User login
Next-generation anti-BCMA CAR T shows promise for RRMM
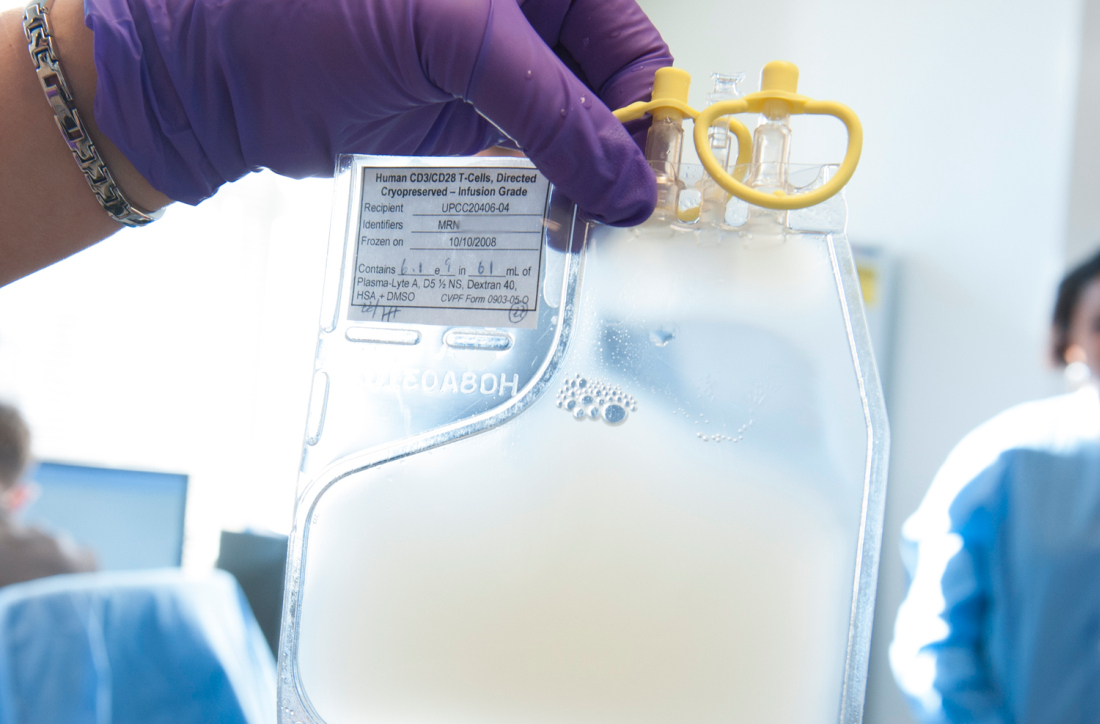
SAN DIEGO – The next-generation anti–B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy bb21217 shows encouraging efficacy for relapsed/refractory multiple myeloma, according to early findings from the phase 1 CRB-402 study.
At a median follow-up of 26 weeks, an objective response was seen in 10 of 12 patients (83%) treated with bb21217 at a dose of 150 x 106 CAR+ T cells, Nina Shah, MD, reported at the annual meeting of the American Society of Hematology.
Immunomodulatory CAR T-cell therapy directed against BCMA has shown promising results for the treatment of relapsed/refractory multiple myeloma (RRMM) in several phase 1 clinical studies in patients with advanced disease; bb21217 is based on the investigational therapy bb2121, said Dr. Shah, a hematologist-oncologist at the University of California, San Francisco.
“It uses the same CAR construct design as bb2121. However, it is cultured in the presence of a pan-[phosphoinositide] 3 kinase inhibitor known as bb007 to enrich for T cells displaying a memory-like phenotype,” she said. “CAR T cells enriched with this phenotype may persist and function longer than nonenriched CAR T cells, and the persistence of functional CAR T cells after infusion may be one determinant of the duration of response.”
Preclinical data have supported this approach and CRB-402 – a first-in-human dose-ranging study – was designed to assess the safety, pharmacokinetics, efficacy, and duration of effect of bb21217, Dr. Shah said.
She presented only the data for the 150 x 106 cell dose.
Study subjects were adults with RRMM who had received at least three prior treatment regimens, including a proteasome inhibitor and an immunomodulatory agent, and who had at least 50% expression of BCMA on the plasma cells in bone marrow samples at screening. They had a median age of 63 years, and 58% had high-risk cytogenetic features.
“Patients tended to be pretty heavily pretreated with a median number of lines of treatment of seven,” Dr. Shah said, noting that almost all patients had prior autologous stem cell transplantation, 58% had been exposed to all five available therapies for RRMM, and 17% were refractory to all five therapies.
The patients underwent collection of peripheral blood mononuclear cells via leukapheresis and underwent lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) daily for 3 days prior to receiving the single bb21217 infusion.
Grade 3 or higher adverse events occurring in more than one patient were predominantly cytopenias, which is to be expected in a clinical trial such as this, Dr. Shah said, adding that some hypophosphatemia also occurred.
In those with cytopenias, 58% recovered their absolute neutrophil count (ANC) to greater than 1,000 by day 32, and of the remaining five patients, four of them recovered by day 65.
“Therefore, 11 out of 12 had full ANC recovery by day 65,” she said.
Thrombocytopenia was seen in half of the patients, and in those six patients, two recovered platelet counts to more than 50,000 by day 32, and two more by day 65.
Overall, 10 out of 12 patients had platelet recovery to greater than 50,000 by day 65, she said.
Other adverse events of clinical interest included cytokine release syndrome (CRS) and neurotoxicity.
CRS was usually grade 1 or 2 and occurred in 8 of the 12 patients (67%). One grade 3 CRS event occurred.
“The median time to onset of the CRS was 4.5 days, and this was fairly manageable with or without tocilizumab,” she said.
Neurotoxicity occurred in 3 of 12 patients (25%), and a dose-limiting grade 4 encephalopathy and prolonged grade 3 CRS occurred in one patient with a high tumor burden and rapidly accelerating disease at baseline.
“Because of this, the dose level was expanded and we included patients equally who had high tumor burden and low tumor burden to further understand the contribution of this to this phenomenon. However, no other [dose-limiting toxicities] occurred,” she noted.
Additionally, one patient experienced a grade 3 catheter-related infection, but no other severe infections have been reported, Dr. Shah said, adding that four patients experienced one or more serious adverse events, but no deaths have occurred to date.
Of the 10 patients who achieved an objective response to bb21217, 3 had a complete response (CR) or stringent CR, and 6 patients achieved at least a very good partial response or better.
Some responses deepened over time, therefore some CRs were achieved as late as month 10. Responses are ongoing in all but one responding patient, and the first patient who was dosed continues to respond more than 1 year after treatment.
Of those with good minimal residual disease (MRD) samples available, four were responders, and all four were MRD negative. In contrast, both nonresponders who had tissue available for MRD analysis were MRD positive.
Correlative data show that bone marrow plasma cell clearance was observed early, by day 15, in these representative samples, Dr. Shah said.
