User login
Wegovy Approved for MASH With Fibrosis, No Cirrhosis
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.
Journal Highlights: May-July 2025
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Data Trends 2025: Obesity
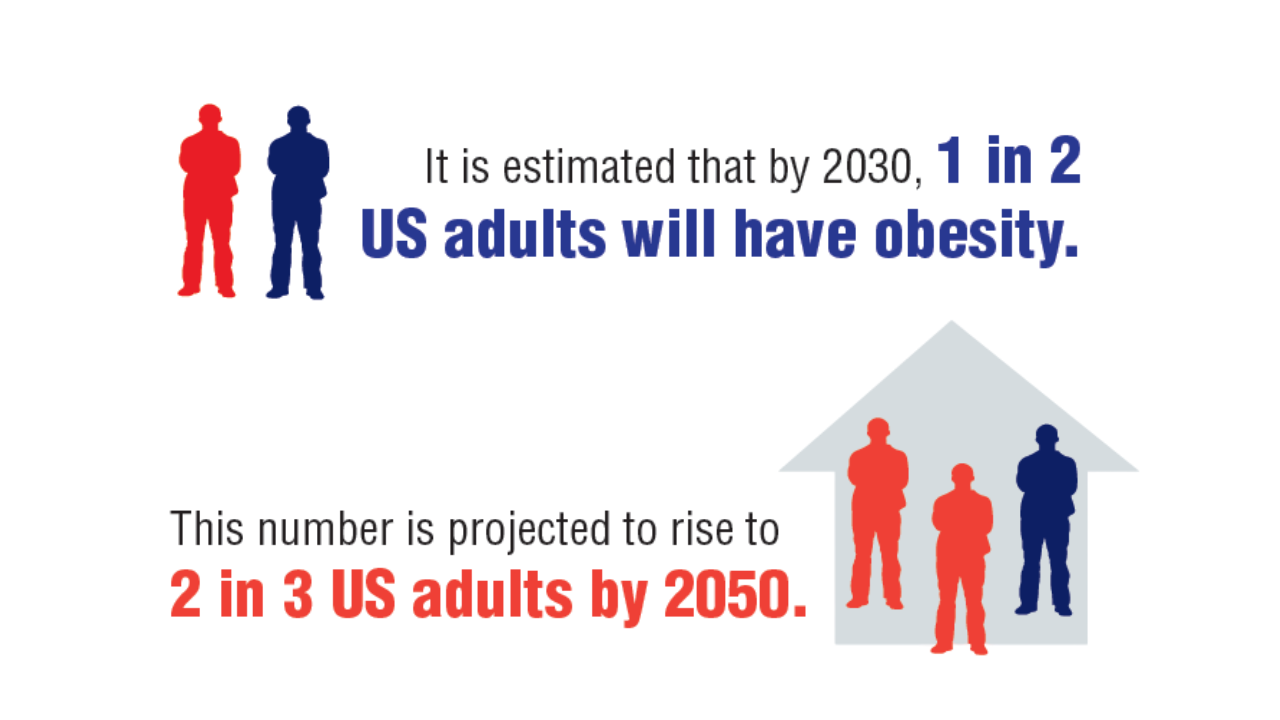
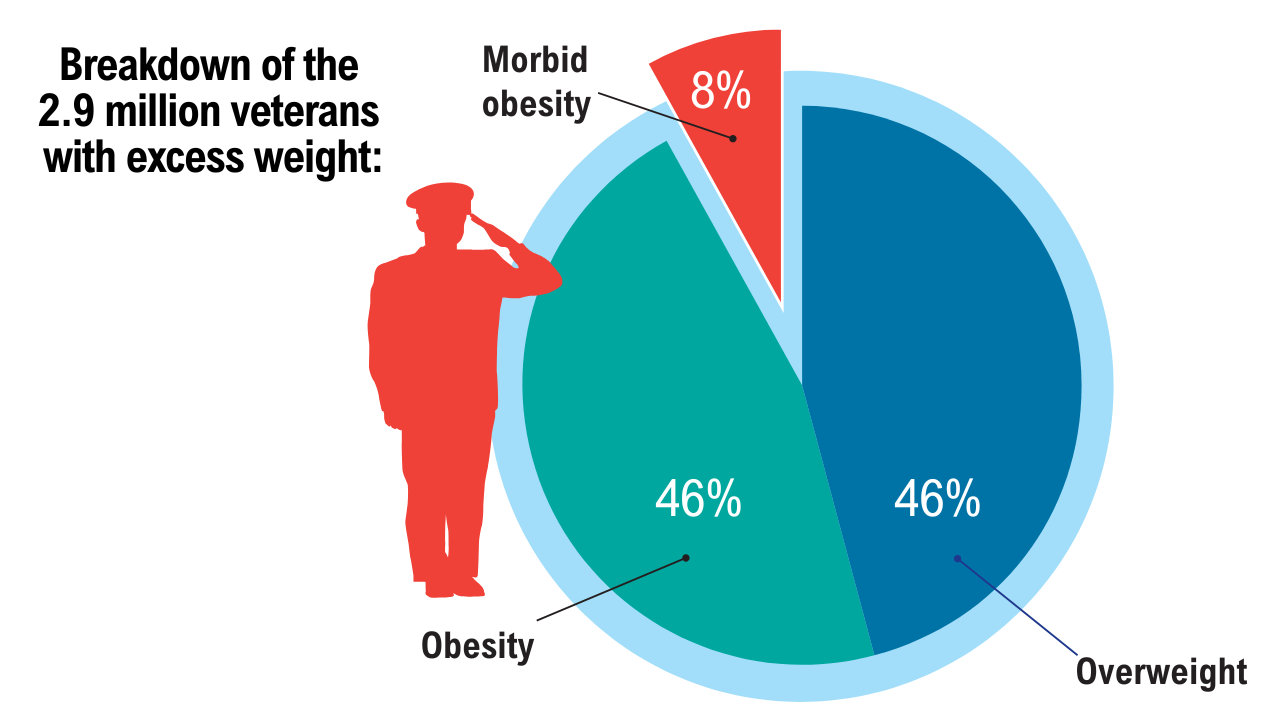
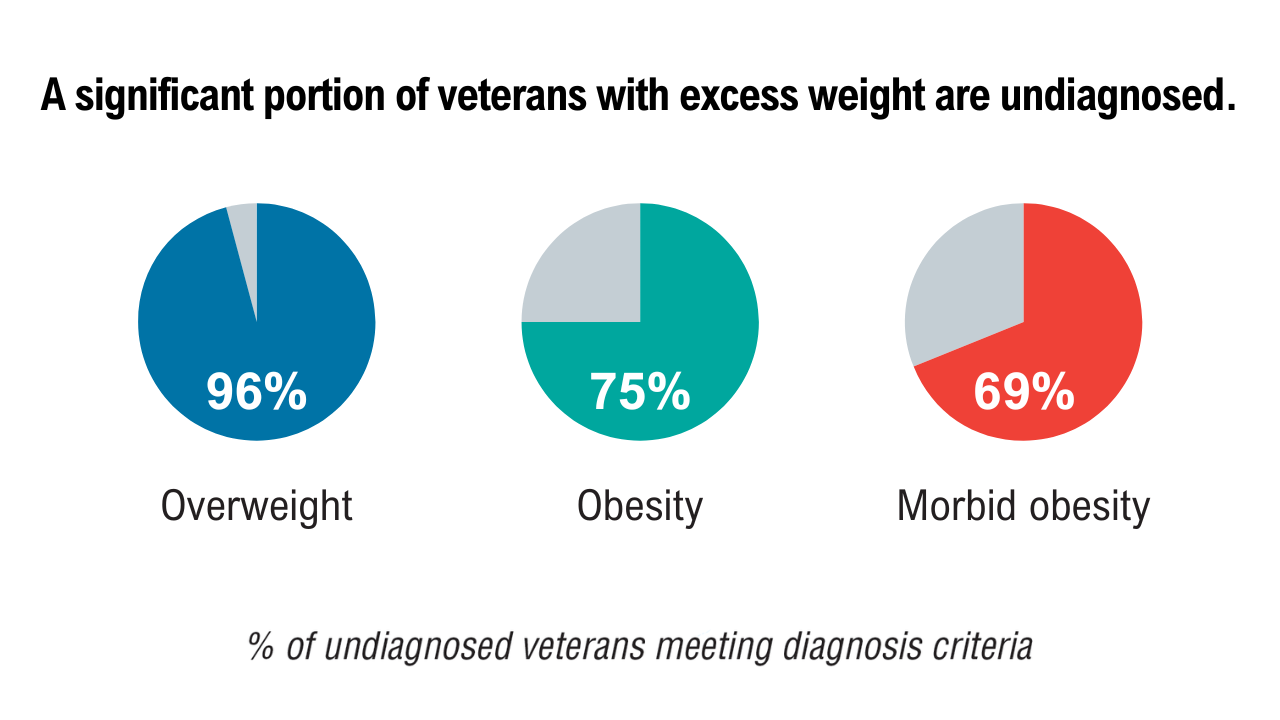
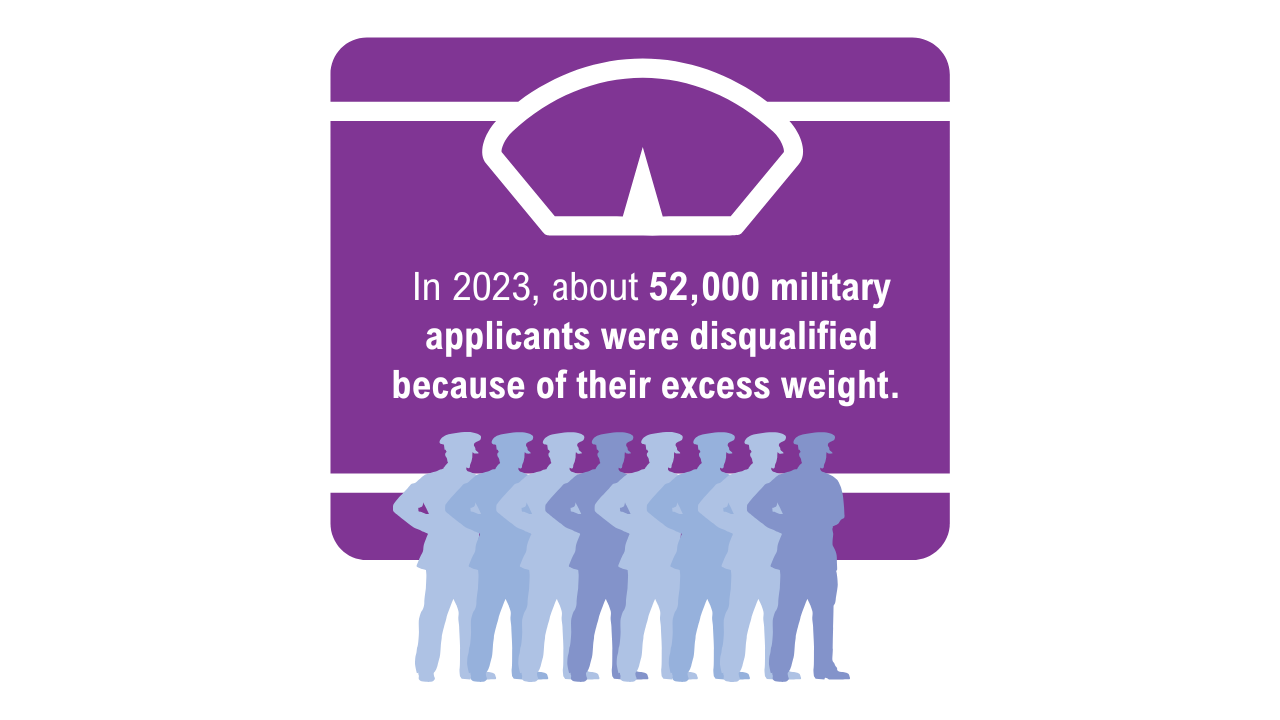
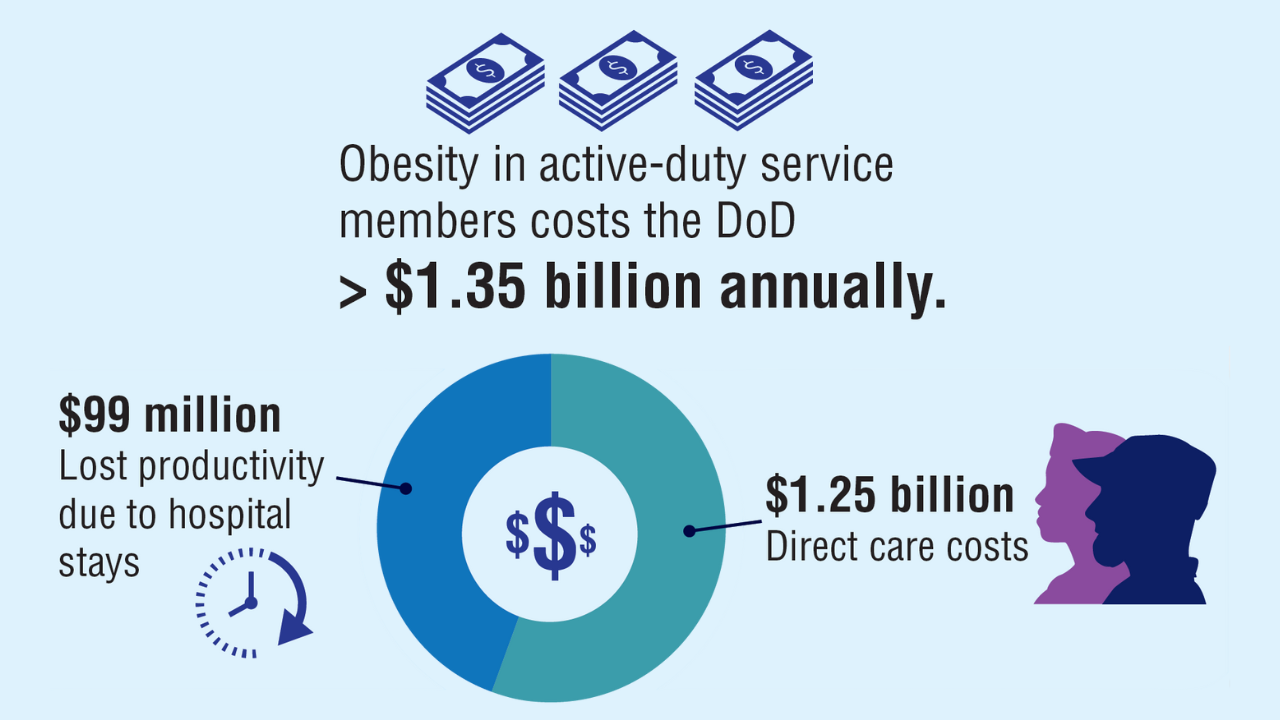
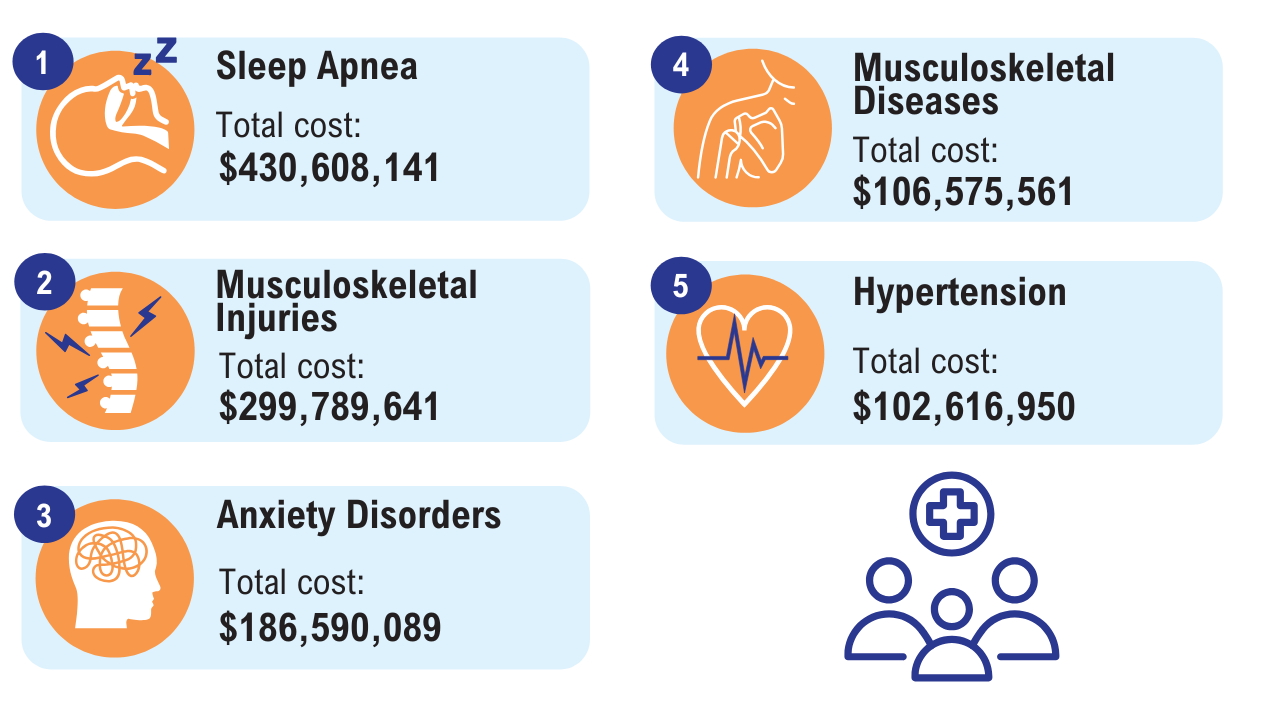
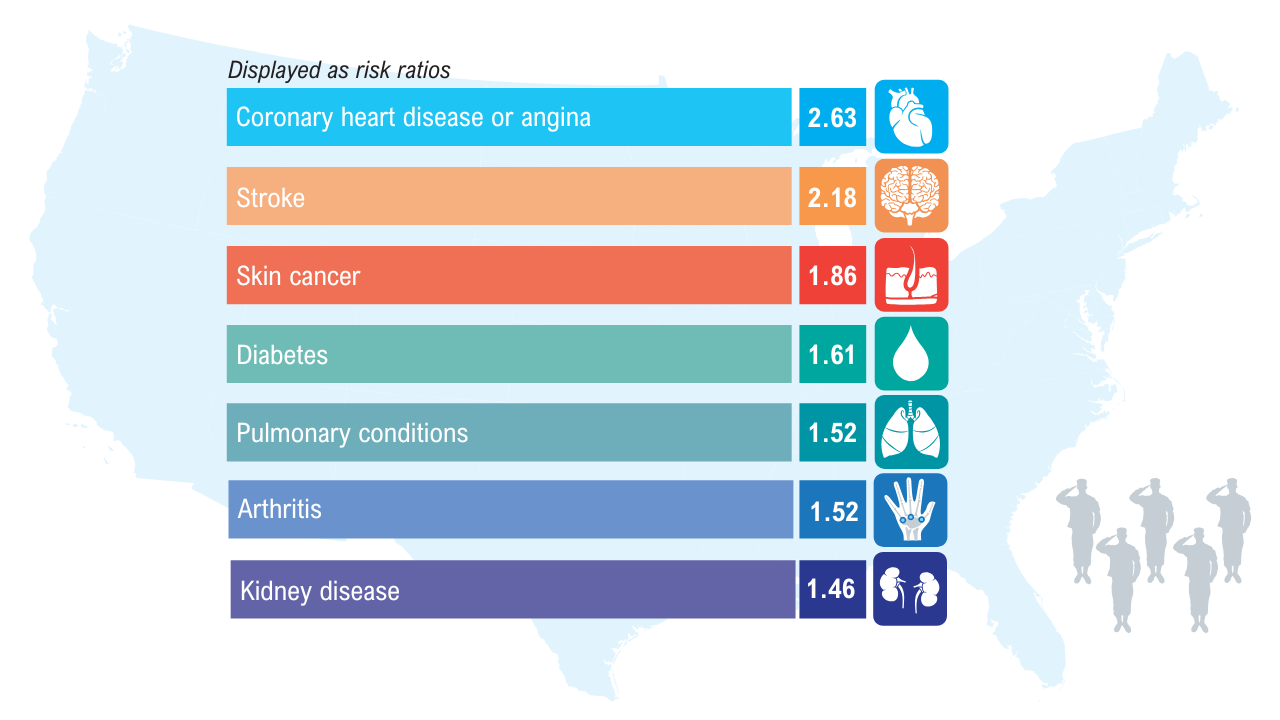
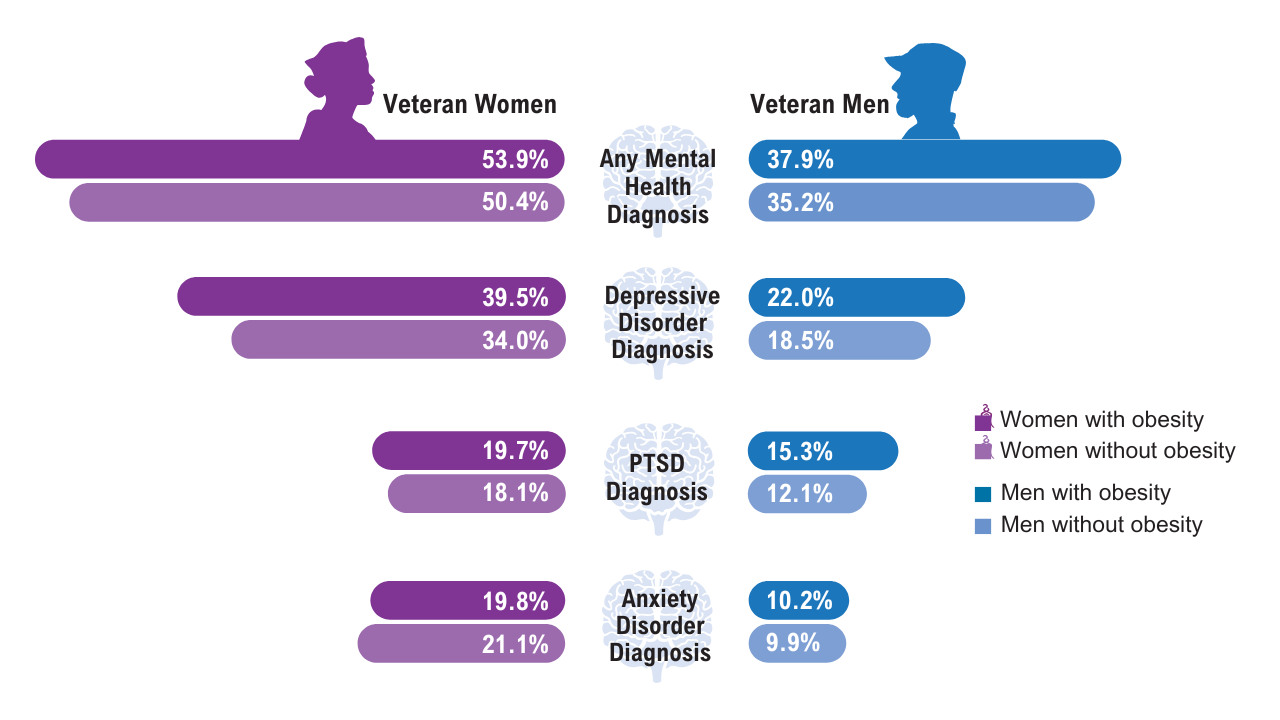
Obesity
Click here to view more from Federal Health Care Data Trends 2025.
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
Obesity
Obesity
Can Nonresponders to Antiobesity Medicines Be Predicted?
, enabling clinicians to better tailor antiobesity medication (AOM) to the patient.
Currently, patient response to AOMs varies widely, with some patients responding robustly to AOMs and others responding weakly or not at all.
For example, trials of the GLP-1 semaglutide found that 32%-39.6% of people are “super responders,” achieving weight loss in excess of 20%, and a subgroup of 10.2%-16.7% of individuals are nonresponders. Similar variability was found with other AOMs, including the GLP-1 liraglutide and tirzepatide, a dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonist.
Studies of semaglutide suggest that people with obesity and type 2 diabetes (T2D) lose less weight on the drug than those without T2D, and men tend to lose less weight than women.
However, little else is known about predictors of response rates for various AOMs, and medication selection is typically based on patient or physician preference, comorbidities, medication interactions, and insurance coverage.
