User login
9/11 responders show increased risk of leukemia, other cancers
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
FROM JNCI CANCER SPECTRUM
Genomic profiling of AML and MDS yields prognostic clues
ORLANDO – A genome-wide study of blood and bone marrow samples from more than 1,300 adults with myeloid disorders has both confirmed the role of known or suspected driver mutations and uncovered new associations that could inform clinical care for patients with acute myeloid leukemia and myelodysplastic syndrome.
“Integration of mutational and expression data is important to refine subytpes and constellations of mutations with prognostic significance,” Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital in Memphis said during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Her team conducted an analysis combining full genomic sequencing and gene-expression profiles in blood and bone marrow samples from 598 adults with acute myeloid leukemia (AML) and 706 with myelodysplastic syndrome (MDS).
The goals of the study were to provide “unbiased analysis of AML and MDS by integrated genomic and transcriptome data and clinico-pathologic features and clinical outcome” and to identify and define myeloid leukemia subtypes with diagnostic, prognostic, and therapeutic significance, she said.
The median age of the MDS cohort was 73.2 years (range 23.3-93.1). According to 2016 World Health Organization criteria, 37% had a diagnosis of MDS with excess blasts, 26.3% had MDS with ring sideroblasts, 20.9% had MDS with multilineage dysplasia, 14.6% had MDS with deletion 5q, and 1.1% had unclassifiable MDS.
The median age of the AML cohort was 68 years. Of this group, 31.7% had a diagnosis of AML not otherwise specified, 29.9% had known cytogenetic alterations, 27.3% had NPM1-mutated AML, and 9.7% had RUNX1-mutated disease.
Samples from all patients underwent tumor whole-genome sequencing and whole-transcriptome sequencing.
The combined sequencing confirmed a diagnosis of AML with recurrent genetic abnormalities in 11% of cases. These patients had disease with distinct gene-expression profiles and favorable prognosis. The sequencing identified combinations of mutations in genes linked with specific AML subtypes.
For example, combinations of mutations in KIT, ZBTB7A, ASXL2, RAD21, CSF3R, and DNM2 were associated with RUNX1-RUNXT1 leukemia, whereas mutations in FLT3, DDX54, WT1, and CALR in promyelocytic leukemia/retinoic acid receptor alpha were associated with promyelocytic leukemia, and KIT and BCORL1 mutations were associated with CBFB-rearranged leukemia.
In addition to rounding up the usual genomic suspects, the investigators also identified combinations that are associated with prognosis. Notably, NPM1 mutations were found in 27.4% of AML and 1% of MDS cases, and these mutations were characterized by four gene-expression signatures that were associated with different combinations of cooperating mutations in cohesin and signaling genes, and with outcome.
They found that patients with co-occurring NPM1 and FLT3 mutations had worse prognosis than those with mutations only in NPM1, whereas patients with NPM1 mutations co-occurring with cohesin gene mutations had better outcomes.
At a briefing prior to her presentation of the data, Dr. Iacobucci explained how her group’s findings might inform treatment, including the possibility of preventing development of AML in patients with MDS.
“What we are doing, in addition to the genomic part, is also establishing a repository of patient-derived xenografts, so in this way we can have the genome information, and we can have the biological material in vivo to test different therapies,” she said.
In an interview, Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne, who was not involved in the genomic study, commented on the role of sequencing in treatment of patients with myeloid malignancies.
“I think the future is that as the leukemia evolves, our therapy will evolve along with it. Furthermore, we now have the potential to measure many of these mutations with much higher sensitivity than just whole-genome sequencing, so we can imagine a future whereby we can track and measure these mutations as they rise in the patient’s bone marrow or blood before the patients becomes sick with florid leukemia, and it gives us the potential to predictably alter our management before they become sick,” he said.
The study was supported by St. Jude Children’s Research Hospital and the Leukemia and Lymphoma Society. Dr. Iacobucci and Dr. Wei reported having no relevant disclosures.
SOURCE: Iacobucci I et al. ASH 2019, Abstract LBA-4.
