User login
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Multiple myeloma treatment produces a similar survival benefit in very elderly patients
More than a third of patients diagnosed with multiple myeloma (MM) are over the age of 80, and yet most treatment successes have been reported in younger patients. However, patients over the age of 80 years received similar benefits as younger patients from MM treatment, according to a study reported online in the Journal of Geriatric Oncology.
Researchers identified 2,155 patients diagnosed with MM at age 80 years or older in the Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database from 2007 to 2013. A cohort of 2,933 similar patients diagnosed with myeloma at age 70-79 was used for comparison.
The researcher found that the number of patients receiving treatment for myeloma within 6 months of diagnosis was significantly lower in the 80 years and older group, compared with the 70-79 years group. Only 51% of patients in the 80 years and older cohort received systemic treatment within 6 months following diagnosis, compared with 71% of patients in the 70-79 years cohort received systemic treatment in the same timeframe (P < .001)
The analysis showed that treatment was associated with an overall 26% decrease in hazard for death, independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. There was no statistically significant difference in treatment benefit based on age cohort (P = .610).
The median overall survival for patients diagnosed at age 80 years or older was 13.4 months, with those patients receiving systemic treatment having a median overall survival of 21.4 months, compared with 6.4 months for those not receiving treatment. In comparison, patients between ages 70 and 79 years had a median overall survival of 30.1 months with treatment.
The population over 80, when myeloma incidence peaks, is projected to triple over the next few decades, according to the researchers. “Antimyeloma treatment in the era of novel therapies seems to have a similar improvement on survival for the oldest-old, those beyond 80 years, as other patients. With growing knowledge of and experience with novel agents in older patients with myeloma, treatment rates have increased, which have in turn improved survival,” they concluded.
The study was funded by the National Cancer Institute. One author reported research funding from Janssen. The other authors had no relevant conflicts of interest.
SOURCE: Fiala MA et al. J Geriatric Oncol. 2020 Mar 10. doi.org/10.1016/j.jgo.2020.03.005.
More than a third of patients diagnosed with multiple myeloma (MM) are over the age of 80, and yet most treatment successes have been reported in younger patients. However, patients over the age of 80 years received similar benefits as younger patients from MM treatment, according to a study reported online in the Journal of Geriatric Oncology.
Researchers identified 2,155 patients diagnosed with MM at age 80 years or older in the Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database from 2007 to 2013. A cohort of 2,933 similar patients diagnosed with myeloma at age 70-79 was used for comparison.
The researcher found that the number of patients receiving treatment for myeloma within 6 months of diagnosis was significantly lower in the 80 years and older group, compared with the 70-79 years group. Only 51% of patients in the 80 years and older cohort received systemic treatment within 6 months following diagnosis, compared with 71% of patients in the 70-79 years cohort received systemic treatment in the same timeframe (P < .001)
The analysis showed that treatment was associated with an overall 26% decrease in hazard for death, independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. There was no statistically significant difference in treatment benefit based on age cohort (P = .610).
The median overall survival for patients diagnosed at age 80 years or older was 13.4 months, with those patients receiving systemic treatment having a median overall survival of 21.4 months, compared with 6.4 months for those not receiving treatment. In comparison, patients between ages 70 and 79 years had a median overall survival of 30.1 months with treatment.
The population over 80, when myeloma incidence peaks, is projected to triple over the next few decades, according to the researchers. “Antimyeloma treatment in the era of novel therapies seems to have a similar improvement on survival for the oldest-old, those beyond 80 years, as other patients. With growing knowledge of and experience with novel agents in older patients with myeloma, treatment rates have increased, which have in turn improved survival,” they concluded.
The study was funded by the National Cancer Institute. One author reported research funding from Janssen. The other authors had no relevant conflicts of interest.
SOURCE: Fiala MA et al. J Geriatric Oncol. 2020 Mar 10. doi.org/10.1016/j.jgo.2020.03.005.
More than a third of patients diagnosed with multiple myeloma (MM) are over the age of 80, and yet most treatment successes have been reported in younger patients. However, patients over the age of 80 years received similar benefits as younger patients from MM treatment, according to a study reported online in the Journal of Geriatric Oncology.