Although definitions of a “nonresponder” vary, the Endocrine Society’s latest guideline, which many clinicians follow, states that an AOM is considered effective if patients lose more than 5% of their body weight within 3 months.
Can nonresponders and lower responders be identified and helped? Yes, but it’s complicated.
“Treating obesity effectively means recognizing that not all patients respond the same way to the same treatment, and that’s not a failure; it’s a signal,” said Andres Acosta, MD, PhD, an obesity expert at Mayo Clinic, Rochester, Minnesota, and a cofounder of Phenomix Sciences, a biotech company in Menlo Park, California.
“Obesity is not a single disease. It’s a complex, multifactorial condition driven by diverse biological pathways,” he told GI & Hepatology News. “Semaglutide and other GLP-1s primarily act by reducing appetite and slowing gastric emptying, but not all patients have obesity that is primarily driven by appetite dysregulation.”
Phenotype-Based Profiling
Figuring out what drives an individual’s obesity is where a phenotype-based profiling test could possibly help.
Acosta and colleagues previously used a variety of validated studies and questionnaires to identify four phenotypes that represent distinct biologic drivers of obesity: hungry brain (abnormal satiation), emotional hunger (hedonic eating), hungry gut (abnormal satiety), and slow burn (decreased metabolic rate). In their pragmatic clinical trial, phenotype-guided AOM selection was associated with 1.75-fold greater weight loss after 12 months than the standard approach to drug selection, with mean weight loss of 15.9% and 9%, respectively.