ORLANDO – A genome-wide study of blood and bone marrow samples from more than 1,300 adults with myeloid disorders has both confirmed the role of known or suspected driver mutations and uncovered new associations that could inform clinical care for patients with acute myeloid leukemia and myelodysplastic syndrome.
“Integration of mutational and expression data is important to refine subytpes and constellations of mutations with prognostic significance,” Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital in Memphis said during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Her team conducted an analysis combining full genomic sequencing and gene-expression profiles in blood and bone marrow samples from 598 adults with acute myeloid leukemia (AML) and 706 with myelodysplastic syndrome (MDS).
The goals of the study were to provide “unbiased analysis of AML and MDS by integrated genomic and transcriptome data and clinico-pathologic features and clinical outcome” and to identify and define myeloid leukemia subtypes with diagnostic, prognostic, and therapeutic significance, she said.
The median age of the MDS cohort was 73.2 years (range 23.3-93.1). According to 2016 World Health Organization criteria, 37% had a diagnosis of MDS with excess blasts, 26.3% had MDS with ring sideroblasts, 20.9% had MDS with multilineage dysplasia, 14.6% had MDS with deletion 5q, and 1.1% had unclassifiable MDS.
The median age of the AML cohort was 68 years. Of this group, 31.7% had a diagnosis of AML not otherwise specified, 29.9% had known cytogenetic alterations, 27.3% had NPM1-mutated AML, and 9.7% had RUNX1-mutated disease.
Samples from all patients underwent tumor whole-genome sequencing and whole-transcriptome sequencing.
The combined sequencing confirmed a diagnosis of AML with recurrent genetic abnormalities in 11% of cases. These patients had disease with distinct gene-expression profiles and favorable prognosis. The sequencing identified combinations of mutations in genes linked with specific AML subtypes.
For example, combinations of mutations in KIT, ZBTB7A, ASXL2, RAD21, CSF3R, and DNM2 were associated with RUNX1-RUNXT1 leukemia, whereas mutations in FLT3, DDX54, WT1, and CALR in promyelocytic leukemia/retinoic acid receptor alpha were associated with promyelocytic leukemia, and KIT and BCORL1 mutations were associated with CBFB-rearranged leukemia.
In addition to rounding up the usual genomic suspects, the investigators also identified combinations that are associated with prognosis. Notably, NPM1 mutations were found in 27.4% of AML and 1% of MDS cases, and these mutations were characterized by four gene-expression signatures that were associated with different combinations of cooperating mutations in cohesin and signaling genes, and with outcome.
They found that patients with co-occurring NPM1 and FLT3 mutations had worse prognosis than those with mutations only in NPM1, whereas patients with NPM1 mutations co-occurring with cohesin gene mutations had better outcomes.
At a briefing prior to her presentation of the data, Dr. Iacobucci explained how her group’s findings might inform treatment, including the possibility of preventing development of AML in patients with MDS.
“What we are doing, in addition to the genomic part, is also establishing a repository of patient-derived xenografts, so in this way we can have the genome information, and we can have the biological material in vivo to test different therapies,” she said.
In an interview, Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne, who was not involved in the genomic study, commented on the role of sequencing in treatment of patients with myeloid malignancies.
“I think the future is that as the leukemia evolves, our therapy will evolve along with it. Furthermore, we now have the potential to measure many of these mutations with much higher sensitivity than just whole-genome sequencing, so we can imagine a future whereby we can track and measure these mutations as they rise in the patient’s bone marrow or blood before the patients becomes sick with florid leukemia, and it gives us the potential to predictably alter our management before they become sick,” he said.
The study was supported by St. Jude Children’s Research Hospital and the Leukemia and Lymphoma Society. Dr. Iacobucci and Dr. Wei reported having no relevant disclosures.
SOURCE: Iacobucci I et al. ASH 2019, Abstract LBA-4.
ORLANDO – A genome-wide study of blood and bone marrow samples from more than 1,300 adults with myeloid disorders has both confirmed the role of known or suspected driver mutations and uncovered new associations that could inform clinical care for patients with acute myeloid leukemia and myelodysplastic syndrome.