Researchers identified 2,155 patients diagnosed with MM at age 80 years or older in the Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database from 2007 to 2013. A cohort of 2,933 similar patients diagnosed with myeloma at age 70-79 was used for comparison.
The researcher found that the number of patients receiving treatment for myeloma within 6 months of diagnosis was significantly lower in the 80 years and older group, compared with the 70-79 years group. Only 51% of patients in the 80 years and older cohort received systemic treatment within 6 months following diagnosis, compared with 71% of patients in the 70-79 years cohort received systemic treatment in the same timeframe (P < .001)
The analysis showed that treatment was associated with an overall 26% decrease in hazard for death, independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. There was no statistically significant difference in treatment benefit based on age cohort (P = .610).
The median overall survival for patients diagnosed at age 80 years or older was 13.4 months, with those patients receiving systemic treatment having a median overall survival of 21.4 months, compared with 6.4 months for those not receiving treatment. In comparison, patients between ages 70 and 79 years had a median overall survival of 30.1 months with treatment.
The population over 80, when myeloma incidence peaks, is projected to triple over the next few decades, according to the researchers. “Antimyeloma treatment in the era of novel therapies seems to have a similar improvement on survival for the oldest-old, those beyond 80 years, as other patients. With growing knowledge of and experience with novel agents in older patients with myeloma, treatment rates have increased, which have in turn improved survival,” they concluded.
The study was funded by the National Cancer Institute. One author reported research funding from Janssen. The other authors had no relevant conflicts of interest.
SOURCE: Fiala MA et al. J Geriatric Oncol. 2020 Mar 10. doi.org/10.1016/j.jgo.2020.03.005.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
Fracture risk for MM patients remains higher despite improvements in treatments, survival
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
FROM BONE
Stored CD34 cells for multiple myeloma patients largely unused
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
ORLANDO – Collecting and storing extra stem cells on the off chance that a patient with multiple myeloma will need a salvage autologous stem cell transplant may not be worth the money or effort, investigators say.
Among patients with multiple myeloma who had adequate collection of mobilized and stored cells, only 3 of 146 eligible patients were given the stored cells in a second autologous stem cell transplant (ASCT), reported Nausheen Ahmed, MD, from the Case Western Reserve Cancer Center and University Hospitals Seidman Cancer Center, both in Cleveland.
“We found overall low utilization of salvage transplants and storage stem cells at our institution, which may not justify the strategy of early collection for all patients fit for transplant,” she said at the Transplantation and Cellular Therapy Meetings.
But Sergio Giralt, MD, a transplant specialist from Memorial Sloan Kettering Cancer Center, New York, who was not involved in the study, warned against changing practice “for the wrong reason, because it’s just a financial reason.”
Get them while they’re fresh
The rationale for collecting and storing extra cells is the risk that mobilization will fail in the future following prolonged maintenance with immunomodulatory agents such as lenalidomide (Revlimid), and the risk for genetic or epigenetic damage to cells from high-dose melphalan used in transplant-conditioning regimens, Dr. Ahmed noted at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“However, there are potential issues with early mobilization and storage, including cost, resources, apheresis scheduling, uncertainty of cell viability, and liability. There’s also risk of side effects with filgrastim and plerixafor use [for mobilization],” she said.
Dr. Ahmed and colleagues conducted a study to determine how stored stem cells for second ASCT were used, describe how second ASCTs are used in patients who meet the Mayo Consensus Stratification for Myeloma & Risk-Adapted Therapy (mSMART) criteria, and the costs of mobilizing and storing stem cells for a second ASCT.
They took a retrospective look at all adults aged 18 years and older with a diagnosis of multiple myeloma who received a first ASCT at their institution from 2009 to 2017. They excluded patients who had amyloidosis without myeloma or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma proliferative disorder, skin changes) syndrome.
Patients were considered eligible for a second ASCT based on mSMART recommendations if they had a relapse either 18 or more months without maintenance therapy or after at least 36 months on maintenance. The investigators defined an extra day of collection as an additional day of apheresis to obtain 2 million or more CD34 cells/kg for storage only.
They estimated costs from the institution’s charge master as the sum of cell processing, leukapheresis costs, additional plerixafor costs, and storage costs, and calculated the total duration of storage as months from the date of collection until the last follow-up.