“There was a dramatic decline in serum free light chain and serum BCMA ... in all responders by month 1. However, the M protein decline did have some delay, which we would expect based on the half-life, and this resulted in an evolving International Myeloma Working Group response over time,” she said.
Sustained serum BCMA suppression was observed up to month 9, which is likely consistent with ongoing plasma cell aplasia resulting from functional CAR T cell persistence, she explained.
An in vivo examination of the phenotype of the infused CAR T cells showed that while the numbers are small, “so far there seems to be an enrichment for memorylike T cells within the CAR T cell population in the blood post infusion – at least by looking at CD62-ligand T cells.”
There also was a robust and consistent CAR T cell expansion post infusion, which was independent of tumor burden.
“CAR T cells have been detectable up to 9 months post infusion,” she said.
However, the follow-up so far has been very short, she noted. “This action may be underrepresenting the true persistence of these T cells,” she added, explaining that there is only one patient at month 9, whereas all three at month 6 were positive for vector copy.
CRB-402 is ongoing with plans to enroll up to 50 patients. A 300 x 106 dosing cohort has been opened, and doses of 450, 800, and 1200 x 106 CAR+ T cells are planned.
“But longer-term follow-up in a larger patient population will further clarify the depth and durability of the bb21217 tumor response and dose response,” she said.
This study is sponsored by Bluebird Bio. Dr. Shah reported research funding from Bluebird Bio and equity ownership in Indapta Therapeutics, as well as research funding and consulting relationships with other companies.
SOURCE: Shah N et al. ASH 2018, Abstract 488.
SAN DIEGO – The next-generation anti–B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy bb21217 shows encouraging efficacy for relapsed/refractory multiple myeloma, according to early findings from the phase 1 CRB-402 study.
At a median follow-up of 26 weeks, an objective response was seen in 10 of 12 patients (83%) treated with bb21217 at a dose of 150 x 106 CAR+ T cells, Nina Shah, MD, reported at the annual meeting of the American Society of Hematology.
Immunomodulatory CAR T-cell therapy directed against BCMA has shown promising results for the treatment of relapsed/refractory multiple myeloma (RRMM) in several phase 1 clinical studies in patients with advanced disease; bb21217 is based on the investigational therapy bb2121, said Dr. Shah, a hematologist-oncologist at the University of California, San Francisco.
“It uses the same CAR construct design as bb2121. However, it is cultured in the presence of a pan-[phosphoinositide] 3 kinase inhibitor known as bb007 to enrich for T cells displaying a memory-like phenotype,” she said. “CAR T cells enriched with this phenotype may persist and function longer than nonenriched CAR T cells, and the persistence of functional CAR T cells after infusion may be one determinant of the duration of response.”
Preclinical data have supported this approach and CRB-402 – a first-in-human dose-ranging study – was designed to assess the safety, pharmacokinetics, efficacy, and duration of effect of bb21217, Dr. Shah said.
She presented only the data for the 150 x 106 cell dose.
Study subjects were adults with RRMM who had received at least three prior treatment regimens, including a proteasome inhibitor and an immunomodulatory agent, and who had at least 50% expression of BCMA on the plasma cells in bone marrow samples at screening. They had a median age of 63 years, and 58% had high-risk cytogenetic features.
“Patients tended to be pretty heavily pretreated with a median number of lines of treatment of seven,” Dr. Shah said, noting that almost all patients had prior autologous stem cell transplantation, 58% had been exposed to all five available therapies for RRMM, and 17% were refractory to all five therapies.
The patients underwent collection of peripheral blood mononuclear cells via leukapheresis and underwent lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) daily for 3 days prior to receiving the single bb21217 infusion.
Grade 3 or higher adverse events occurring in more than one patient were predominantly cytopenias, which is to be expected in a clinical trial such as this, Dr. Shah said, adding that some hypophosphatemia also occurred.
In those with cytopenias, 58% recovered their absolute neutrophil count (ANC) to greater than 1,000 by day 32, and of the remaining five patients, four of them recovered by day 65.
“Therefore, 11 out of 12 had full ANC recovery by day 65,” she said.
Thrombocytopenia was seen in half of the patients, and in those six patients, two recovered platelet counts to more than 50,000 by day 32, and two more by day 65.
Overall, 10 out of 12 patients had platelet recovery to greater than 50,000 by day 65, she said.
Other adverse events of clinical interest included cytokine release syndrome (CRS) and neurotoxicity.
CRS was usually grade 1 or 2 and occurred in 8 of the 12 patients (67%). One grade 3 CRS event occurred.
“The median time to onset of the CRS was 4.5 days, and this was fairly manageable with or without tocilizumab,” she said.
Neurotoxicity occurred in 3 of 12 patients (25%), and a dose-limiting grade 4 encephalopathy and prolonged grade 3 CRS occurred in one patient with a high tumor burden and rapidly accelerating disease at baseline.
“Because of this, the dose level was expanded and we included patients equally who had high tumor burden and low tumor burden to further understand the contribution of this to this phenomenon. However, no other [dose-limiting toxicities] occurred,” she noted.
Additionally, one patient experienced a grade 3 catheter-related infection, but no other severe infections have been reported, Dr. Shah said, adding that four patients experienced one or more serious adverse events, but no deaths have occurred to date.
Of the 10 patients who achieved an objective response to bb21217, 3 had a complete response (CR) or stringent CR, and 6 patients achieved at least a very good partial response or better.
Some responses deepened over time, therefore some CRs were achieved as late as month 10. Responses are ongoing in all but one responding patient, and the first patient who was dosed continues to respond more than 1 year after treatment.
Of those with good minimal residual disease (MRD) samples available, four were responders, and all four were MRD negative. In contrast, both nonresponders who had tissue available for MRD analysis were MRD positive.
Correlative data show that bone marrow plasma cell clearance was observed early, by day 15, in these representative samples, Dr. Shah said.
“There was a dramatic decline in serum free light chain and serum BCMA ... in all responders by month 1. However, the M protein decline did have some delay, which we would expect based on the half-life, and this resulted in an evolving International Myeloma Working Group response over time,” she said.
Sustained serum BCMA suppression was observed up to month 9, which is likely consistent with ongoing plasma cell aplasia resulting from functional CAR T cell persistence, she explained.
An in vivo examination of the phenotype of the infused CAR T cells showed that while the numbers are small, “so far there seems to be an enrichment for memorylike T cells within the CAR T cell population in the blood post infusion – at least by looking at CD62-ligand T cells.”
There also was a robust and consistent CAR T cell expansion post infusion, which was independent of tumor burden.
“CAR T cells have been detectable up to 9 months post infusion,” she said.
However, the follow-up so far has been very short, she noted. “This action may be underrepresenting the true persistence of these T cells,” she added, explaining that there is only one patient at month 9, whereas all three at month 6 were positive for vector copy.
CRB-402 is ongoing with plans to enroll up to 50 patients. A 300 x 106 dosing cohort has been opened, and doses of 450, 800, and 1200 x 106 CAR+ T cells are planned.
“But longer-term follow-up in a larger patient population will further clarify the depth and durability of the bb21217 tumor response and dose response,” she said.