“Integration of mutational and expression data is important to refine subytpes and constellations of mutations with prognostic significance,” Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital in Memphis said during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Her team conducted an analysis combining full genomic sequencing and gene-expression profiles in blood and bone marrow samples from 598 adults with acute myeloid leukemia (AML) and 706 with myelodysplastic syndrome (MDS).
The goals of the study were to provide “unbiased analysis of AML and MDS by integrated genomic and transcriptome data and clinico-pathologic features and clinical outcome” and to identify and define myeloid leukemia subtypes with diagnostic, prognostic, and therapeutic significance, she said.
The median age of the MDS cohort was 73.2 years (range 23.3-93.1). According to 2016 World Health Organization criteria, 37% had a diagnosis of MDS with excess blasts, 26.3% had MDS with ring sideroblasts, 20.9% had MDS with multilineage dysplasia, 14.6% had MDS with deletion 5q, and 1.1% had unclassifiable MDS.
The median age of the AML cohort was 68 years. Of this group, 31.7% had a diagnosis of AML not otherwise specified, 29.9% had known cytogenetic alterations, 27.3% had NPM1-mutated AML, and 9.7% had RUNX1-mutated disease.
Samples from all patients underwent tumor whole-genome sequencing and whole-transcriptome sequencing.
The combined sequencing confirmed a diagnosis of AML with recurrent genetic abnormalities in 11% of cases. These patients had disease with distinct gene-expression profiles and favorable prognosis. The sequencing identified combinations of mutations in genes linked with specific AML subtypes.
For example, combinations of mutations in KIT, ZBTB7A, ASXL2, RAD21, CSF3R, and DNM2 were associated with RUNX1-RUNXT1 leukemia, whereas mutations in FLT3, DDX54, WT1, and CALR in promyelocytic leukemia/retinoic acid receptor alpha were associated with promyelocytic leukemia, and KIT and BCORL1 mutations were associated with CBFB-rearranged leukemia.
In addition to rounding up the usual genomic suspects, the investigators also identified combinations that are associated with prognosis. Notably, NPM1 mutations were found in 27.4% of AML and 1% of MDS cases, and these mutations were characterized by four gene-expression signatures that were associated with different combinations of cooperating mutations in cohesin and signaling genes, and with outcome.
They found that patients with co-occurring NPM1 and FLT3 mutations had worse prognosis than those with mutations only in NPM1, whereas patients with NPM1 mutations co-occurring with cohesin gene mutations had better outcomes.
At a briefing prior to her presentation of the data, Dr. Iacobucci explained how her group’s findings might inform treatment, including the possibility of preventing development of AML in patients with MDS.
“What we are doing, in addition to the genomic part, is also establishing a repository of patient-derived xenografts, so in this way we can have the genome information, and we can have the biological material in vivo to test different therapies,” she said.
In an interview, Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne, who was not involved in the genomic study, commented on the role of sequencing in treatment of patients with myeloid malignancies.
“I think the future is that as the leukemia evolves, our therapy will evolve along with it. Furthermore, we now have the potential to measure many of these mutations with much higher sensitivity than just whole-genome sequencing, so we can imagine a future whereby we can track and measure these mutations as they rise in the patient’s bone marrow or blood before the patients becomes sick with florid leukemia, and it gives us the potential to predictably alter our management before they become sick,” he said.
The study was supported by St. Jude Children’s Research Hospital and the Leukemia and Lymphoma Society. Dr. Iacobucci and Dr. Wei reported having no relevant disclosures.
SOURCE: Iacobucci I et al. ASH 2019, Abstract LBA-4.
REPORTING FROM ASH 2019
High response, survival rates with ponatinib/hCVAD in Ph-positive ALL
ORLANDO – For adults with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL), the combination of hyper-CVAD chemotherapy and ponatinib is associated with high complete molecular response and 5-year overall survival rates, investigators reported.