The median age of the total study population of 179 patients was 61 years, with a majority of male and white patients. Of this group, 98% had an Eastern Cooperative Oncology Group performance score of 0-1. In all, 63.7% of the patients had standard-risk cytogenetics, 22.4% had high-risk disease, and the remainder had unknown cytogenetic risk.
At a median follow-up of 56.5 months, 95 patients (53.1%) had experienced a relapse after transplant with a median time to progression of 47.5 months. The majority of patients (166; 92.7%) had received a single transplant, 10 (5.6%) had received tandem transplants, and only 3 (1.6%) had a second transplant at relapse.
Looking at the use of second transplant in patients who met the criteria for salvage transplant based on mSMART (excluding patients who had undergone tandem transplant) and whose maintenance status was known, they identified 61 patients on maintenance therapy and 24 with no maintenance. A total of 31 patients (18 in the maintenance group and 13 in the no-maintenance group) met mSMART criteria for salvage ASCT.
Dr. Ahmed and colleagues next looked at the 146 patients who had at least 2 million stored cells/kg, and found that the stored cells were used for only three patients. Of the 146 patients, 66 had 1 extra collection day, 17 had 2 extra days, and 4 had 3 extra days, for an average additional cost per patient of $16,859.
‘Woefully underutilized’
Discussing the study, Dr. Giralt asked: “How valid are the SMART criteria of 36 months? And the answer is there is no data to support it, and if we actually go back to our oncology, any patient who has had more than 18 months without exposure to a drug can continue to have sensitivity to that drug, and that’s why if we used the ASBMT criteria of greater than 18 months you’d have a larger population” of patients eligible for salvage transplant.
He stated that, “we know these patients exist, we know they have cells in the freezer, but we’re not using those cells. Second transplant is woefully underutilized in myeloma patients,” and he added that stored cells could also be used to support those patients who develop cytopenias following chimeric antigen receptor (CAR) T-cell therapy.
Yago Nieto, MD, from the University of Texas MD Anderson Cancer Center, Houston, who comoderated the session where the data were presented, agreed with Dr. Giralt that stored stem cells are underutilized in the treatment of patients with multiple myeloma.
“I don’t think that the experience from Case Western, where the percentage of patients who are eligible for salvage transplant and actually got it was less than 10%, can be extrapolated to many other centers. I think that in most centers the actual percentage is higher than that,” he said in an interview.