This study is sponsored by Bluebird Bio. Dr. Shah reported research funding from Bluebird Bio and equity ownership in Indapta Therapeutics, as well as research funding and consulting relationships with other companies.
SOURCE: Shah N et al. ASH 2018, Abstract 488.
SAN DIEGO – The next-generation anti–B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy bb21217 shows encouraging efficacy for relapsed/refractory multiple myeloma, according to early findings from the phase 1 CRB-402 study.
At a median follow-up of 26 weeks, an objective response was seen in 10 of 12 patients (83%) treated with bb21217 at a dose of 150 x 106 CAR+ T cells, Nina Shah, MD, reported at the annual meeting of the American Society of Hematology.
Immunomodulatory CAR T-cell therapy directed against BCMA has shown promising results for the treatment of relapsed/refractory multiple myeloma (RRMM) in several phase 1 clinical studies in patients with advanced disease; bb21217 is based on the investigational therapy bb2121, said Dr. Shah, a hematologist-oncologist at the University of California, San Francisco.
“It uses the same CAR construct design as bb2121. However, it is cultured in the presence of a pan-[phosphoinositide] 3 kinase inhibitor known as bb007 to enrich for T cells displaying a memory-like phenotype,” she said. “CAR T cells enriched with this phenotype may persist and function longer than nonenriched CAR T cells, and the persistence of functional CAR T cells after infusion may be one determinant of the duration of response.”
Preclinical data have supported this approach and CRB-402 – a first-in-human dose-ranging study – was designed to assess the safety, pharmacokinetics, efficacy, and duration of effect of bb21217, Dr. Shah said.
She presented only the data for the 150 x 106 cell dose.
Study subjects were adults with RRMM who had received at least three prior treatment regimens, including a proteasome inhibitor and an immunomodulatory agent, and who had at least 50% expression of BCMA on the plasma cells in bone marrow samples at screening. They had a median age of 63 years, and 58% had high-risk cytogenetic features.
“Patients tended to be pretty heavily pretreated with a median number of lines of treatment of seven,” Dr. Shah said, noting that almost all patients had prior autologous stem cell transplantation, 58% had been exposed to all five available therapies for RRMM, and 17% were refractory to all five therapies.
The patients underwent collection of peripheral blood mononuclear cells via leukapheresis and underwent lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) daily for 3 days prior to receiving the single bb21217 infusion.
Grade 3 or higher adverse events occurring in more than one patient were predominantly cytopenias, which is to be expected in a clinical trial such as this, Dr. Shah said, adding that some hypophosphatemia also occurred.
In those with cytopenias, 58% recovered their absolute neutrophil count (ANC) to greater than 1,000 by day 32, and of the remaining five patients, four of them recovered by day 65.
“Therefore, 11 out of 12 had full ANC recovery by day 65,” she said.
Thrombocytopenia was seen in half of the patients, and in those six patients, two recovered platelet counts to more than 50,000 by day 32, and two more by day 65.
Overall, 10 out of 12 patients had platelet recovery to greater than 50,000 by day 65, she said.
Other adverse events of clinical interest included cytokine release syndrome (CRS) and neurotoxicity.
CRS was usually grade 1 or 2 and occurred in 8 of the 12 patients (67%). One grade 3 CRS event occurred.
“The median time to onset of the CRS was 4.5 days, and this was fairly manageable with or without tocilizumab,” she said.
Neurotoxicity occurred in 3 of 12 patients (25%), and a dose-limiting grade 4 encephalopathy and prolonged grade 3 CRS occurred in one patient with a high tumor burden and rapidly accelerating disease at baseline.
“Because of this, the dose level was expanded and we included patients equally who had high tumor burden and low tumor burden to further understand the contribution of this to this phenomenon. However, no other [dose-limiting toxicities] occurred,” she noted.
Additionally, one patient experienced a grade 3 catheter-related infection, but no other severe infections have been reported, Dr. Shah said, adding that four patients experienced one or more serious adverse events, but no deaths have occurred to date.
Of the 10 patients who achieved an objective response to bb21217, 3 had a complete response (CR) or stringent CR, and 6 patients achieved at least a very good partial response or better.
Some responses deepened over time, therefore some CRs were achieved as late as month 10. Responses are ongoing in all but one responding patient, and the first patient who was dosed continues to respond more than 1 year after treatment.
Of those with good minimal residual disease (MRD) samples available, four were responders, and all four were MRD negative. In contrast, both nonresponders who had tissue available for MRD analysis were MRD positive.
Correlative data show that bone marrow plasma cell clearance was observed early, by day 15, in these representative samples, Dr. Shah said.
“There was a dramatic decline in serum free light chain and serum BCMA ... in all responders by month 1. However, the M protein decline did have some delay, which we would expect based on the half-life, and this resulted in an evolving International Myeloma Working Group response over time,” she said.
Sustained serum BCMA suppression was observed up to month 9, which is likely consistent with ongoing plasma cell aplasia resulting from functional CAR T cell persistence, she explained.
An in vivo examination of the phenotype of the infused CAR T cells showed that while the numbers are small, “so far there seems to be an enrichment for memorylike T cells within the CAR T cell population in the blood post infusion – at least by looking at CD62-ligand T cells.”
There also was a robust and consistent CAR T cell expansion post infusion, which was independent of tumor burden.
“CAR T cells have been detectable up to 9 months post infusion,” she said.
However, the follow-up so far has been very short, she noted. “This action may be underrepresenting the true persistence of these T cells,” she added, explaining that there is only one patient at month 9, whereas all three at month 6 were positive for vector copy.
CRB-402 is ongoing with plans to enroll up to 50 patients. A 300 x 106 dosing cohort has been opened, and doses of 450, 800, and 1200 x 106 CAR+ T cells are planned.
“But longer-term follow-up in a larger patient population will further clarify the depth and durability of the bb21217 tumor response and dose response,” she said.
This study is sponsored by Bluebird Bio. Dr. Shah reported research funding from Bluebird Bio and equity ownership in Indapta Therapeutics, as well as research funding and consulting relationships with other companies.
SOURCE: Shah N et al. ASH 2018, Abstract 488.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Objective response rate was 83% in the first 12 treated patients.
Study details: The CRB-402 phase 1 dose-escalation of 12 patients (up to 50 planned).
Disclosures: This study is sponsored by Bluebird Bio. Dr. Shah reported research funding from Bluebird Bio and equity ownership in Indapta Therapeutics, as well as research funding and consulting relationships with other companies.
Source: Shah N et al. ASH 2018, Abstract 488.
Group proposes new grading systems for CRS, neurotoxicity
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
EC approves split dosing regimen for daratumumab
The European Commission (EC) has granted marketing authorization for a split dosing regimen for daratumumab (Darzalex®).
The approval provides healthcare professionals with the option to split the first infusion of daratumumab over 2 consecutive days.
“We are hopeful that the availability of this more flexible dosing option will make the first infusion of Darzalex more convenient for European multiple myeloma patients,” said Jan van de Winkel, PhD, chief executive officer of Genmab, which licensed daratumumab to Janssen Biotech, Inc.