“If a patient’s obesity isn’t primarily rooted in the mechanisms targeted by a particular drug, their response will naturally be limited,” Acosta said. “It’s not that they’re failing the medication; the medication simply isn’t the right match for their biology.”
For their new study, published online in Cell Metabolism, Acosta and colleagues built on their previous research by analyzing the genetic and nongenetic factors that influenced calories needed to reach satiation (Calories to Satiation [CTS]) in adults with obesity. They then used machine learning techniques to develop a CTS gene risk score (CTS-GRS) that could be measured by a DNA saliva test.
The study included 717 adults with obesity (mean age, 41; 75% women) with marked variability in satiation, ranging from 140 to 2166 kcals to reach satiation.
CTS was assessed through an ad libitum meal, combined with physiological and behavioral evaluations, including calorimetry, imaging, blood sampling, and gastric emptying tests. The largest contributors to CTS variability were sex and genetic factors, while other anthropometric measurements played lesser roles.
Various analyses and assessments of participants’ CTS-GRS scores showed that individuals with a high CTS-GRS, or hungry brain phenotype, experienced significantly greater weight loss when treated with phentermine/topiramate than those with a low CTS-GRS, or hungry gut, phenotype. After 52 weeks of treatment, individuals with the hungry brain phenotype lost an average of 17.4% of their body weight compared with 11.2% in those with the hungry gut phenotype.
An analysis of a separate 16-week study showed that patients with the hungry gut phenotype responded better to the GLP-1 liraglutide, losing 6.4% total body weight, compared to 3.3% for those with the hungry brain phenotype.