“There are going to be therapies like CAR T that will compete with salvage transplants, but I think more patients should be considered for this salvage procedure,” he added.
No funding source for the story was disclosed. Dr. Ahmed reported no financial disclosures. Dr. Giralt reported consulting/advisory activities and receiving research funding from multiple companies. Dr. Nieto disclosed research funding from, and consultancy for, several companies.
SOURCE: Ahmed N et al. TCT 2020, Abstract 28.
REPORTING FROM TCT 2020
FDA approves new drug for relapsed/refractory multiple myeloma
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
New CAR T-cell therapy eliminates MM and tumor propagating cells without fratricide in lab study
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
FROM NATURE COMMUNICATIONS
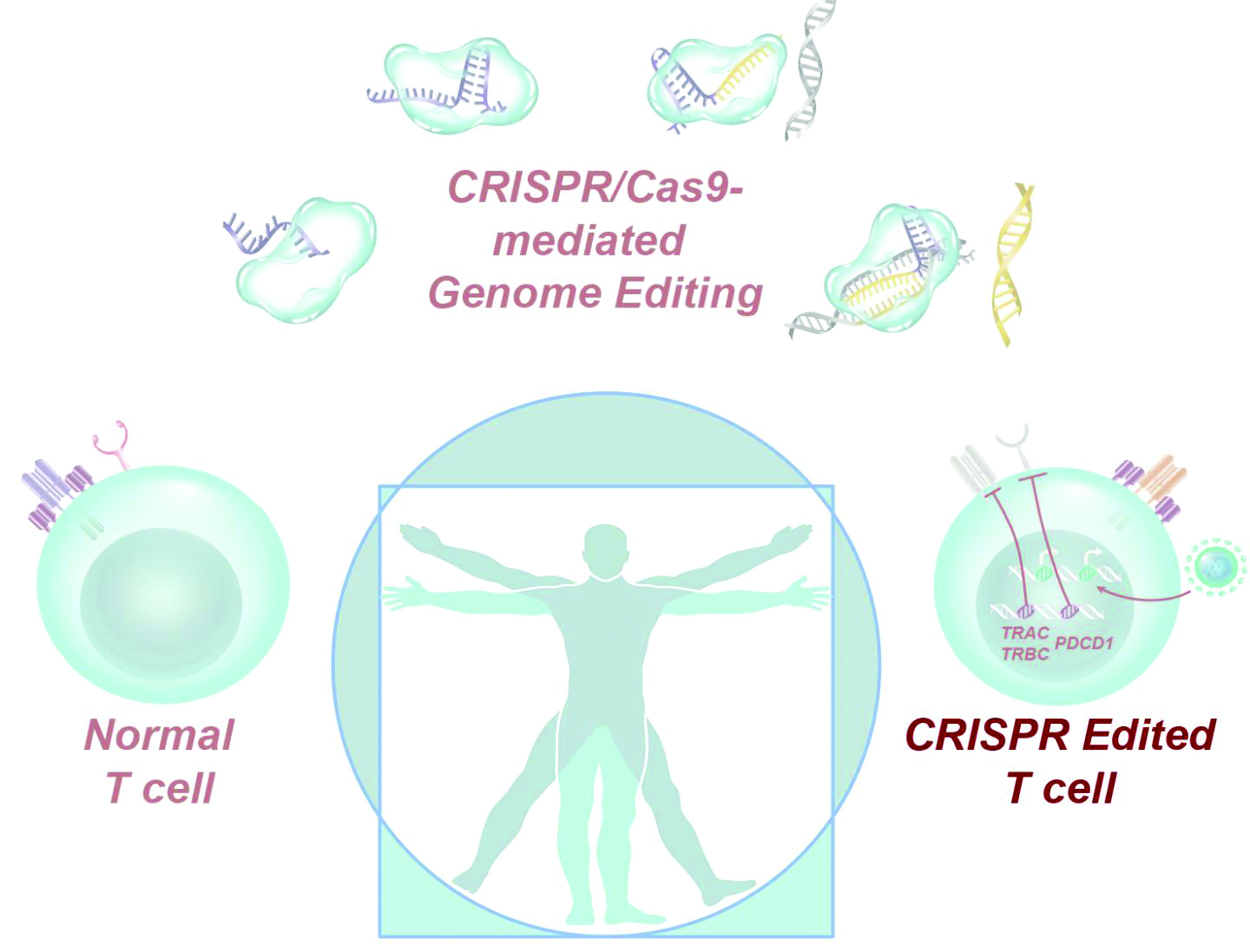
CRISPR-engineered T cells may be safe for cancer, but do they work?
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
FROM SCIENCE
Start of myeloma therapy may be delayed for women, minorities
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
FROM JCO ONCOLOGY PRACTICE
Key clinical point:
Major finding: Patients with delays in treatment were more likely to be women (odds ratio, 1.15) and more likely to be non-Hispanic blacks (OR, 1.21).
Study details: Retrospective analysis of 74,722 patients in the National Cancer Database diagnosed with multiple myeloma between 2004 and 2015.
Disclosures: Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
Source: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Inhibitor appears to strengthen anti-BCMA CAR T cells in myeloma patients
ORLANDO – A gamma secretase inhibitor could enhance the efficacy of B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cells in patients with relapsed or refractory multiple myeloma, a phase 1 trial suggests.
The inhibitor, JSMD194, increased BCMA expression in all 10 patients studied. All patients responded to anti-BCMA CAR T-cell therapy, including three patients who had previously failed BCMA-directed therapy.
Nine patients remain alive and in response at a median follow-up of 20 weeks, with two patients being followed for more than a year. One patient experienced dose-limiting toxicity and died, which prompted a change to the study’s eligibility criteria.
Andrew J. Cowan, MD, of the University of Washington and Fred Hutchinson Cancer Research Center in Seattle, presented these results at the annual meeting of the American Society of Hematology.