Daratumumab is currently EC-approved for the following indications:
- For use in combination with bortezomib, melphalan, and prednisone to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant
- For use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adults with MM who have received at least one prior therapy
- As monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s approval of a split dosing regimen for daratumumab was based on data from the phase 1b EQUULEUS trial (MMY1001, NCT01998971), which was sponsored by Janssen.
This trial was designed to evaluate daratumumab in combination with bortezomib-dexamethasone, bortezomib-melphalan-prednisone, bortezomib-thalidomide-dexamethasone, pomalidomide-dexamethasone, carfilzomib-dexamethasone, and carfilzomib-lenalidomide-dexamethasone.
At the 2018 ASH Annual Meeting (abstract 1970), researchers presented data from this trial in MM patients who received their first 16 mg/kg daratumumab dose as a split dose of 8 mg/kg on day 1 of cycle 1 and 8 mg/kg on day 2 of cycle 1, compared to patients who received the full 16 mg/kg dose on day 1 of cycle 1.
The researchers said they observed “virtually identical” pharmacokinetics between the dosing groups.
Cmax on the first day of cycle 1 was lower in the split-dose group than in the full-dose group. However, after patients in the split-dose group received the second 8 mg/kg dose on day 2, concentrations were similar between the groups.
The researchers said they do not expect the initial difference they observed to have any impact on clinical outcomes.
The team also pointed out that there was no increase in infusion-related reactions among patients who received the split dose.
The researchers said split dosing of daratumumab is still being investigated in ongoing studies of MM patients, including CANDOR (NCT03158688) and LYRA (NCT02951819).
The European Commission (EC) has granted marketing authorization for a split dosing regimen for daratumumab (Darzalex®).
The approval provides healthcare professionals with the option to split the first infusion of daratumumab over 2 consecutive days.
“We are hopeful that the availability of this more flexible dosing option will make the first infusion of Darzalex more convenient for European multiple myeloma patients,” said Jan van de Winkel, PhD, chief executive officer of Genmab, which licensed daratumumab to Janssen Biotech, Inc.
Daratumumab is currently EC-approved for the following indications:
- For use in combination with bortezomib, melphalan, and prednisone to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant
- For use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adults with MM who have received at least one prior therapy
- As monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s approval of a split dosing regimen for daratumumab was based on data from the phase 1b EQUULEUS trial (MMY1001, NCT01998971), which was sponsored by Janssen.
This trial was designed to evaluate daratumumab in combination with bortezomib-dexamethasone, bortezomib-melphalan-prednisone, bortezomib-thalidomide-dexamethasone, pomalidomide-dexamethasone, carfilzomib-dexamethasone, and carfilzomib-lenalidomide-dexamethasone.
At the 2018 ASH Annual Meeting (abstract 1970), researchers presented data from this trial in MM patients who received their first 16 mg/kg daratumumab dose as a split dose of 8 mg/kg on day 1 of cycle 1 and 8 mg/kg on day 2 of cycle 1, compared to patients who received the full 16 mg/kg dose on day 1 of cycle 1.
The researchers said they observed “virtually identical” pharmacokinetics between the dosing groups.
Cmax on the first day of cycle 1 was lower in the split-dose group than in the full-dose group. However, after patients in the split-dose group received the second 8 mg/kg dose on day 2, concentrations were similar between the groups.
The researchers said they do not expect the initial difference they observed to have any impact on clinical outcomes.
The team also pointed out that there was no increase in infusion-related reactions among patients who received the split dose.
The researchers said split dosing of daratumumab is still being investigated in ongoing studies of MM patients, including CANDOR (NCT03158688) and LYRA (NCT02951819).
The European Commission (EC) has granted marketing authorization for a split dosing regimen for daratumumab (Darzalex®).
The approval provides healthcare professionals with the option to split the first infusion of daratumumab over 2 consecutive days.
“We are hopeful that the availability of this more flexible dosing option will make the first infusion of Darzalex more convenient for European multiple myeloma patients,” said Jan van de Winkel, PhD, chief executive officer of Genmab, which licensed daratumumab to Janssen Biotech, Inc.
Daratumumab is currently EC-approved for the following indications:
- For use in combination with bortezomib, melphalan, and prednisone to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant
- For use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adults with MM who have received at least one prior therapy
- As monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s approval of a split dosing regimen for daratumumab was based on data from the phase 1b EQUULEUS trial (MMY1001, NCT01998971), which was sponsored by Janssen.
This trial was designed to evaluate daratumumab in combination with bortezomib-dexamethasone, bortezomib-melphalan-prednisone, bortezomib-thalidomide-dexamethasone, pomalidomide-dexamethasone, carfilzomib-dexamethasone, and carfilzomib-lenalidomide-dexamethasone.
At the 2018 ASH Annual Meeting (abstract 1970), researchers presented data from this trial in MM patients who received their first 16 mg/kg daratumumab dose as a split dose of 8 mg/kg on day 1 of cycle 1 and 8 mg/kg on day 2 of cycle 1, compared to patients who received the full 16 mg/kg dose on day 1 of cycle 1.
The researchers said they observed “virtually identical” pharmacokinetics between the dosing groups.
Cmax on the first day of cycle 1 was lower in the split-dose group than in the full-dose group. However, after patients in the split-dose group received the second 8 mg/kg dose on day 2, concentrations were similar between the groups.
The researchers said they do not expect the initial difference they observed to have any impact on clinical outcomes.
The team also pointed out that there was no increase in infusion-related reactions among patients who received the split dose.
The researchers said split dosing of daratumumab is still being investigated in ongoing studies of MM patients, including CANDOR (NCT03158688) and LYRA (NCT02951819).
DRd improves PFS in transplant-ineligible MM
SAN DIEGO—An interim analysis from the MAIA trial showed that adding daratumumab to lenalidomide and dexamethasone could significantly improve progression-free survival (PFS) in older patients with multiple myeloma (MM) who were ineligible for transplant.
The 30-month PFS rate was 71% in patients who received daratumumab plus lenalidomide and dexamethasone (DRd) and 56% in patients who received only lenalidomide and dexamethasone (Rd).
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” said Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille in France.
Dr. Facon presented results from MAIA during the late-breaking abstracts session at the 2018 ASH Annual Meeting (abstract LBA-2).
The phase 3 trial (NCT02252172) enrolled 737 transplant-ineligible patients with newly diagnosed MM.
The patients were randomly assigned to DRd or Rd. Daratumumab was given at 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression.
Lenalidomide was given at 25 mg orally per day on days 1-21 until disease progression, and dexamethasone was given at 40 mg orally or intravenously weekly until disease progression.
The median patient age was 73 years, and 99% of all patients were 65 or older. Demographic and clinical characteristics were well balanced between the treatment arms.
Results
The primary endpoint of PFS was superior with DRd.
At a median follow-up of 28 months, the median PFS had not been reached in the DRd arm and was 31.9 months in the Rd arm.