Overall, the CTS-GRS test predicted drug response with up to 84% accuracy (area under the curve, 0.76 in men and 0.84 in women). The authors acknowledged that these results need to be replicated prospectively and in more diverse populations to validate the test’s predictive ability.
“This kind of phenotype-based profiling allows us to predict which patients are more likely to respond and who might need a different intervention,” Acosta said. “It’s a critical step toward eliminating trial-and-error in obesity treatment.”
The test (MyPhenome test) is used at more than 80 healthcare clinics in the United States, according to Phenomix Sciences, which manufactures it. A company spokesperson said the test does not require FDA approval because it is used to predict obesity phenotypes to help inform treatment, but not to identify specific medications or other interventions. “If it were to do the latter,” the spokesperson said, “it would be considered a ‘companion diagnostic’ and subject to the FDA clearance process.”
What to Do if an AOM Isn’t Working?
It’s one thing to predict whether an individual might do better on one drug vs another, but what should clinicians do meanwhile to optimize weight loss for their patients who may be struggling on a particular drug?
“Efforts to predict the response to GLP-1 therapy have been a hot topic,” noted Sriram Machineni, MD, associate professor at Montefiore Medical Center, Bronx, New York, and founding director of the Fleischer Institute Medical Weight Center at Montefiore Einstein. Although the current study showed that genetic testing could predict responders, like Acosta, he agreed that the results need to be replicated in a prospective manner.
“In the absence of a validated tool for predicting response to specific medications, we use a prioritization process for trialing medications,” Machineni told GI & Hepatology News. “The prioritization is based on the suitability of the side-effect profile to the specific patient, including contraindications; benefits independent of weight loss, such as cardiovascular protection for semaglutide; average efficacy; and financial accessibility for patients.”
Predicting responders isn’t straightforward, said Robert Kushner, MD, professor of medicine and medical education at the Feinberg School of Medicine at Northwestern University and medical director of the Wellness Institute at Northwestern Memorial Hospital in Chicago.
“Despite looking at baseline demographic data such as race, ethnicity, age, weight, and BMI, we are unable to predict who will lose more or less weight,” he told GI & Hepatology News. The one exception is that women generally lose more weight than men. “However, even among females, we cannot discern which females will lose more weight than other females,” he said.
If an individual is not showing sufficient weight loss on a particular medication, “we first explore potential reasons that can be addressed, such as the patient is not taking the medication or is skipping doses,” Kushner said. If need be, they discuss changing to a different drug to improve compliance. He also stresses the importance of making lifestyle changes in diet and physical activity for patients taking AOMs.
Often patients who do not lose at least 5% of their weight within 3 months are not likely to respond well to that medication even if they remain on it. “So, early response rates determine longer-term success,” Kushner said.
Acosta said that if a patient isn’t responding to one class of medication, he pivots to a treatment better aligned with their phenotype. “That could mean switching from a GLP-1 to a medication like [naltrexone/bupropion] or trying a new method altogether,” he said. “The key is that the treatment decision is rooted in the patient’s biology, not just a reaction to short-term results. We also emphasize the importance of long-term follow-up and support.”
The goal isn’t just weight loss but also improved health and quality of life, Acosta said. “Whether through medication, surgery, or behavior change, what matters most is tailoring the care plan to each individual’s unique biology and needs.”
The new study received support from the Mayo Clinic Clinical Research Trials Unit, Vivus Inc., and Phenomix Sciences. Acosta is supported by a National Institutes of Health grant.
Acosta is a co-founder and inventor of intellectual property licensed to Phenomix Sciences Inc.; has served as a consultant for Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Boehringer Ingelheim, Currax Pharmaceuticals, Nestlé, Bausch Health, and Rare Diseases; and has received research support or had contracts with Vivus Inc., Satiogen Pharmaceuticals, Boehringer Ingelheim, and Rhythm Pharmaceuticals. Machineni has been involved in semaglutide and tirzepatide clinical trials and has been a consultant to Novo Nordisk, Eli Lilly and Company, and Rhythm Pharmaceuticals. Kushner is on the scientific advisory board for Novo Nordisk.
A version of this article appeared on Medscape.com.
, enabling clinicians to better tailor antiobesity medication (AOM) to the patient.
Currently, patient response to AOMs varies widely, with some patients responding robustly to AOMs and others responding weakly or not at all.
For example, trials of the GLP-1 semaglutide found that 32%-39.6% of people are “super responders,” achieving weight loss in excess of 20%, and a subgroup of 10.2%-16.7% of individuals are nonresponders. Similar variability was found with other AOMs, including the GLP-1 liraglutide and tirzepatide, a dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonist.
Studies of semaglutide suggest that people with obesity and type 2 diabetes (T2D) lose less weight on the drug than those without T2D, and men tend to lose less weight than women.
However, little else is known about predictors of response rates for various AOMs, and medication selection is typically based on patient or physician preference, comorbidities, medication interactions, and insurance coverage.
Although definitions of a “nonresponder” vary, the Endocrine Society’s latest guideline, which many clinicians follow, states that an AOM is considered effective if patients lose more than 5% of their body weight within 3 months.
Can nonresponders and lower responders be identified and helped? Yes, but it’s complicated.
“Treating obesity effectively means recognizing that not all patients respond the same way to the same treatment, and that’s not a failure; it’s a signal,” said Andres Acosta, MD, PhD, an obesity expert at Mayo Clinic, Rochester, Minnesota, and a cofounder of Phenomix Sciences, a biotech company in Menlo Park, California.
“Obesity is not a single disease. It’s a complex, multifactorial condition driven by diverse biological pathways,” he told GI & Hepatology News. “Semaglutide and other GLP-1s primarily act by reducing appetite and slowing gastric emptying, but not all patients have obesity that is primarily driven by appetite dysregulation.”
Phenotype-Based Profiling
Figuring out what drives an individual’s obesity is where a phenotype-based profiling test could possibly help.
Acosta and colleagues previously used a variety of validated studies and questionnaires to identify four phenotypes that represent distinct biologic drivers of obesity: hungry brain (abnormal satiation), emotional hunger (hedonic eating), hungry gut (abnormal satiety), and slow burn (decreased metabolic rate). In their pragmatic clinical trial, phenotype-guided AOM selection was associated with 1.75-fold greater weight loss after 12 months than the standard approach to drug selection, with mean weight loss of 15.9% and 9%, respectively.
“If a patient’s obesity isn’t primarily rooted in the mechanisms targeted by a particular drug, their response will naturally be limited,” Acosta said. “It’s not that they’re failing the medication; the medication simply isn’t the right match for their biology.”
For their new study, published online in Cell Metabolism, Acosta and colleagues built on their previous research by analyzing the genetic and nongenetic factors that influenced calories needed to reach satiation (Calories to Satiation [CTS]) in adults with obesity. They then used machine learning techniques to develop a CTS gene risk score (CTS-GRS) that could be measured by a DNA saliva test.
The study included 717 adults with obesity (mean age, 41; 75% women) with marked variability in satiation, ranging from 140 to 2166 kcals to reach satiation.
CTS was assessed through an ad libitum meal, combined with physiological and behavioral evaluations, including calorimetry, imaging, blood sampling, and gastric emptying tests. The largest contributors to CTS variability were sex and genetic factors, while other anthropometric measurements played lesser roles.
Various analyses and assessments of participants’ CTS-GRS scores showed that individuals with a high CTS-GRS, or hungry brain phenotype, experienced significantly greater weight loss when treated with phentermine/topiramate than those with a low CTS-GRS, or hungry gut, phenotype. After 52 weeks of treatment, individuals with the hungry brain phenotype lost an average of 17.4% of their body weight compared with 11.2% in those with the hungry gut phenotype.
An analysis of a separate 16-week study showed that patients with the hungry gut phenotype responded better to the GLP-1 liraglutide, losing 6.4% total body weight, compared to 3.3% for those with the hungry brain phenotype.
Overall, the CTS-GRS test predicted drug response with up to 84% accuracy (area under the curve, 0.76 in men and 0.84 in women). The authors acknowledged that these results need to be replicated prospectively and in more diverse populations to validate the test’s predictive ability.
“This kind of phenotype-based profiling allows us to predict which patients are more likely to respond and who might need a different intervention,” Acosta said. “It’s a critical step toward eliminating trial-and-error in obesity treatment.”
The test (MyPhenome test) is used at more than 80 healthcare clinics in the United States, according to Phenomix Sciences, which manufactures it. A company spokesperson said the test does not require FDA approval because it is used to predict obesity phenotypes to help inform treatment, but not to identify specific medications or other interventions. “If it were to do the latter,” the spokesperson said, “it would be considered a ‘companion diagnostic’ and subject to the FDA clearance process.”
What to Do if an AOM Isn’t Working?
It’s one thing to predict whether an individual might do better on one drug vs another, but what should clinicians do meanwhile to optimize weight loss for their patients who may be struggling on a particular drug?
“Efforts to predict the response to GLP-1 therapy have been a hot topic,” noted Sriram Machineni, MD, associate professor at Montefiore Medical Center, Bronx, New York, and founding director of the Fleischer Institute Medical Weight Center at Montefiore Einstein. Although the current study showed that genetic testing could predict responders, like Acosta, he agreed that the results need to be replicated in a prospective manner.
“In the absence of a validated tool for predicting response to specific medications, we use a prioritization process for trialing medications,” Machineni told GI & Hepatology News. “The prioritization is based on the suitability of the side-effect profile to the specific patient, including contraindications; benefits independent of weight loss, such as cardiovascular protection for semaglutide; average efficacy; and financial accessibility for patients.”