Dr. Cowan and colleagues previously showed that treatment with a gamma secretase inhibitor increased BCMA expression on tumor cells and improved the efficacy of BCMA-targeted CAR T cells in a mouse model of multiple myeloma. The team also showed that a gamma secretase inhibitor could “markedly” increase the percentage of BCMA-positive tumor cells in myeloma patients (Blood. 2019 Nov 7;134[19]:1585-97).
To expand upon these findings, the researchers began a phase 1 trial of BCMA-directed CAR T cells and the oral gamma secretase inhibitor JSMD194 in patients with relapsed/refractory multiple myeloma.
Ten patients have been treated, five men and five women. The patients’ median age at baseline was 66 years (range, 44-74 years). They received a median of 10 prior therapies (range, 4-23). Nine patients had received at least one autologous stem cell transplant, and one patient had two. One patient underwent allogeneic transplant (as well as autologous transplant).
Three patients had received prior BCMA-directed therapy. Two patients had received BCMA-directed CAR T cells. One of them did not respond, and the other responded but relapsed. The third patient received a BCMA-targeted bispecific T-cell engager and did not respond.
Study treatment
Patients had BCMA expression measured at baseline, then underwent apheresis for CAR T-cell production.
Patients received JSMD194 at 25 mg on days 1, 3, and 5. Then, they received cyclophosphamide at 300 mg and fludarabine at 25 mg for 3 days.
Next, patients received a single CAR T-cell infusion at a dose of 50 x 106 (n = 5), 150 x 106 (n = 3), or 300 x 106 (n = 2). They also received JSMD194 at 25 mg three times a week for 3 weeks.
Safety
“Nearly all patients had a serious adverse event, which was typically admission to the hospital for neutropenic fever,” Dr. Cowan said.
One patient experienced dose-limiting toxicity and died at day 33. The patient had a disseminating fungal infection, grade 4 cytokine release syndrome (CRS), and neurotoxicity. The patient’s death prompted the researchers to include performance status in the study’s eligibility criteria.
All patients developed CRS. Only the aforementioned patient had grade 4 CRS, and three patients had grade 3 CRS. Six patients experienced neurotoxicity. There were no cases of tumor lysis syndrome.
Efficacy
“All patients experienced an increase of cells expressing BCMA,” Dr. Cowan said. “While there was significant variability in BCMA expression at baseline, all cells expressed BCMA after three doses of the gamma secretase inhibitor.”
The median BCMA expression after JSMD194 treatment was 99% (range, 96%-100%), and there was a median 20-fold (range, 8- to 157-fold) increase in BCMA surface density.
The overall response rate was 100%. Two patients achieved a stringent complete response (CR), one achieved a CR, five patients had a very good partial response, and two had a partial response.
The patient with a CR received the 50 x 106 dose of CAR T cells, and the patients with stringent CRs received the 150 x 106 and 300 x 106 doses.
Of the three patients who previously received BCMA-directed therapy, two achieved a very good partial response, and one had a partial response.
Nine of the 10 patients are still alive and in response, with a median follow-up of 20 weeks. The longest follow-up is 444 days.
“To date, all patients have evidence of durable responses,” Dr. Cowan said. “Moreover, all patients had dramatic reductions in involved serum free light chain ... and serum monoclonal proteins.”
Dr. Cowan noted that longer follow-up is needed to assess CAR T-cell persistence and the durability of response.
This trial is sponsored by the Fred Hutchinson Cancer Research Center in collaboration with the National Cancer Institute. Two researchers involved in this work are employees of Juno Therapeutics. Dr. Cowan reported relationships with Juno Therapeutics, Janssen, Celgene, AbbVie, Cellectar, and Sanofi.
SOURCE: Cowan AJ et al. ASH 2019. Abstract 204.
ORLANDO – A gamma secretase inhibitor could enhance the efficacy of B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cells in patients with relapsed or refractory multiple myeloma, a phase 1 trial suggests.
The inhibitor, JSMD194, increased BCMA expression in all 10 patients studied. All patients responded to anti-BCMA CAR T-cell therapy, including three patients who had previously failed BCMA-directed therapy.
Nine patients remain alive and in response at a median follow-up of 20 weeks, with two patients being followed for more than a year. One patient experienced dose-limiting toxicity and died, which prompted a change to the study’s eligibility criteria.
Andrew J. Cowan, MD, of the University of Washington and Fred Hutchinson Cancer Research Center in Seattle, presented these results at the annual meeting of the American Society of Hematology.