The 30-month PFS rate was 71% in the DRd arm and 56% in the Rd arm (hazard ratio [HR]=0.56; P<0.0001).
DRd was associated with a significantly higher overall response rate than Rd—93% and 81%, respectively (P<0.0001).
The complete response rates were 48% and 25%, respectively (P<0.0001). The rates of very good partial response or better were 79% and 53%, respectively (P<0.0001). And the rates of minimal residual disease negativity were 24% and 7%, respectively (P<0.0001).
DRd was associated with infusion-related reactions in 41% of patients, and 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events of grade 3 or higher that were more common with DRd than Rd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%).
Conversely, grade 3/4 thrombocytopenia (7% vs. 9%) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic treatment-emergent adverse events that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia.
Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the treatment arms.
Janssen funded this study. Dr. Facon reported relationships with Celgene, Janssen, Takeda, Sanofi, Amgen, Karyopharm, and Oncopeptides.
SAN DIEGO—An interim analysis from the MAIA trial showed that adding daratumumab to lenalidomide and dexamethasone could significantly improve progression-free survival (PFS) in older patients with multiple myeloma (MM) who were ineligible for transplant.
The 30-month PFS rate was 71% in patients who received daratumumab plus lenalidomide and dexamethasone (DRd) and 56% in patients who received only lenalidomide and dexamethasone (Rd).
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” said Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille in France.
Dr. Facon presented results from MAIA during the late-breaking abstracts session at the 2018 ASH Annual Meeting (abstract LBA-2).
The phase 3 trial (NCT02252172) enrolled 737 transplant-ineligible patients with newly diagnosed MM.
The patients were randomly assigned to DRd or Rd. Daratumumab was given at 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression.
Lenalidomide was given at 25 mg orally per day on days 1-21 until disease progression, and dexamethasone was given at 40 mg orally or intravenously weekly until disease progression.
The median patient age was 73 years, and 99% of all patients were 65 or older. Demographic and clinical characteristics were well balanced between the treatment arms.
Results
The primary endpoint of PFS was superior with DRd.
At a median follow-up of 28 months, the median PFS had not been reached in the DRd arm and was 31.9 months in the Rd arm.
The 30-month PFS rate was 71% in the DRd arm and 56% in the Rd arm (hazard ratio [HR]=0.56; P<0.0001).
DRd was associated with a significantly higher overall response rate than Rd—93% and 81%, respectively (P<0.0001).
The complete response rates were 48% and 25%, respectively (P<0.0001). The rates of very good partial response or better were 79% and 53%, respectively (P<0.0001). And the rates of minimal residual disease negativity were 24% and 7%, respectively (P<0.0001).
DRd was associated with infusion-related reactions in 41% of patients, and 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events of grade 3 or higher that were more common with DRd than Rd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%).
Conversely, grade 3/4 thrombocytopenia (7% vs. 9%) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic treatment-emergent adverse events that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia.
Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the treatment arms.
Janssen funded this study. Dr. Facon reported relationships with Celgene, Janssen, Takeda, Sanofi, Amgen, Karyopharm, and Oncopeptides.
SAN DIEGO—An interim analysis from the MAIA trial showed that adding daratumumab to lenalidomide and dexamethasone could significantly improve progression-free survival (PFS) in older patients with multiple myeloma (MM) who were ineligible for transplant.
The 30-month PFS rate was 71% in patients who received daratumumab plus lenalidomide and dexamethasone (DRd) and 56% in patients who received only lenalidomide and dexamethasone (Rd).
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” said Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille in France.
Dr. Facon presented results from MAIA during the late-breaking abstracts session at the 2018 ASH Annual Meeting (abstract LBA-2).
The phase 3 trial (NCT02252172) enrolled 737 transplant-ineligible patients with newly diagnosed MM.
The patients were randomly assigned to DRd or Rd. Daratumumab was given at 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression.
Lenalidomide was given at 25 mg orally per day on days 1-21 until disease progression, and dexamethasone was given at 40 mg orally or intravenously weekly until disease progression.
The median patient age was 73 years, and 99% of all patients were 65 or older. Demographic and clinical characteristics were well balanced between the treatment arms.
Results
The primary endpoint of PFS was superior with DRd.
At a median follow-up of 28 months, the median PFS had not been reached in the DRd arm and was 31.9 months in the Rd arm.
The 30-month PFS rate was 71% in the DRd arm and 56% in the Rd arm (hazard ratio [HR]=0.56; P<0.0001).
DRd was associated with a significantly higher overall response rate than Rd—93% and 81%, respectively (P<0.0001).
The complete response rates were 48% and 25%, respectively (P<0.0001). The rates of very good partial response or better were 79% and 53%, respectively (P<0.0001). And the rates of minimal residual disease negativity were 24% and 7%, respectively (P<0.0001).
DRd was associated with infusion-related reactions in 41% of patients, and 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events of grade 3 or higher that were more common with DRd than Rd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%).
Conversely, grade 3/4 thrombocytopenia (7% vs. 9%) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic treatment-emergent adverse events that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia.
Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the treatment arms.
Janssen funded this study. Dr. Facon reported relationships with Celgene, Janssen, Takeda, Sanofi, Amgen, Karyopharm, and Oncopeptides.
Frailty-adjusted treatment strategy emerges in myeloma
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Median event-free survival was 9.3 months for the Rd induction followed by lenalidomide maintenance, compared with 6.6 months for continuous Rd (P = .044).
Study details: Results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed multiple myeloma patients of intermediate fitness.
Disclosures: Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
Source: Larocca A et al. ASH 2018, Abstract 305.
Health Canada approves new indication for daratumumab
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
10 Important VA Studies You Might Have Missed at ASH
With hundreds of sessions and thousands of abstracts, it can be difficult to wade through all the new findings to find the most significant and relevant findings. Federal Practitioner consulted with Association of VA Hematology/Oncology members who attended the meeting, VA researchers, and other sources to provide these nuggets you might have missed on lymphomas, white blood cells, leukemias, and multiple myeloma:
Lymphomas
This retrospective analysis of diffuse large B cell lymphoma (DCBL) patients who received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) at the VA Audie Murphy Hospital in San Antonio, Texas was compared with patients with DLBCL who received R-CHOP in a community setting. According to the researchers, the response to initial treatment was inferior in the veteran population when compared with a patient population with similar demographics and having similar time from diagnosis to treatment. Veteran patients also had worse outcomes when compared with uninsured patients.
This retrospective analysis study identified 2,290 patients with follicular lymphoma treated in the Veterans Health Administration between 2006–2014 and detailed their staging, demographics, and comorbidities. The researchers found that maintenance therapy with rituximab was associated with an improvement in overall survival.
Another retrospective analysis of DBCL using VHA data examined the effectiveness of second-line chemotherapy and chemoimmunotherapy in patients aged ≥ 65 years. The researchers found 230 patients from 2001 to 2015 that met the inclusion criteria. According to the researchers, the overall survival was < 1 year and about half of the patients "did not receive or were not candidates for regimens typically used with intent for high-dose therapy and autologous transplant."