Predicting responders isn’t straightforward, said Robert Kushner, MD, professor of medicine and medical education at the Feinberg School of Medicine at Northwestern University and medical director of the Wellness Institute at Northwestern Memorial Hospital in Chicago.
“Despite looking at baseline demographic data such as race, ethnicity, age, weight, and BMI, we are unable to predict who will lose more or less weight,” he told GI & Hepatology News. The one exception is that women generally lose more weight than men. “However, even among females, we cannot discern which females will lose more weight than other females,” he said.
If an individual is not showing sufficient weight loss on a particular medication, “we first explore potential reasons that can be addressed, such as the patient is not taking the medication or is skipping doses,” Kushner said. If need be, they discuss changing to a different drug to improve compliance. He also stresses the importance of making lifestyle changes in diet and physical activity for patients taking AOMs.
Often patients who do not lose at least 5% of their weight within 3 months are not likely to respond well to that medication even if they remain on it. “So, early response rates determine longer-term success,” Kushner said.
Acosta said that if a patient isn’t responding to one class of medication, he pivots to a treatment better aligned with their phenotype. “That could mean switching from a GLP-1 to a medication like [naltrexone/bupropion] or trying a new method altogether,” he said. “The key is that the treatment decision is rooted in the patient’s biology, not just a reaction to short-term results. We also emphasize the importance of long-term follow-up and support.”
The goal isn’t just weight loss but also improved health and quality of life, Acosta said. “Whether through medication, surgery, or behavior change, what matters most is tailoring the care plan to each individual’s unique biology and needs.”
The new study received support from the Mayo Clinic Clinical Research Trials Unit, Vivus Inc., and Phenomix Sciences. Acosta is supported by a National Institutes of Health grant.
Acosta is a co-founder and inventor of intellectual property licensed to Phenomix Sciences Inc.; has served as a consultant for Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Boehringer Ingelheim, Currax Pharmaceuticals, Nestlé, Bausch Health, and Rare Diseases; and has received research support or had contracts with Vivus Inc., Satiogen Pharmaceuticals, Boehringer Ingelheim, and Rhythm Pharmaceuticals. Machineni has been involved in semaglutide and tirzepatide clinical trials and has been a consultant to Novo Nordisk, Eli Lilly and Company, and Rhythm Pharmaceuticals. Kushner is on the scientific advisory board for Novo Nordisk.
A version of this article appeared on Medscape.com.
, enabling clinicians to better tailor antiobesity medication (AOM) to the patient.
Currently, patient response to AOMs varies widely, with some patients responding robustly to AOMs and others responding weakly or not at all.
For example, trials of the GLP-1 semaglutide found that 32%-39.6% of people are “super responders,” achieving weight loss in excess of 20%, and a subgroup of 10.2%-16.7% of individuals are nonresponders. Similar variability was found with other AOMs, including the GLP-1 liraglutide and tirzepatide, a dual GLP-1/glucose-dependent insulinotropic polypeptide receptor agonist.
Studies of semaglutide suggest that people with obesity and type 2 diabetes (T2D) lose less weight on the drug than those without T2D, and men tend to lose less weight than women.
However, little else is known about predictors of response rates for various AOMs, and medication selection is typically based on patient or physician preference, comorbidities, medication interactions, and insurance coverage.
Although definitions of a “nonresponder” vary, the Endocrine Society’s latest guideline, which many clinicians follow, states that an AOM is considered effective if patients lose more than 5% of their body weight within 3 months.
Can nonresponders and lower responders be identified and helped? Yes, but it’s complicated.
“Treating obesity effectively means recognizing that not all patients respond the same way to the same treatment, and that’s not a failure; it’s a signal,” said Andres Acosta, MD, PhD, an obesity expert at Mayo Clinic, Rochester, Minnesota, and a cofounder of Phenomix Sciences, a biotech company in Menlo Park, California.
“Obesity is not a single disease. It’s a complex, multifactorial condition driven by diverse biological pathways,” he told GI & Hepatology News. “Semaglutide and other GLP-1s primarily act by reducing appetite and slowing gastric emptying, but not all patients have obesity that is primarily driven by appetite dysregulation.”
Phenotype-Based Profiling
Figuring out what drives an individual’s obesity is where a phenotype-based profiling test could possibly help.
Acosta and colleagues previously used a variety of validated studies and questionnaires to identify four phenotypes that represent distinct biologic drivers of obesity: hungry brain (abnormal satiation), emotional hunger (hedonic eating), hungry gut (abnormal satiety), and slow burn (decreased metabolic rate). In their pragmatic clinical trial, phenotype-guided AOM selection was associated with 1.75-fold greater weight loss after 12 months than the standard approach to drug selection, with mean weight loss of 15.9% and 9%, respectively.
“If a patient’s obesity isn’t primarily rooted in the mechanisms targeted by a particular drug, their response will naturally be limited,” Acosta said. “It’s not that they’re failing the medication; the medication simply isn’t the right match for their biology.”
For their new study, published online in Cell Metabolism, Acosta and colleagues built on their previous research by analyzing the genetic and nongenetic factors that influenced calories needed to reach satiation (Calories to Satiation [CTS]) in adults with obesity. They then used machine learning techniques to develop a CTS gene risk score (CTS-GRS) that could be measured by a DNA saliva test.
The study included 717 adults with obesity (mean age, 41; 75% women) with marked variability in satiation, ranging from 140 to 2166 kcals to reach satiation.
CTS was assessed through an ad libitum meal, combined with physiological and behavioral evaluations, including calorimetry, imaging, blood sampling, and gastric emptying tests. The largest contributors to CTS variability were sex and genetic factors, while other anthropometric measurements played lesser roles.
Various analyses and assessments of participants’ CTS-GRS scores showed that individuals with a high CTS-GRS, or hungry brain phenotype, experienced significantly greater weight loss when treated with phentermine/topiramate than those with a low CTS-GRS, or hungry gut, phenotype. After 52 weeks of treatment, individuals with the hungry brain phenotype lost an average of 17.4% of their body weight compared with 11.2% in those with the hungry gut phenotype.
An analysis of a separate 16-week study showed that patients with the hungry gut phenotype responded better to the GLP-1 liraglutide, losing 6.4% total body weight, compared to 3.3% for those with the hungry brain phenotype.
Overall, the CTS-GRS test predicted drug response with up to 84% accuracy (area under the curve, 0.76 in men and 0.84 in women). The authors acknowledged that these results need to be replicated prospectively and in more diverse populations to validate the test’s predictive ability.
“This kind of phenotype-based profiling allows us to predict which patients are more likely to respond and who might need a different intervention,” Acosta said. “It’s a critical step toward eliminating trial-and-error in obesity treatment.”
The test (MyPhenome test) is used at more than 80 healthcare clinics in the United States, according to Phenomix Sciences, which manufactures it. A company spokesperson said the test does not require FDA approval because it is used to predict obesity phenotypes to help inform treatment, but not to identify specific medications or other interventions. “If it were to do the latter,” the spokesperson said, “it would be considered a ‘companion diagnostic’ and subject to the FDA clearance process.”
What to Do if an AOM Isn’t Working?
It’s one thing to predict whether an individual might do better on one drug vs another, but what should clinicians do meanwhile to optimize weight loss for their patients who may be struggling on a particular drug?
“Efforts to predict the response to GLP-1 therapy have been a hot topic,” noted Sriram Machineni, MD, associate professor at Montefiore Medical Center, Bronx, New York, and founding director of the Fleischer Institute Medical Weight Center at Montefiore Einstein. Although the current study showed that genetic testing could predict responders, like Acosta, he agreed that the results need to be replicated in a prospective manner.
“In the absence of a validated tool for predicting response to specific medications, we use a prioritization process for trialing medications,” Machineni told GI & Hepatology News. “The prioritization is based on the suitability of the side-effect profile to the specific patient, including contraindications; benefits independent of weight loss, such as cardiovascular protection for semaglutide; average efficacy; and financial accessibility for patients.”
Predicting responders isn’t straightforward, said Robert Kushner, MD, professor of medicine and medical education at the Feinberg School of Medicine at Northwestern University and medical director of the Wellness Institute at Northwestern Memorial Hospital in Chicago.
“Despite looking at baseline demographic data such as race, ethnicity, age, weight, and BMI, we are unable to predict who will lose more or less weight,” he told GI & Hepatology News. The one exception is that women generally lose more weight than men. “However, even among females, we cannot discern which females will lose more weight than other females,” he said.
If an individual is not showing sufficient weight loss on a particular medication, “we first explore potential reasons that can be addressed, such as the patient is not taking the medication or is skipping doses,” Kushner said. If need be, they discuss changing to a different drug to improve compliance. He also stresses the importance of making lifestyle changes in diet and physical activity for patients taking AOMs.
Often patients who do not lose at least 5% of their weight within 3 months are not likely to respond well to that medication even if they remain on it. “So, early response rates determine longer-term success,” Kushner said.
Acosta said that if a patient isn’t responding to one class of medication, he pivots to a treatment better aligned with their phenotype. “That could mean switching from a GLP-1 to a medication like [naltrexone/bupropion] or trying a new method altogether,” he said. “The key is that the treatment decision is rooted in the patient’s biology, not just a reaction to short-term results. We also emphasize the importance of long-term follow-up and support.”