Long-term follow-up of 86 adults with Ph+ALL treated in the front line with chemotherapy plus ponatinib (Iclusig), a third-generation tyrosine kinase inhibitor (TKI), showed a complete remission (CR) rate of 100%, complete molecular remission (CMR) rate of 86%, and a 5-year overall survival (OS) rate of 74%, reported Nicholas J. Short, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Although we observed two treatment-related cardiovascular deaths with the original trial design, with almost 50 patients treated since instituting a risk-adapted dosing schedule with lower doses of ponatinib, no additional ponatinib-related deaths have been observed,” he said at the annual meeting of the American Society of Hematology.
The standard of care for adults with Ph+ALL is chemotherapy plus a TKI. With a first- or second-generation TKI plus chemotherapy, reported 5-year OS rates range from 35% to 50%.
“However, relapses are still common, and these are usually driven by the development of new resistance mutations in the ABL gene, particularly the T315I gatekeeper mutation which has been reported in up to 75% of patients at the time of relapse,” he said.
Ponatinib is a pan-BCR-ABL TKI with activity against ALL with T315I mutations, and the combination of this agent with hyper-CVAD chemotherapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) has been associated with higher response rates than those seen with earlier-generation TKIs, as well as higher levels of minimal residual disease (MRD) negativity, he noted.
Dr. Short and colleagues hypothesized that, compared with the standard of care, hyper-CVAD plus ponatinib would be associated with higher MRD levels, low relapse rates by suppression of T315I subclones, decreased reliance on stem cell transplantation in first remission, and improved long-term survival.
To test this, they treated 86 adults with newly diagnosed Ph+ALL, including those who had undergone one or two previous courses of chemotherapy with a TKI other than ponatinib. The patients had Eastern Cooperative Oncology Group performance status 0-2, adequate organ function, and no clinically significant cardiovascular disease.
The patients underwent eight cycles of hyper-CVAD alternating with high-dose methotrexate/cytarabine approximately every 21 days. The first 37 patients were treated with, ponatinib 45 mg daily for the first 14 days of cycle 1, then continuously for subsequent cycles. Patients with CD20 expression of 20% or greater also received rituximab during the first four cycles. CNS prophylaxis was also administered with 12 doses of intrathecal chemotherapy with alternating methotrexate and cytarabine.
Patients who had a CR received maintenance with ponatinib and vincristine/prednisone monthly for 2 years, followed by ponatinib indefinitely.
Out of concern for vascular toxicity with long-term use of high-dose ponatinib, including the two deaths mentioned before, the protocol was amended after the first 37 patients were treated. The amended protocol reduced ponatinib to 30 mg starting at cycle 2, with further reduction to 15 mg once a CMR (absence of BCR-ABL on polymerase chain reaction) was achieved.
At a median follow-up of 44 months, the event-free survival rates – the primary endpoint – were 71% at 3 years and 68% at 5 years. The 3-year OS rate was 78%, and the 5-year OS rate was 74%.
All patients had complete remission and complete cytogenetic remission as assessed by conventional karyotyping. Additionally, 73 of 85 evaluable patients (86%) achieved a CMR at some point during therapy.
“We had previously reported that achievement of a complete molecular response by 3 months is associated with superior outcomes. Approximately three quarters of patients achieved this milestone,” Dr. Short said.
Grade 3 or greater adverse events of particular concern included transaminase elevations in 29% of patients, elevated bilirubin and pancreatitis in 15% each, and hypertension in 14%.
Four patients had grade 3 or greater venous thromboembolic or arterial events, including the two previously noted deaths from myocardial infarction, both of which occurred prior to the protocol amendment.
At the most recent follow-up, 11 patients had experienced relapse (no CNS-only relapses), and of this group, 5 died and 6 were still alive. Nineteen patients underwent hematopoietic stem cell transplant, and of this group, 13 were still alive and 6 died.
Causes of death in the nine patients who died while in CR included the two myocardial infarction deaths on study, three deaths from sepsis during consolidation, one from lung cancer, one from a head injury after a fall, one from myocardial infarction in a 79-year-old patient 4 years after stopping ponatinib (off study), and one from preexisting congestive heart failure in a 74-year-old patient.