Dr. Cowan and colleagues previously showed that treatment with a gamma secretase inhibitor increased BCMA expression on tumor cells and improved the efficacy of BCMA-targeted CAR T cells in a mouse model of multiple myeloma. The team also showed that a gamma secretase inhibitor could “markedly” increase the percentage of BCMA-positive tumor cells in myeloma patients (Blood. 2019 Nov 7;134[19]:1585-97).
To expand upon these findings, the researchers began a phase 1 trial of BCMA-directed CAR T cells and the oral gamma secretase inhibitor JSMD194 in patients with relapsed/refractory multiple myeloma.
Ten patients have been treated, five men and five women. The patients’ median age at baseline was 66 years (range, 44-74 years). They received a median of 10 prior therapies (range, 4-23). Nine patients had received at least one autologous stem cell transplant, and one patient had two. One patient underwent allogeneic transplant (as well as autologous transplant).
Three patients had received prior BCMA-directed therapy. Two patients had received BCMA-directed CAR T cells. One of them did not respond, and the other responded but relapsed. The third patient received a BCMA-targeted bispecific T-cell engager and did not respond.
Study treatment
Patients had BCMA expression measured at baseline, then underwent apheresis for CAR T-cell production.
Patients received JSMD194 at 25 mg on days 1, 3, and 5. Then, they received cyclophosphamide at 300 mg and fludarabine at 25 mg for 3 days.
Next, patients received a single CAR T-cell infusion at a dose of 50 x 106 (n = 5), 150 x 106 (n = 3), or 300 x 106 (n = 2). They also received JSMD194 at 25 mg three times a week for 3 weeks.
Safety
“Nearly all patients had a serious adverse event, which was typically admission to the hospital for neutropenic fever,” Dr. Cowan said.
One patient experienced dose-limiting toxicity and died at day 33. The patient had a disseminating fungal infection, grade 4 cytokine release syndrome (CRS), and neurotoxicity. The patient’s death prompted the researchers to include performance status in the study’s eligibility criteria.
All patients developed CRS. Only the aforementioned patient had grade 4 CRS, and three patients had grade 3 CRS. Six patients experienced neurotoxicity. There were no cases of tumor lysis syndrome.
Efficacy
“All patients experienced an increase of cells expressing BCMA,” Dr. Cowan said. “While there was significant variability in BCMA expression at baseline, all cells expressed BCMA after three doses of the gamma secretase inhibitor.”
The median BCMA expression after JSMD194 treatment was 99% (range, 96%-100%), and there was a median 20-fold (range, 8- to 157-fold) increase in BCMA surface density.
The overall response rate was 100%. Two patients achieved a stringent complete response (CR), one achieved a CR, five patients had a very good partial response, and two had a partial response.
The patient with a CR received the 50 x 106 dose of CAR T cells, and the patients with stringent CRs received the 150 x 106 and 300 x 106 doses.
Of the three patients who previously received BCMA-directed therapy, two achieved a very good partial response, and one had a partial response.
Nine of the 10 patients are still alive and in response, with a median follow-up of 20 weeks. The longest follow-up is 444 days.
“To date, all patients have evidence of durable responses,” Dr. Cowan said. “Moreover, all patients had dramatic reductions in involved serum free light chain ... and serum monoclonal proteins.”
Dr. Cowan noted that longer follow-up is needed to assess CAR T-cell persistence and the durability of response.
This trial is sponsored by the Fred Hutchinson Cancer Research Center in collaboration with the National Cancer Institute. Two researchers involved in this work are employees of Juno Therapeutics. Dr. Cowan reported relationships with Juno Therapeutics, Janssen, Celgene, AbbVie, Cellectar, and Sanofi.
SOURCE: Cowan AJ et al. ASH 2019. Abstract 204.
ORLANDO – A gamma secretase inhibitor could enhance the efficacy of B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cells in patients with relapsed or refractory multiple myeloma, a phase 1 trial suggests.
The inhibitor, JSMD194, increased BCMA expression in all 10 patients studied. All patients responded to anti-BCMA CAR T-cell therapy, including three patients who had previously failed BCMA-directed therapy.