White Blood Cells
Cost-Effective Use of White Blood Cell Growth Factors in the Veterans Administration
This study analyzed the use of granulocyte colony-stimulating (G-CSF) vs pegfilgrastim in the UD Department of Veterans Affairs (VA) health care system. The researchers looked at the relative frequency of use of filgrastim, Tbo-filgrastim, Filgrastim-sndz and pegfilgrastim at 23 VA sites and found that uptake of biosimilar G-CSF has been extremely rapid. All sites are using biosimilar GCSF for all new patients; 6 of 23 sites were comfortable shifting current patients on branded G-CSF to the biosimilar.However, switching to a Tbo-filgrastim brought a cost savings of 2.2% that was "small compared to other clinical changes."
This analysis described the association between white blood cell (EBC) levels and occurrence of thrombotic events among patients with polycythemia vera (PV) using Veterans Health Administration claims data collected between 2005 and 2012. The researchers found A significant, positive association between increased WBC counts and occurrence of thrombotic events in patients with PV was observed in this study. Patients with WBC counts ≥ 8.5 × 109/L had a significantly increased risk of thrombotic events, and those with counts ≥ 11.0 × 109/L were at greatest risk. Effective control of WBC counts is an important component of disease management and may reduce risk of thrombotic events in patients with PV.
Leukemias
Black patients with chronic lymphocytic leukemia tend to have worse overall survival and an earlier age at diagnosis with higher rates of adverse cytogenetics. This retrospective analysis of VHA patients compared black and nonblack patients. It found that black patients had worse survival compared to nonblack patients in a single health care delivery system, which limits differences in access to care. Black patients were younger and had shorter periods of observation and were more frequently given first-line fludarabine.
Induction chemotherapy (7+3) or high-dose ara-C-based (HIDAC) for acute myeloid leukemia (AML) results in prolonged neutropenia with a high risk of serious infection and attendant morbidity, and prolonged hospitalization. Researchers, including former AVAHO president Suman Kambhampati developed a randomized, open-label, controlled Phase 2 trial to study the effect of romyelocel-L in de novo patients receiving HIDAC or 7+3 induction therapy for reduction of fever and infection. The results from pooled and 7+3 cohorts, were previously presented, showing decrease in infections and days in hospital. The results from cohorts receiving HIDAC chemotherapy are presented here. The incidence of infections was decreased during the day 15-28 period and a decrease of three days in hospital stay was observed in de novo HIDAC AML subjects receiving romyelocel-L. Romyelocel-L may provide a new option to reduce infections in AML patients undergoing HIDAC induction therapy.
Multiple Myeloma
Two studies focused on racial disparities in multiple myeloma (MM), while another reported phase 2 data on a relapsed/refractory option.
This group of researchers found an absence of disparity in use of novel agents, no racial disparity was observed in overall survival between black and white patients with MM. Among patients aged < 65 years at diagnosis, the researchers observed a significantly lower age-adjusted risk of death for black patients compared with white patients. The difference in the younger population was not explained by access or utilization of resources. This analysis suggests that when healthcare access is neutralized, younger black patients may even have improved overall survival, which may indicate the possibility of genetic differences that may drive the disease biology and therapeutic outcome in AA patients.
Outcomes of Black Patients with Multiple Myeloma in the Veterans Health Administration
The second study found survival of black patients with MM was improved compared to non-blacks in the VHA, a national comprehensive care delivery system. Black patients also received similar therapies compared to non-blacks, while presenting at a younger age with more comorbidities. These results are strengthened after adjusting for treatments and patient characteristics not available in other large data studies. Despite increased incidence of MM in the black population, outcomes are improved, similar to other large studies of patients in the United States.
Multiple myeloma clinical trial CC-4047-MM-014 (NCT01946477) is a phase 2 study designed to test the safety and efficacy of pomalidomide and low-dose dexamethasone alone (arm A) or in combination with daratumumab, an anti-CD38 antibody, (arm B) subjects with relapsed or refractory MM who have received a first- or second-line treatment of lenalidomide-based therapy. In this trial, researchers (including those from VA facilities, Celgene, and multiple other locations) sought to characterize on-treatment pharmacodynamic changes of immune biomarkers associated with POM + LoDEX + DARA administration (arm B) using multicolor flow cytometry panels designed to characterize T-cell subsets and CD38+ expressing cells. The researchers reported that the triplet regimen POM + LoDEX + DARA has shown notable clinical activity with deep and durable responses in relapsed MM patients progressed and are or refractory to lenalidomide. According to the researchers the results demonstrate that patients treated with the POM + LoDEX + DARA combination do not demonstrate impairment in the innate and adaptive immune compartments and, in contrast, show significant proliferative activity in the subsets of CD4, CD8 and NK cells following treatment.
With hundreds of sessions and thousands of abstracts, it can be difficult to wade through all the new findings to find the most significant and relevant findings. Federal Practitioner consulted with Association of VA Hematology/Oncology members who attended the meeting, VA researchers, and other sources to provide these nuggets you might have missed on lymphomas, white blood cells, leukemias, and multiple myeloma:
Lymphomas
This retrospective analysis of diffuse large B cell lymphoma (DCBL) patients who received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) at the VA Audie Murphy Hospital in San Antonio, Texas was compared with patients with DLBCL who received R-CHOP in a community setting. According to the researchers, the response to initial treatment was inferior in the veteran population when compared with a patient population with similar demographics and having similar time from diagnosis to treatment. Veteran patients also had worse outcomes when compared with uninsured patients.
This retrospective analysis study identified 2,290 patients with follicular lymphoma treated in the Veterans Health Administration between 2006–2014 and detailed their staging, demographics, and comorbidities. The researchers found that maintenance therapy with rituximab was associated with an improvement in overall survival.
Another retrospective analysis of DBCL using VHA data examined the effectiveness of second-line chemotherapy and chemoimmunotherapy in patients aged ≥ 65 years. The researchers found 230 patients from 2001 to 2015 that met the inclusion criteria. According to the researchers, the overall survival was < 1 year and about half of the patients "did not receive or were not candidates for regimens typically used with intent for high-dose therapy and autologous transplant."
White Blood Cells
Cost-Effective Use of White Blood Cell Growth Factors in the Veterans Administration
This study analyzed the use of granulocyte colony-stimulating (G-CSF) vs pegfilgrastim in the UD Department of Veterans Affairs (VA) health care system. The researchers looked at the relative frequency of use of filgrastim, Tbo-filgrastim, Filgrastim-sndz and pegfilgrastim at 23 VA sites and found that uptake of biosimilar G-CSF has been extremely rapid. All sites are using biosimilar GCSF for all new patients; 6 of 23 sites were comfortable shifting current patients on branded G-CSF to the biosimilar.However, switching to a Tbo-filgrastim brought a cost savings of 2.2% that was "small compared to other clinical changes."