In all, 47 patients were either in ongoing therapy or observation at last follow-up, including three patients who were transitioned to MRD-directed therapy including blinatumomab (Blincyto).
“As a next step, we are now evaluating lower-intensity regimens with ponatinib and blinatumomab in both the frontline and relapsed/refractory settings, with the goals of decreased chemotherapy-related toxicity, increased MRD-negativity rates, further decreased reliance on transplant, and improved long-term outcomes,” Dr. Short said.
The study was sponsored by MD Anderson with support from the National Cancer Institute. Dr. Short reported consulting for AstraZenca, honoraria from Amgen, and consulting and receiving research funding from Takeda Oncology.
SOURCE: Short NJ et al. ASH 2019, Abstract 283.
ORLANDO – For adults with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL), the combination of hyper-CVAD chemotherapy and ponatinib is associated with high complete molecular response and 5-year overall survival rates, investigators reported.
Long-term follow-up of 86 adults with Ph+ALL treated in the front line with chemotherapy plus ponatinib (Iclusig), a third-generation tyrosine kinase inhibitor (TKI), showed a complete remission (CR) rate of 100%, complete molecular remission (CMR) rate of 86%, and a 5-year overall survival (OS) rate of 74%, reported Nicholas J. Short, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Although we observed two treatment-related cardiovascular deaths with the original trial design, with almost 50 patients treated since instituting a risk-adapted dosing schedule with lower doses of ponatinib, no additional ponatinib-related deaths have been observed,” he said at the annual meeting of the American Society of Hematology.
The standard of care for adults with Ph+ALL is chemotherapy plus a TKI. With a first- or second-generation TKI plus chemotherapy, reported 5-year OS rates range from 35% to 50%.
“However, relapses are still common, and these are usually driven by the development of new resistance mutations in the ABL gene, particularly the T315I gatekeeper mutation which has been reported in up to 75% of patients at the time of relapse,” he said.
Ponatinib is a pan-BCR-ABL TKI with activity against ALL with T315I mutations, and the combination of this agent with hyper-CVAD chemotherapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) has been associated with higher response rates than those seen with earlier-generation TKIs, as well as higher levels of minimal residual disease (MRD) negativity, he noted.
Dr. Short and colleagues hypothesized that, compared with the standard of care, hyper-CVAD plus ponatinib would be associated with higher MRD levels, low relapse rates by suppression of T315I subclones, decreased reliance on stem cell transplantation in first remission, and improved long-term survival.
To test this, they treated 86 adults with newly diagnosed Ph+ALL, including those who had undergone one or two previous courses of chemotherapy with a TKI other than ponatinib. The patients had Eastern Cooperative Oncology Group performance status 0-2, adequate organ function, and no clinically significant cardiovascular disease.
The patients underwent eight cycles of hyper-CVAD alternating with high-dose methotrexate/cytarabine approximately every 21 days. The first 37 patients were treated with, ponatinib 45 mg daily for the first 14 days of cycle 1, then continuously for subsequent cycles. Patients with CD20 expression of 20% or greater also received rituximab during the first four cycles. CNS prophylaxis was also administered with 12 doses of intrathecal chemotherapy with alternating methotrexate and cytarabine.
Patients who had a CR received maintenance with ponatinib and vincristine/prednisone monthly for 2 years, followed by ponatinib indefinitely.
Out of concern for vascular toxicity with long-term use of high-dose ponatinib, including the two deaths mentioned before, the protocol was amended after the first 37 patients were treated. The amended protocol reduced ponatinib to 30 mg starting at cycle 2, with further reduction to 15 mg once a CMR (absence of BCR-ABL on polymerase chain reaction) was achieved.
At a median follow-up of 44 months, the event-free survival rates – the primary endpoint – were 71% at 3 years and 68% at 5 years. The 3-year OS rate was 78%, and the 5-year OS rate was 74%.
All patients had complete remission and complete cytogenetic remission as assessed by conventional karyotyping. Additionally, 73 of 85 evaluable patients (86%) achieved a CMR at some point during therapy.