The goal isn’t just weight loss but also improved health and quality of life, Acosta said. “Whether through medication, surgery, or behavior change, what matters most is tailoring the care plan to each individual’s unique biology and needs.”
The new study received support from the Mayo Clinic Clinical Research Trials Unit, Vivus Inc., and Phenomix Sciences. Acosta is supported by a National Institutes of Health grant.
Acosta is a co-founder and inventor of intellectual property licensed to Phenomix Sciences Inc.; has served as a consultant for Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Boehringer Ingelheim, Currax Pharmaceuticals, Nestlé, Bausch Health, and Rare Diseases; and has received research support or had contracts with Vivus Inc., Satiogen Pharmaceuticals, Boehringer Ingelheim, and Rhythm Pharmaceuticals. Machineni has been involved in semaglutide and tirzepatide clinical trials and has been a consultant to Novo Nordisk, Eli Lilly and Company, and Rhythm Pharmaceuticals. Kushner is on the scientific advisory board for Novo Nordisk.
A version of this article appeared on Medscape.com.
You Are When You Eat: Microbiome Rhythm and Metabolic Health
Similar to circadian rhythms that help regulate when we naturally fall asleep and wake up, microbial rhythms in our gut are naturally active at certain times of the day to help regulate our digestion.
Investigators from the University of California, San Diego sought out to track these microbial rhythms to determine whether aligning the times we eat to when our gut microbes are most active – time-restricted feeding (TRF) – can bolster our metabolic health. Their research was published recently in Cell Host & Microbe.
“Microbial rhythms are daily fluctuations in the composition and function of microbes living in our gut. Much like how our bodies follow an internal clock (circadian rhythm), gut microbes also have their own rhythms, adjusting their activities based on the time of day and when we eat,” said Amir Zarrinpar, MD, PhD, a gastroenterologist at UC San Diego School of Medicine, and senior author of the study.
Zarrinpar and his team were particularly interested in observing whether adopting the TRF approach counteracted the harmful metabolic effects often associated with consuming a high-fat diet.
The study is also notable for the team’s use of technology able to observe real-time microbial changes in the gut — something not previously attainable with existing metagenomics.
How the Study Evolved With New Tech
Researchers separated three groups of mice to analyze their microbiome activity: one on a high-fat diet with unrestricted access, another on the same high-fat diet within a TRF window of 8 hours per day, and a control group on a normal chow diet with unrestricted access.
“In mice, [their] microbial rhythms are well-aligned with their nocturnal lifestyle. For example, during their active (nighttime) period, certain beneficial microbial activities increase, helping digest food, absorb nutrients, and regulate metabolism,” said Zarrinpar. As a result, the team made sure the mice’s TRF window was at night or when they would normally be awake.
“We chose an 8-hour feeding window based on earlier research showing this time period allows mice to consume the same total calories as those with unlimited food access,” said Zarrinpar. “By controlling [the] calories in this way, we ensure any metabolic or microbial benefits we observe are specifically due to the timing of eating, rather than differences in total food intake.”
But before any observations could be made, the team first needed a way to see real-time changes in the animals’ gut microbiomes.
Zarrinpar and his team were able to uncover this, thanks to metatranscriptomics, a technique used to capture real-time microbial activity by profiling RNA transcripts. Compared with the more traditional technique of metagenomics, which could only be used to identify which genes were present, metatranscriptomics provided more in-depth temporal and activity-related context, allowing the team to observe dynamic microbial changes.
“[Metatranscriptomics] helps us understand not just which microbes are present, but specifically what they are doing at any given moment,” said Zarrinpar. “In contrast, metagenomics looks only at microbial DNA, which provides information about what microbes are potentially capable of doing, but doesn’t tell us if those genes are actively expressed. By comparing microbial gene expression (using metatranscriptomics) and microbial gene abundance (using metagenomics) across different diet and feeding conditions in [light and dark] phases, we aimed to identify how feeding timing might influence microbial activity.”
Because metagenomics focuses on stable genetic material, this technique cannot capture the real-time microbial responses to dietary timing presented in rapidly changing, short-lived RNA. At the same time, the instability of the RNA makes it difficult to test hypotheses experimentally and explains why researchers haven’t more widely relied on metatranscriptomics.
To overcome this difficulty, Zarrinpar and his team had to wait to take advantage of improved bioinformatics tools to simplify their analysis of complex datasets. “It took several years for us to analyze this dataset because robust computational tools for metatranscriptomic analysis were not widely available when we initially collected our samples. Additionally, sequencing costs were very high. To clearly identify microbial activity, we needed deep sequencing coverage to distinguish species-level differences in gene expression, especially for genes that are common across multiple types of microbes,” said Zarrinpar.
What They Found
After monitoring these groups of mice for 8 weeks, the results were revealed.
As predicted, “When mice have free access to a high-fat diet, their normal eating behavior changes significantly. Instead of limiting their activity and feeding to their active nighttime period, these mice begin to stay awake and eat during the day, which is their typical rest phase,” Zarrinpar explained.
“This unusual daytime activity interferes with important physiological processes. Consequently, the animals experience circadian misalignment, a condition similar to what human shift workers experience when their sleep-wake and eating cycles don’t match their internal biological clocks,” he continued. “This misalignment can negatively affect metabolism, immunity, and overall health, potentially leading to metabolic diseases.”
For the mice that consumed a high-fat diet within a TRF window, metabolic phenotyping demonstrated that their specific diet regimen had protected them from harmful high-fat induced effects including adiposity, inflammation, and insulin resistance.
Even more promising, the mice not only were protected from metabolic disruption but also experienced physiological improvements including glucose homeostasis and the partial restoration of the daily microbial rhythms absent in the mice with unrestricted access to a high-fat diet.
While the TRF approach did not fully restore the normal, healthy rhythmicity seen in the control mice, the researchers noted distinct shifts in microbial patterns that indicated time-dependent enrichment in genes attributed to lipid and carbohydrate metabolism.
Better Metabolic Health — and Better Tools for Researching It
Thankfully, the latest advancements in sequencing technology, including long-read sequencing methods, are making metatranscriptomics easier for research. “These newer platforms offer greater resolution at a lower cost, making metatranscriptomics increasingly accessible,” said Zarrinpar. With these emerging technologies, he believes metatranscriptomics will become a more standard, widely used method for researchers to better understand the influence of microbial activity on our health.
These tools, for example, enabled Zarrinpar and the team to delve deeper and focus on the transcription of a particular enzyme they identified as a pivotal influence in observable metabolic improvements: bile salt hydrolase (BSH), known to regulate lipid and glucose metabolism. The TRF approach notably enhanced the expression of the BSH gene during the daytime in the gut microbe Dubosiella newyorkensis, which has a functional human equivalent.
To determine why this happened, the team leveraged genetic engineering to insert several active BSH gene variants into a benign strain of gut bacteria to administer to the mice. The only variant to produce metabolic improvements was the one derived from Dubosiella newyorkensis; the mice who were given this BSH-expressing engineered native bacteria (ENB) had increased lean muscle mass, less body fat, lower insulin levels, enhanced insulin sensitivity, and better blood glucose regulation.
“It is still early to know the full clinical potential of this new BSH-expressing engineered native bacterium,” said Zarrinpar. “However, our long-term goal is to develop a therapeutic that can be administered as a single dose, stably colonize the gut, and provide long-lasting metabolic benefits.” Testing the engineered bacteria in obese and diabetic mice on a high-fat diet would be a next step to determine whether its potential indeed holds up. If proven successful, it could then be used to develop future targeted therapies and interventions to treat common metabolic disorders.
With this engineered bacteria, Zarrinpar and his team are hopeful that it alone can replicate the microbial benefits associated with following a TRF dietary schedule. “In our study, the engineered bacterium continuously expressed the enzyme DnBSH1, independently of dietary or environmental factors. As a result, the bacterium provided metabolic benefits similar to those seen with TRF, even without requiring the mice to strictly adhere to a TRF schedule,” said Zarrinpar.
“This suggests the exciting possibility that this engineered microbe might serve either as a replacement for TRF or as a way to enhance its beneficial effects,” he continued. “Further studies will help determine whether combining this ENB with TRF could provide additional or synergistic improvements in metabolic health.”
Looking Ahead
“As the pioneer of the single anastomosis duodenal switch which separates bile from food until halfway down the GI tract, I agree that bile is very important in controlling metabolism and glucose,” said Mitchell Roslin, MD, chief director of bariatric and metabolic surgery at Lenox Hill Hospital, and the Donald and Barbara Zucker School of Medicine, Hempstead, New York, who was not involved in the study. “Using enzymes or medications that work in the GI tract without absorption into the body is very interesting and has great potential. It is an early but exciting prospect.”
However, Roslin expressed some reservations. “I think we are still trying to understand whether the difference in microbiomes is the cause or effect/association. Is the microbiome the difference or is a different microbiome representative of a diet that has more fiber and less processed foods? Thus, while I find this academically fascinating, I think that there are very basic questions that need better answers, before we look at the transcription of bacteria.”
Furthermore, translating the metabolic results observed in mice to humans might not be as straightforward. “Small animal research is mandatory, but how the findings convert to humans is highly speculative,” said Roslin. “Mice that are studied are usually bred for medical research, with reduced genetic variation. Many animal models are more sensitive to time-restricted eating and caloric restriction than humans.”