This analysis described the association between white blood cell (EBC) levels and occurrence of thrombotic events among patients with polycythemia vera (PV) using Veterans Health Administration claims data collected between 2005 and 2012. The researchers found A significant, positive association between increased WBC counts and occurrence of thrombotic events in patients with PV was observed in this study. Patients with WBC counts ≥ 8.5 × 109/L had a significantly increased risk of thrombotic events, and those with counts ≥ 11.0 × 109/L were at greatest risk. Effective control of WBC counts is an important component of disease management and may reduce risk of thrombotic events in patients with PV.
Leukemias
Black patients with chronic lymphocytic leukemia tend to have worse overall survival and an earlier age at diagnosis with higher rates of adverse cytogenetics. This retrospective analysis of VHA patients compared black and nonblack patients. It found that black patients had worse survival compared to nonblack patients in a single health care delivery system, which limits differences in access to care. Black patients were younger and had shorter periods of observation and were more frequently given first-line fludarabine.
Induction chemotherapy (7+3) or high-dose ara-C-based (HIDAC) for acute myeloid leukemia (AML) results in prolonged neutropenia with a high risk of serious infection and attendant morbidity, and prolonged hospitalization. Researchers, including former AVAHO president Suman Kambhampati developed a randomized, open-label, controlled Phase 2 trial to study the effect of romyelocel-L in de novo patients receiving HIDAC or 7+3 induction therapy for reduction of fever and infection. The results from pooled and 7+3 cohorts, were previously presented, showing decrease in infections and days in hospital. The results from cohorts receiving HIDAC chemotherapy are presented here. The incidence of infections was decreased during the day 15-28 period and a decrease of three days in hospital stay was observed in de novo HIDAC AML subjects receiving romyelocel-L. Romyelocel-L may provide a new option to reduce infections in AML patients undergoing HIDAC induction therapy.
Multiple Myeloma
Two studies focused on racial disparities in multiple myeloma (MM), while another reported phase 2 data on a relapsed/refractory option.
This group of researchers found an absence of disparity in use of novel agents, no racial disparity was observed in overall survival between black and white patients with MM. Among patients aged < 65 years at diagnosis, the researchers observed a significantly lower age-adjusted risk of death for black patients compared with white patients. The difference in the younger population was not explained by access or utilization of resources. This analysis suggests that when healthcare access is neutralized, younger black patients may even have improved overall survival, which may indicate the possibility of genetic differences that may drive the disease biology and therapeutic outcome in AA patients.
Outcomes of Black Patients with Multiple Myeloma in the Veterans Health Administration
The second study found survival of black patients with MM was improved compared to non-blacks in the VHA, a national comprehensive care delivery system. Black patients also received similar therapies compared to non-blacks, while presenting at a younger age with more comorbidities. These results are strengthened after adjusting for treatments and patient characteristics not available in other large data studies. Despite increased incidence of MM in the black population, outcomes are improved, similar to other large studies of patients in the United States.
Multiple myeloma clinical trial CC-4047-MM-014 (NCT01946477) is a phase 2 study designed to test the safety and efficacy of pomalidomide and low-dose dexamethasone alone (arm A) or in combination with daratumumab, an anti-CD38 antibody, (arm B) subjects with relapsed or refractory MM who have received a first- or second-line treatment of lenalidomide-based therapy. In this trial, researchers (including those from VA facilities, Celgene, and multiple other locations) sought to characterize on-treatment pharmacodynamic changes of immune biomarkers associated with POM + LoDEX + DARA administration (arm B) using multicolor flow cytometry panels designed to characterize T-cell subsets and CD38+ expressing cells. The researchers reported that the triplet regimen POM + LoDEX + DARA has shown notable clinical activity with deep and durable responses in relapsed MM patients progressed and are or refractory to lenalidomide. According to the researchers the results demonstrate that patients treated with the POM + LoDEX + DARA combination do not demonstrate impairment in the innate and adaptive immune compartments and, in contrast, show significant proliferative activity in the subsets of CD4, CD8 and NK cells following treatment.
With hundreds of sessions and thousands of abstracts, it can be difficult to wade through all the new findings to find the most significant and relevant findings. Federal Practitioner consulted with Association of VA Hematology/Oncology members who attended the meeting, VA researchers, and other sources to provide these nuggets you might have missed on lymphomas, white blood cells, leukemias, and multiple myeloma:
Lymphomas
This retrospective analysis of diffuse large B cell lymphoma (DCBL) patients who received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) at the VA Audie Murphy Hospital in San Antonio, Texas was compared with patients with DLBCL who received R-CHOP in a community setting. According to the researchers, the response to initial treatment was inferior in the veteran population when compared with a patient population with similar demographics and having similar time from diagnosis to treatment. Veteran patients also had worse outcomes when compared with uninsured patients.
This retrospective analysis study identified 2,290 patients with follicular lymphoma treated in the Veterans Health Administration between 2006–2014 and detailed their staging, demographics, and comorbidities. The researchers found that maintenance therapy with rituximab was associated with an improvement in overall survival.
Another retrospective analysis of DBCL using VHA data examined the effectiveness of second-line chemotherapy and chemoimmunotherapy in patients aged ≥ 65 years. The researchers found 230 patients from 2001 to 2015 that met the inclusion criteria. According to the researchers, the overall survival was < 1 year and about half of the patients "did not receive or were not candidates for regimens typically used with intent for high-dose therapy and autologous transplant."
White Blood Cells
Cost-Effective Use of White Blood Cell Growth Factors in the Veterans Administration
This study analyzed the use of granulocyte colony-stimulating (G-CSF) vs pegfilgrastim in the UD Department of Veterans Affairs (VA) health care system. The researchers looked at the relative frequency of use of filgrastim, Tbo-filgrastim, Filgrastim-sndz and pegfilgrastim at 23 VA sites and found that uptake of biosimilar G-CSF has been extremely rapid. All sites are using biosimilar GCSF for all new patients; 6 of 23 sites were comfortable shifting current patients on branded G-CSF to the biosimilar.However, switching to a Tbo-filgrastim brought a cost savings of 2.2% that was "small compared to other clinical changes."
This analysis described the association between white blood cell (EBC) levels and occurrence of thrombotic events among patients with polycythemia vera (PV) using Veterans Health Administration claims data collected between 2005 and 2012. The researchers found A significant, positive association between increased WBC counts and occurrence of thrombotic events in patients with PV was observed in this study. Patients with WBC counts ≥ 8.5 × 109/L had a significantly increased risk of thrombotic events, and those with counts ≥ 11.0 × 109/L were at greatest risk. Effective control of WBC counts is an important component of disease management and may reduce risk of thrombotic events in patients with PV.
Leukemias
Black patients with chronic lymphocytic leukemia tend to have worse overall survival and an earlier age at diagnosis with higher rates of adverse cytogenetics. This retrospective analysis of VHA patients compared black and nonblack patients. It found that black patients had worse survival compared to nonblack patients in a single health care delivery system, which limits differences in access to care. Black patients were younger and had shorter periods of observation and were more frequently given first-line fludarabine.