Nine patients remain alive and in response at a median follow-up of 20 weeks, with two patients being followed for more than a year. One patient experienced dose-limiting toxicity and died, which prompted a change to the study’s eligibility criteria.
Andrew J. Cowan, MD, of the University of Washington and Fred Hutchinson Cancer Research Center in Seattle, presented these results at the annual meeting of the American Society of Hematology.
Dr. Cowan and colleagues previously showed that treatment with a gamma secretase inhibitor increased BCMA expression on tumor cells and improved the efficacy of BCMA-targeted CAR T cells in a mouse model of multiple myeloma. The team also showed that a gamma secretase inhibitor could “markedly” increase the percentage of BCMA-positive tumor cells in myeloma patients (Blood. 2019 Nov 7;134[19]:1585-97).
To expand upon these findings, the researchers began a phase 1 trial of BCMA-directed CAR T cells and the oral gamma secretase inhibitor JSMD194 in patients with relapsed/refractory multiple myeloma.
Ten patients have been treated, five men and five women. The patients’ median age at baseline was 66 years (range, 44-74 years). They received a median of 10 prior therapies (range, 4-23). Nine patients had received at least one autologous stem cell transplant, and one patient had two. One patient underwent allogeneic transplant (as well as autologous transplant).
Three patients had received prior BCMA-directed therapy. Two patients had received BCMA-directed CAR T cells. One of them did not respond, and the other responded but relapsed. The third patient received a BCMA-targeted bispecific T-cell engager and did not respond.
Study treatment
Patients had BCMA expression measured at baseline, then underwent apheresis for CAR T-cell production.
Patients received JSMD194 at 25 mg on days 1, 3, and 5. Then, they received cyclophosphamide at 300 mg and fludarabine at 25 mg for 3 days.
Next, patients received a single CAR T-cell infusion at a dose of 50 x 106 (n = 5), 150 x 106 (n = 3), or 300 x 106 (n = 2). They also received JSMD194 at 25 mg three times a week for 3 weeks.
Safety
“Nearly all patients had a serious adverse event, which was typically admission to the hospital for neutropenic fever,” Dr. Cowan said.
One patient experienced dose-limiting toxicity and died at day 33. The patient had a disseminating fungal infection, grade 4 cytokine release syndrome (CRS), and neurotoxicity. The patient’s death prompted the researchers to include performance status in the study’s eligibility criteria.
All patients developed CRS. Only the aforementioned patient had grade 4 CRS, and three patients had grade 3 CRS. Six patients experienced neurotoxicity. There were no cases of tumor lysis syndrome.
Efficacy
“All patients experienced an increase of cells expressing BCMA,” Dr. Cowan said. “While there was significant variability in BCMA expression at baseline, all cells expressed BCMA after three doses of the gamma secretase inhibitor.”
The median BCMA expression after JSMD194 treatment was 99% (range, 96%-100%), and there was a median 20-fold (range, 8- to 157-fold) increase in BCMA surface density.
The overall response rate was 100%. Two patients achieved a stringent complete response (CR), one achieved a CR, five patients had a very good partial response, and two had a partial response.
The patient with a CR received the 50 x 106 dose of CAR T cells, and the patients with stringent CRs received the 150 x 106 and 300 x 106 doses.
Of the three patients who previously received BCMA-directed therapy, two achieved a very good partial response, and one had a partial response.
Nine of the 10 patients are still alive and in response, with a median follow-up of 20 weeks. The longest follow-up is 444 days.
“To date, all patients have evidence of durable responses,” Dr. Cowan said. “Moreover, all patients had dramatic reductions in involved serum free light chain ... and serum monoclonal proteins.”
Dr. Cowan noted that longer follow-up is needed to assess CAR T-cell persistence and the durability of response.
This trial is sponsored by the Fred Hutchinson Cancer Research Center in collaboration with the National Cancer Institute. Two researchers involved in this work are employees of Juno Therapeutics. Dr. Cowan reported relationships with Juno Therapeutics, Janssen, Celgene, AbbVie, Cellectar, and Sanofi.
SOURCE: Cowan AJ et al. ASH 2019. Abstract 204.
REPORTING FROM ASH 2019
D-RVd for frontline myeloma looks robust in GRIFFIN trial update
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
REPORTING FROM ASH 2019