While it requires further research and validation, this UC San Diego study nevertheless contributes to our overall understanding of host-microbe interactions. “We demonstrate that host circadian rhythms significantly influence microbial function, and conversely, these microbial functions can directly impact host metabolism,” said Zarrinpar. “Importantly, we now have a method to test how specific microbial activities affect host physiology by engineering native gut bacteria.”
Roslin similarly emphasized the importance of continued investment in exploring the microbial ecosystem inside us all. “There is wider evidence that bacteria and microbes are not just passengers using us for a ride but perhaps manipulating every action we take.”
A version of this article appeared on Medscape.com.
Similar to circadian rhythms that help regulate when we naturally fall asleep and wake up, microbial rhythms in our gut are naturally active at certain times of the day to help regulate our digestion.
Investigators from the University of California, San Diego sought out to track these microbial rhythms to determine whether aligning the times we eat to when our gut microbes are most active – time-restricted feeding (TRF) – can bolster our metabolic health. Their research was published recently in Cell Host & Microbe.
“Microbial rhythms are daily fluctuations in the composition and function of microbes living in our gut. Much like how our bodies follow an internal clock (circadian rhythm), gut microbes also have their own rhythms, adjusting their activities based on the time of day and when we eat,” said Amir Zarrinpar, MD, PhD, a gastroenterologist at UC San Diego School of Medicine, and senior author of the study.
Zarrinpar and his team were particularly interested in observing whether adopting the TRF approach counteracted the harmful metabolic effects often associated with consuming a high-fat diet.
The study is also notable for the team’s use of technology able to observe real-time microbial changes in the gut — something not previously attainable with existing metagenomics.
How the Study Evolved With New Tech
Researchers separated three groups of mice to analyze their microbiome activity: one on a high-fat diet with unrestricted access, another on the same high-fat diet within a TRF window of 8 hours per day, and a control group on a normal chow diet with unrestricted access.
“In mice, [their] microbial rhythms are well-aligned with their nocturnal lifestyle. For example, during their active (nighttime) period, certain beneficial microbial activities increase, helping digest food, absorb nutrients, and regulate metabolism,” said Zarrinpar. As a result, the team made sure the mice’s TRF window was at night or when they would normally be awake.
“We chose an 8-hour feeding window based on earlier research showing this time period allows mice to consume the same total calories as those with unlimited food access,” said Zarrinpar. “By controlling [the] calories in this way, we ensure any metabolic or microbial benefits we observe are specifically due to the timing of eating, rather than differences in total food intake.”
But before any observations could be made, the team first needed a way to see real-time changes in the animals’ gut microbiomes.
Zarrinpar and his team were able to uncover this, thanks to metatranscriptomics, a technique used to capture real-time microbial activity by profiling RNA transcripts. Compared with the more traditional technique of metagenomics, which could only be used to identify which genes were present, metatranscriptomics provided more in-depth temporal and activity-related context, allowing the team to observe dynamic microbial changes.
“[Metatranscriptomics] helps us understand not just which microbes are present, but specifically what they are doing at any given moment,” said Zarrinpar. “In contrast, metagenomics looks only at microbial DNA, which provides information about what microbes are potentially capable of doing, but doesn’t tell us if those genes are actively expressed. By comparing microbial gene expression (using metatranscriptomics) and microbial gene abundance (using metagenomics) across different diet and feeding conditions in [light and dark] phases, we aimed to identify how feeding timing might influence microbial activity.”
Because metagenomics focuses on stable genetic material, this technique cannot capture the real-time microbial responses to dietary timing presented in rapidly changing, short-lived RNA. At the same time, the instability of the RNA makes it difficult to test hypotheses experimentally and explains why researchers haven’t more widely relied on metatranscriptomics.
To overcome this difficulty, Zarrinpar and his team had to wait to take advantage of improved bioinformatics tools to simplify their analysis of complex datasets. “It took several years for us to analyze this dataset because robust computational tools for metatranscriptomic analysis were not widely available when we initially collected our samples. Additionally, sequencing costs were very high. To clearly identify microbial activity, we needed deep sequencing coverage to distinguish species-level differences in gene expression, especially for genes that are common across multiple types of microbes,” said Zarrinpar.
What They Found
After monitoring these groups of mice for 8 weeks, the results were revealed.
As predicted, “When mice have free access to a high-fat diet, their normal eating behavior changes significantly. Instead of limiting their activity and feeding to their active nighttime period, these mice begin to stay awake and eat during the day, which is their typical rest phase,” Zarrinpar explained.
“This unusual daytime activity interferes with important physiological processes. Consequently, the animals experience circadian misalignment, a condition similar to what human shift workers experience when their sleep-wake and eating cycles don’t match their internal biological clocks,” he continued. “This misalignment can negatively affect metabolism, immunity, and overall health, potentially leading to metabolic diseases.”
For the mice that consumed a high-fat diet within a TRF window, metabolic phenotyping demonstrated that their specific diet regimen had protected them from harmful high-fat induced effects including adiposity, inflammation, and insulin resistance.
Even more promising, the mice not only were protected from metabolic disruption but also experienced physiological improvements including glucose homeostasis and the partial restoration of the daily microbial rhythms absent in the mice with unrestricted access to a high-fat diet.
While the TRF approach did not fully restore the normal, healthy rhythmicity seen in the control mice, the researchers noted distinct shifts in microbial patterns that indicated time-dependent enrichment in genes attributed to lipid and carbohydrate metabolism.
Better Metabolic Health — and Better Tools for Researching It
Thankfully, the latest advancements in sequencing technology, including long-read sequencing methods, are making metatranscriptomics easier for research. “These newer platforms offer greater resolution at a lower cost, making metatranscriptomics increasingly accessible,” said Zarrinpar. With these emerging technologies, he believes metatranscriptomics will become a more standard, widely used method for researchers to better understand the influence of microbial activity on our health.
These tools, for example, enabled Zarrinpar and the team to delve deeper and focus on the transcription of a particular enzyme they identified as a pivotal influence in observable metabolic improvements: bile salt hydrolase (BSH), known to regulate lipid and glucose metabolism. The TRF approach notably enhanced the expression of the BSH gene during the daytime in the gut microbe Dubosiella newyorkensis, which has a functional human equivalent.
To determine why this happened, the team leveraged genetic engineering to insert several active BSH gene variants into a benign strain of gut bacteria to administer to the mice. The only variant to produce metabolic improvements was the one derived from Dubosiella newyorkensis; the mice who were given this BSH-expressing engineered native bacteria (ENB) had increased lean muscle mass, less body fat, lower insulin levels, enhanced insulin sensitivity, and better blood glucose regulation.
“It is still early to know the full clinical potential of this new BSH-expressing engineered native bacterium,” said Zarrinpar. “However, our long-term goal is to develop a therapeutic that can be administered as a single dose, stably colonize the gut, and provide long-lasting metabolic benefits.” Testing the engineered bacteria in obese and diabetic mice on a high-fat diet would be a next step to determine whether its potential indeed holds up. If proven successful, it could then be used to develop future targeted therapies and interventions to treat common metabolic disorders.
With this engineered bacteria, Zarrinpar and his team are hopeful that it alone can replicate the microbial benefits associated with following a TRF dietary schedule. “In our study, the engineered bacterium continuously expressed the enzyme DnBSH1, independently of dietary or environmental factors. As a result, the bacterium provided metabolic benefits similar to those seen with TRF, even without requiring the mice to strictly adhere to a TRF schedule,” said Zarrinpar.
“This suggests the exciting possibility that this engineered microbe might serve either as a replacement for TRF or as a way to enhance its beneficial effects,” he continued. “Further studies will help determine whether combining this ENB with TRF could provide additional or synergistic improvements in metabolic health.”
Looking Ahead
“As the pioneer of the single anastomosis duodenal switch which separates bile from food until halfway down the GI tract, I agree that bile is very important in controlling metabolism and glucose,” said Mitchell Roslin, MD, chief director of bariatric and metabolic surgery at Lenox Hill Hospital, and the Donald and Barbara Zucker School of Medicine, Hempstead, New York, who was not involved in the study. “Using enzymes or medications that work in the GI tract without absorption into the body is very interesting and has great potential. It is an early but exciting prospect.”
However, Roslin expressed some reservations. “I think we are still trying to understand whether the difference in microbiomes is the cause or effect/association. Is the microbiome the difference or is a different microbiome representative of a diet that has more fiber and less processed foods? Thus, while I find this academically fascinating, I think that there are very basic questions that need better answers, before we look at the transcription of bacteria.”
Furthermore, translating the metabolic results observed in mice to humans might not be as straightforward. “Small animal research is mandatory, but how the findings convert to humans is highly speculative,” said Roslin. “Mice that are studied are usually bred for medical research, with reduced genetic variation. Many animal models are more sensitive to time-restricted eating and caloric restriction than humans.”
While it requires further research and validation, this UC San Diego study nevertheless contributes to our overall understanding of host-microbe interactions. “We demonstrate that host circadian rhythms significantly influence microbial function, and conversely, these microbial functions can directly impact host metabolism,” said Zarrinpar. “Importantly, we now have a method to test how specific microbial activities affect host physiology by engineering native gut bacteria.”