Induction chemotherapy (7+3) or high-dose ara-C-based (HIDAC) for acute myeloid leukemia (AML) results in prolonged neutropenia with a high risk of serious infection and attendant morbidity, and prolonged hospitalization. Researchers, including former AVAHO president Suman Kambhampati developed a randomized, open-label, controlled Phase 2 trial to study the effect of romyelocel-L in de novo patients receiving HIDAC or 7+3 induction therapy for reduction of fever and infection. The results from pooled and 7+3 cohorts, were previously presented, showing decrease in infections and days in hospital. The results from cohorts receiving HIDAC chemotherapy are presented here. The incidence of infections was decreased during the day 15-28 period and a decrease of three days in hospital stay was observed in de novo HIDAC AML subjects receiving romyelocel-L. Romyelocel-L may provide a new option to reduce infections in AML patients undergoing HIDAC induction therapy.
Multiple Myeloma
Two studies focused on racial disparities in multiple myeloma (MM), while another reported phase 2 data on a relapsed/refractory option.
This group of researchers found an absence of disparity in use of novel agents, no racial disparity was observed in overall survival between black and white patients with MM. Among patients aged < 65 years at diagnosis, the researchers observed a significantly lower age-adjusted risk of death for black patients compared with white patients. The difference in the younger population was not explained by access or utilization of resources. This analysis suggests that when healthcare access is neutralized, younger black patients may even have improved overall survival, which may indicate the possibility of genetic differences that may drive the disease biology and therapeutic outcome in AA patients.
Outcomes of Black Patients with Multiple Myeloma in the Veterans Health Administration
The second study found survival of black patients with MM was improved compared to non-blacks in the VHA, a national comprehensive care delivery system. Black patients also received similar therapies compared to non-blacks, while presenting at a younger age with more comorbidities. These results are strengthened after adjusting for treatments and patient characteristics not available in other large data studies. Despite increased incidence of MM in the black population, outcomes are improved, similar to other large studies of patients in the United States.
Multiple myeloma clinical trial CC-4047-MM-014 (NCT01946477) is a phase 2 study designed to test the safety and efficacy of pomalidomide and low-dose dexamethasone alone (arm A) or in combination with daratumumab, an anti-CD38 antibody, (arm B) subjects with relapsed or refractory MM who have received a first- or second-line treatment of lenalidomide-based therapy. In this trial, researchers (including those from VA facilities, Celgene, and multiple other locations) sought to characterize on-treatment pharmacodynamic changes of immune biomarkers associated with POM + LoDEX + DARA administration (arm B) using multicolor flow cytometry panels designed to characterize T-cell subsets and CD38+ expressing cells. The researchers reported that the triplet regimen POM + LoDEX + DARA has shown notable clinical activity with deep and durable responses in relapsed MM patients progressed and are or refractory to lenalidomide. According to the researchers the results demonstrate that patients treated with the POM + LoDEX + DARA combination do not demonstrate impairment in the innate and adaptive immune compartments and, in contrast, show significant proliferative activity in the subsets of CD4, CD8 and NK cells following treatment.
MAIA: Daratumumab plus len-dex improves myeloma PFS
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
SAN DIEGO – Patients with newly diagnosed multiple myeloma who were ineligible for transplant had a 44% reduction in the risk of disease progression or death when they were treated with the anti-CD38 monoclonal antibody daratumumab (Darzalex) added to lenalidomide (Revlimid) and dexamethasone, compared with lenalidomide-dexamethasone alone, an interim analysis from the MAIA trial showed.
Among 737 patients in a phase 3 trial, median progression-free survival – the primary endpoint – had not been reached after a median follow-up of 28 months for patients randomized to daratumumab, lenalidomide, and dexamethasone (DRd), versus 31.9 months for patients randomized to lenalidomide and dexamethasone (Rd).
The 30-month PFS rate in the DRd arm was 71%, compared with 56% for the Rd arm. This difference translated into a hazard ratio (HR) for progression of 0.56 (P less than .0001), reported Thierry Facon, MD, of Hôpital Claude Huriez and the University of Lille, France.
“These results support DRd as a new standard of care for elderly patients with myeloma who are ineligible for transplant,” he said at the annual meeting of the American Society of Hematology.
Dr. Facon and his colleagues had previously shown in the FIRST trial that in newly diagnosed transplant-ineligible patients, continuous treatment with lenalidomide and low-dose dexamethasone was associated with significant overall survival and PFS benefits, compared with melphalan-prednisone-thalidomide.
In the POLLUX trial, investigators reported that in patients with multiple myeloma that was refractory or had relapsed after at least one prior line of therapy, the DRd combination was associated with a 63% reduction in the risk for disease progression or death, compared with Rd alone.
MAIA details
The MAIA trial is a pivotal, phase 3 study pitting DRd against Rd in transplant-ineligible patients with newly diagnosed multiple myeloma.
Patients with untreated disease who had Eastern Cooperative Oncology Group (ECOG) status of 0-2 and creatinine clearance rates of at least 30 mL/min were enrolled. Patients were randomly assigned to either DRd, with intravenous daratumumab 16 mg/kg weekly for cycles 1 and 2, every other week for cycles 3 through 6, and every 4 weeks from cycle 7 until disease progression, plus lenalidomide 25 mg orally per day on days 1-21 until disease progression, and dexamethasone 40 mg orally or intravenously weekly until disease progression; or to the same regimen without daratumumab.
The median patient age was 73 years and 99% of all patients were aged 65 years or older. Demographic and clinical characteristics were well balanced between the treatment arms.
The primary endpoint of progression-free survival was superior with DRd.
DRd also was associated with a significantly higher overall response rate (93% vs. 81%), complete response rate (48% vs. 25%), very good partial response or better rate (79% vs. 53%), and minimal residual disease (MRD) negativity rate (24% vs. 7%; P less than .0001 for all comparisons).
The DRd combination was associated with infusion-related reactions in 41% of patients, but only 3% were grade 3 or 4 in severity.
Hematologic treatment-emergent adverse events (TEAE) grade 3 or greater that were more common with DRd included neutropenia (50% vs. 35%) and lymphopenia (15% vs. 11%). Conversely, thrombocytopenia (7% vs. 9%, grade 3 or 4) and anemia (12% vs. 20%) were more frequent with Rd.
Nonhematologic TEAEs that were more frequent with DRd included diarrhea, constipation, fatigue, peripheral edema, and pneumonia. Rates of asthenia, back pain, nausea, and deep vein thrombosis/pulmonary embolism were similar between the study arms.
Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
SOURCE: Facon T et al. ASH 2018, Abstract LBA-2.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: At 30 months of follow-up, DRd was associated with a 44% reduction in the risk of death, compared with Rd.
Study details: Randomized phase 3 trial of 737 patients with newly diagnosed multiple myeloma who were ineligible for transplant.
Disclosures: Janssen funded the study. Dr. Facon reported speakers bureau and advisory board participation for Celgene, Janssen, and Takeda; and advisory board participation for Sanofi, Amgen, Karyopharm, and Oncopeptides.
Source: Facon T et al. ASH 2018, Abstract LBA-2.
Melflufen-dex proves active in multi-resistant MM
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
Tom Brokaw opens up on surviving multiple myeloma
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
EXPERT ANALYSIS FROM ASH 2018