty magical & for that I’m grateful. You’re a change maker and you are so very loved. Love, Mama.” Before she slipped the folded paper into a sea of more than 20,000 baby hats, Rogen added his own message: “Hope you are having a good time – Rogen.”
Reese would have turned 8 this month.
Before Amanda spoke, she took a deep breath and silenced her nerves. She walked onto the stage and called on Congress to pass the stillbirth legislation before it. She didn’t ask. She demanded.
“It’s time to empower pregnant people and their care providers with information that leads to prevention,” she insisted.
With the afternoon sun bearing down, Amanda and Rogen disappeared into the crowd of families marching toward the Capitol. Many carried signs. Some pushed empty strollers. Amanda was still wearing the orange-scented oil she had rubbed on her wrists that morning.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published
Maternal hypertensive disorders of pregnancy increase mortality risk in offspring
Key clinical point: Prenatal exposure to hypertensive disorders during pregnancy (HDP), particularly preeclampsia and eclampsia, increased the risk for all-cause mortality in offspring from birth to young adulthood, with early-onset and severe preeclampsia exposure notably increasing the risk.
Major finding: Offspring exposed vs not exposed to maternal HDP were at a 26% higher risk for all-cause mortality (adjusted hazard ratio [aHR] 1.26; 95% CI 1.18-1.34), with the risk being 29% (aHR 1.29; 95% CI 1.20-1.38) and 188% (aHR 2.88; 95% CI 1.79-4.63) higher on exposure to preeclampsia and eclampsia, respectively. The all-cause mortality risk was much higher in offspring prenatally exposed to early-onset and severe preeclampsia (aHR 6.06; 95% CI 5.35-6.86).
Study details: This population-based cohort study included 2,437,718 offspring born between 1978 and 2018, of which 102,095 were prenatally exposed to maternal HDP.
Disclosures: This study was supported by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Shanghai Municipal Natural Science Foundation, Shanghai Municipal Science and Technology Major Project, Independent Research Fund Denmark, Nordic Cancer Union, Karen Elise Jensens Fond, and Novo Nordisk Fonden. The authors declared receiving support from the sources funding the study.
Source: Huang C et al. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: National population based cohort study. BMJ. 2022;379:e072157 (Oct 19) Erratum: 2022;379:o2726. Doi: 10.1136/bmj-2022-072157
Key clinical point: Prenatal exposure to hypertensive disorders during pregnancy (HDP), particularly preeclampsia and eclampsia, increased the risk for all-cause mortality in offspring from birth to young adulthood, with early-onset and severe preeclampsia exposure notably increasing the risk.
Major finding: Offspring exposed vs not exposed to maternal HDP were at a 26% higher risk for all-cause mortality (adjusted hazard ratio [aHR] 1.26; 95% CI 1.18-1.34), with the risk being 29% (aHR 1.29; 95% CI 1.20-1.38) and 188% (aHR 2.88; 95% CI 1.79-4.63) higher on exposure to preeclampsia and eclampsia, respectively. The all-cause mortality risk was much higher in offspring prenatally exposed to early-onset and severe preeclampsia (aHR 6.06; 95% CI 5.35-6.86).
Study details: This population-based cohort study included 2,437,718 offspring born between 1978 and 2018, of which 102,095 were prenatally exposed to maternal HDP.
Disclosures: This study was supported by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Shanghai Municipal Natural Science Foundation, Shanghai Municipal Science and Technology Major Project, Independent Research Fund Denmark, Nordic Cancer Union, Karen Elise Jensens Fond, and Novo Nordisk Fonden. The authors declared receiving support from the sources funding the study.
Source: Huang C et al. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: National population based cohort study. BMJ. 2022;379:e072157 (Oct 19) Erratum: 2022;379:o2726. Doi: 10.1136/bmj-2022-072157
Key clinical point: Prenatal exposure to hypertensive disorders during pregnancy (HDP), particularly preeclampsia and eclampsia, increased the risk for all-cause mortality in offspring from birth to young adulthood, with early-onset and severe preeclampsia exposure notably increasing the risk.
Major finding: Offspring exposed vs not exposed to maternal HDP were at a 26% higher risk for all-cause mortality (adjusted hazard ratio [aHR] 1.26; 95% CI 1.18-1.34), with the risk being 29% (aHR 1.29; 95% CI 1.20-1.38) and 188% (aHR 2.88; 95% CI 1.79-4.63) higher on exposure to preeclampsia and eclampsia, respectively. The all-cause mortality risk was much higher in offspring prenatally exposed to early-onset and severe preeclampsia (aHR 6.06; 95% CI 5.35-6.86).
Study details: This population-based cohort study included 2,437,718 offspring born between 1978 and 2018, of which 102,095 were prenatally exposed to maternal HDP.
Disclosures: This study was supported by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Shanghai Municipal Natural Science Foundation, Shanghai Municipal Science and Technology Major Project, Independent Research Fund Denmark, Nordic Cancer Union, Karen Elise Jensens Fond, and Novo Nordisk Fonden. The authors declared receiving support from the sources funding the study.
Source: Huang C et al. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: National population based cohort study. BMJ. 2022;379:e072157 (Oct 19) Erratum: 2022;379:o2726. Doi: 10.1136/bmj-2022-072157