Roslin similarly emphasized the importance of continued investment in exploring the microbial ecosystem inside us all. “There is wider evidence that bacteria and microbes are not just passengers using us for a ride but perhaps manipulating every action we take.”
A version of this article appeared on Medscape.com.
Similar to circadian rhythms that help regulate when we naturally fall asleep and wake up, microbial rhythms in our gut are naturally active at certain times of the day to help regulate our digestion.
Investigators from the University of California, San Diego sought out to track these microbial rhythms to determine whether aligning the times we eat to when our gut microbes are most active – time-restricted feeding (TRF) – can bolster our metabolic health. Their research was published recently in Cell Host & Microbe.
“Microbial rhythms are daily fluctuations in the composition and function of microbes living in our gut. Much like how our bodies follow an internal clock (circadian rhythm), gut microbes also have their own rhythms, adjusting their activities based on the time of day and when we eat,” said Amir Zarrinpar, MD, PhD, a gastroenterologist at UC San Diego School of Medicine, and senior author of the study.
Zarrinpar and his team were particularly interested in observing whether adopting the TRF approach counteracted the harmful metabolic effects often associated with consuming a high-fat diet.
The study is also notable for the team’s use of technology able to observe real-time microbial changes in the gut — something not previously attainable with existing metagenomics.
How the Study Evolved With New Tech
Researchers separated three groups of mice to analyze their microbiome activity: one on a high-fat diet with unrestricted access, another on the same high-fat diet within a TRF window of 8 hours per day, and a control group on a normal chow diet with unrestricted access.
“In mice, [their] microbial rhythms are well-aligned with their nocturnal lifestyle. For example, during their active (nighttime) period, certain beneficial microbial activities increase, helping digest food, absorb nutrients, and regulate metabolism,” said Zarrinpar. As a result, the team made sure the mice’s TRF window was at night or when they would normally be awake.
“We chose an 8-hour feeding window based on earlier research showing this time period allows mice to consume the same total calories as those with unlimited food access,” said Zarrinpar. “By controlling [the] calories in this way, we ensure any metabolic or microbial benefits we observe are specifically due to the timing of eating, rather than differences in total food intake.”
But before any observations could be made, the team first needed a way to see real-time changes in the animals’ gut microbiomes.
Zarrinpar and his team were able to uncover this, thanks to metatranscriptomics, a technique used to capture real-time microbial activity by profiling RNA transcripts. Compared with the more traditional technique of metagenomics, which could only be used to identify which genes were present, metatranscriptomics provided more in-depth temporal and activity-related context, allowing the team to observe dynamic microbial changes.
“[Metatranscriptomics] helps us understand not just which microbes are present, but specifically what they are doing at any given moment,” said Zarrinpar. “In contrast, metagenomics looks only at microbial DNA, which provides information about what microbes are potentially capable of doing, but doesn’t tell us if those genes are actively expressed. By comparing microbial gene expression (using metatranscriptomics) and microbial gene abundance (using metagenomics) across different diet and feeding conditions in [light and dark] phases, we aimed to identify how feeding timing might influence microbial activity.”
Because metagenomics focuses on stable genetic material, this technique cannot capture the real-time microbial responses to dietary timing presented in rapidly changing, short-lived RNA. At the same time, the instability of the RNA makes it difficult to test hypotheses experimentally and explains why researchers haven’t more widely relied on metatranscriptomics.
To overcome this difficulty, Zarrinpar and his team had to wait to take advantage of improved bioinformatics tools to simplify their analysis of complex datasets. “It took several years for us to analyze this dataset because robust computational tools for metatranscriptomic analysis were not widely available when we initially collected our samples. Additionally, sequencing costs were very high. To clearly identify microbial activity, we needed deep sequencing coverage to distinguish species-level differences in gene expression, especially for genes that are common across multiple types of microbes,” said Zarrinpar.
What They Found
After monitoring these groups of mice for 8 weeks, the results were revealed.
As predicted, “When mice have free access to a high-fat diet, their normal eating behavior changes significantly. Instead of limiting their activity and feeding to their active nighttime period, these mice begin to stay awake and eat during the day, which is their typical rest phase,” Zarrinpar explained.
“This unusual daytime activity interferes with important physiological processes. Consequently, the animals experience circadian misalignment, a condition similar to what human shift workers experience when their sleep-wake and eating cycles don’t match their internal biological clocks,” he continued. “This misalignment can negatively affect metabolism, immunity, and overall health, potentially leading to metabolic diseases.”
For the mice that consumed a high-fat diet within a TRF window, metabolic phenotyping demonstrated that their specific diet regimen had protected them from harmful high-fat induced effects including adiposity, inflammation, and insulin resistance.
Even more promising, the mice not only were protected from metabolic disruption but also experienced physiological improvements including glucose homeostasis and the partial restoration of the daily microbial rhythms absent in the mice with unrestricted access to a high-fat diet.
While the TRF approach did not fully restore the normal, healthy rhythmicity seen in the control mice, the researchers noted distinct shifts in microbial patterns that indicated time-dependent enrichment in genes attributed to lipid and carbohydrate metabolism.
Better Metabolic Health — and Better Tools for Researching It
Thankfully, the latest advancements in sequencing technology, including long-read sequencing methods, are making metatranscriptomics easier for research. “These newer platforms offer greater resolution at a lower cost, making metatranscriptomics increasingly accessible,” said Zarrinpar. With these emerging technologies, he believes metatranscriptomics will become a more standard, widely used method for researchers to better understand the influence of microbial activity on our health.
These tools, for example, enabled Zarrinpar and the team to delve deeper and focus on the transcription of a particular enzyme they identified as a pivotal influence in observable metabolic improvements: bile salt hydrolase (BSH), known to regulate lipid and glucose metabolism. The TRF approach notably enhanced the expression of the BSH gene during the daytime in the gut microbe Dubosiella newyorkensis, which has a functional human equivalent.
To determine why this happened, the team leveraged genetic engineering to insert several active BSH gene variants into a benign strain of gut bacteria to administer to the mice. The only variant to produce metabolic improvements was the one derived from Dubosiella newyorkensis; the mice who were given this BSH-expressing engineered native bacteria (ENB) had increased lean muscle mass, less body fat, lower insulin levels, enhanced insulin sensitivity, and better blood glucose regulation.
“It is still early to know the full clinical potential of this new BSH-expressing engineered native bacterium,” said Zarrinpar. “However, our long-term goal is to develop a therapeutic that can be administered as a single dose, stably colonize the gut, and provide long-lasting metabolic benefits.” Testing the engineered bacteria in obese and diabetic mice on a high-fat diet would be a next step to determine whether its potential indeed holds up. If proven successful, it could then be used to develop future targeted therapies and interventions to treat common metabolic disorders.
With this engineered bacteria, Zarrinpar and his team are hopeful that it alone can replicate the microbial benefits associated with following a TRF dietary schedule. “In our study, the engineered bacterium continuously expressed the enzyme DnBSH1, independently of dietary or environmental factors. As a result, the bacterium provided metabolic benefits similar to those seen with TRF, even without requiring the mice to strictly adhere to a TRF schedule,” said Zarrinpar.
“This suggests the exciting possibility that this engineered microbe might serve either as a replacement for TRF or as a way to enhance its beneficial effects,” he continued. “Further studies will help determine whether combining this ENB with TRF could provide additional or synergistic improvements in metabolic health.”
Looking Ahead
“As the pioneer of the single anastomosis duodenal switch which separates bile from food until halfway down the GI tract, I agree that bile is very important in controlling metabolism and glucose,” said Mitchell Roslin, MD, chief director of bariatric and metabolic surgery at Lenox Hill Hospital, and the Donald and Barbara Zucker School of Medicine, Hempstead, New York, who was not involved in the study. “Using enzymes or medications that work in the GI tract without absorption into the body is very interesting and has great potential. It is an early but exciting prospect.”
However, Roslin expressed some reservations. “I think we are still trying to understand whether the difference in microbiomes is the cause or effect/association. Is the microbiome the difference or is a different microbiome representative of a diet that has more fiber and less processed foods? Thus, while I find this academically fascinating, I think that there are very basic questions that need better answers, before we look at the transcription of bacteria.”
Furthermore, translating the metabolic results observed in mice to humans might not be as straightforward. “Small animal research is mandatory, but how the findings convert to humans is highly speculative,” said Roslin. “Mice that are studied are usually bred for medical research, with reduced genetic variation. Many animal models are more sensitive to time-restricted eating and caloric restriction than humans.”
While it requires further research and validation, this UC San Diego study nevertheless contributes to our overall understanding of host-microbe interactions. “We demonstrate that host circadian rhythms significantly influence microbial function, and conversely, these microbial functions can directly impact host metabolism,” said Zarrinpar. “Importantly, we now have a method to test how specific microbial activities affect host physiology by engineering native gut bacteria.”
Roslin similarly emphasized the importance of continued investment in exploring the microbial ecosystem inside us all. “There is wider evidence that bacteria and microbes are not just passengers using us for a ride but perhaps manipulating every action we take.”
A version of this article appeared on Medscape.com.
Novel Gene Risk Score Predicts Outcomes After RYGB Surgery
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Walnuts Cut Gut Permeability in Obesity
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
GLP-1s Treat and Even Reverse Some Forms of Liver Disease
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
Do GLP-1s Lower CRC Risk in Patients With Obesity and T2D?
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM DDW 2025