“We had previously reported that achievement of a complete molecular response by 3 months is associated with superior outcomes. Approximately three quarters of patients achieved this milestone,” Dr. Short said.
Grade 3 or greater adverse events of particular concern included transaminase elevations in 29% of patients, elevated bilirubin and pancreatitis in 15% each, and hypertension in 14%.
Four patients had grade 3 or greater venous thromboembolic or arterial events, including the two previously noted deaths from myocardial infarction, both of which occurred prior to the protocol amendment.
At the most recent follow-up, 11 patients had experienced relapse (no CNS-only relapses), and of this group, 5 died and 6 were still alive. Nineteen patients underwent hematopoietic stem cell transplant, and of this group, 13 were still alive and 6 died.
Causes of death in the nine patients who died while in CR included the two myocardial infarction deaths on study, three deaths from sepsis during consolidation, one from lung cancer, one from a head injury after a fall, one from myocardial infarction in a 79-year-old patient 4 years after stopping ponatinib (off study), and one from preexisting congestive heart failure in a 74-year-old patient.
In all, 47 patients were either in ongoing therapy or observation at last follow-up, including three patients who were transitioned to MRD-directed therapy including blinatumomab (Blincyto).
“As a next step, we are now evaluating lower-intensity regimens with ponatinib and blinatumomab in both the frontline and relapsed/refractory settings, with the goals of decreased chemotherapy-related toxicity, increased MRD-negativity rates, further decreased reliance on transplant, and improved long-term outcomes,” Dr. Short said.
The study was sponsored by MD Anderson with support from the National Cancer Institute. Dr. Short reported consulting for AstraZenca, honoraria from Amgen, and consulting and receiving research funding from Takeda Oncology.
SOURCE: Short NJ et al. ASH 2019, Abstract 283.
ORLANDO – For adults with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL), the combination of hyper-CVAD chemotherapy and ponatinib is associated with high complete molecular response and 5-year overall survival rates, investigators reported.
Long-term follow-up of 86 adults with Ph+ALL treated in the front line with chemotherapy plus ponatinib (Iclusig), a third-generation tyrosine kinase inhibitor (TKI), showed a complete remission (CR) rate of 100%, complete molecular remission (CMR) rate of 86%, and a 5-year overall survival (OS) rate of 74%, reported Nicholas J. Short, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Although we observed two treatment-related cardiovascular deaths with the original trial design, with almost 50 patients treated since instituting a risk-adapted dosing schedule with lower doses of ponatinib, no additional ponatinib-related deaths have been observed,” he said at the annual meeting of the American Society of Hematology.
The standard of care for adults with Ph+ALL is chemotherapy plus a TKI. With a first- or second-generation TKI plus chemotherapy, reported 5-year OS rates range from 35% to 50%.
“However, relapses are still common, and these are usually driven by the development of new resistance mutations in the ABL gene, particularly the T315I gatekeeper mutation which has been reported in up to 75% of patients at the time of relapse,” he said.
Ponatinib is a pan-BCR-ABL TKI with activity against ALL with T315I mutations, and the combination of this agent with hyper-CVAD chemotherapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) has been associated with higher response rates than those seen with earlier-generation TKIs, as well as higher levels of minimal residual disease (MRD) negativity, he noted.
Dr. Short and colleagues hypothesized that, compared with the standard of care, hyper-CVAD plus ponatinib would be associated with higher MRD levels, low relapse rates by suppression of T315I subclones, decreased reliance on stem cell transplantation in first remission, and improved long-term survival.
To test this, they treated 86 adults with newly diagnosed Ph+ALL, including those who had undergone one or two previous courses of chemotherapy with a TKI other than ponatinib. The patients had Eastern Cooperative Oncology Group performance status 0-2, adequate organ function, and no clinically significant cardiovascular disease.
The patients underwent eight cycles of hyper-CVAD alternating with high-dose methotrexate/cytarabine approximately every 21 days. The first 37 patients were treated with, ponatinib 45 mg daily for the first 14 days of cycle 1, then continuously for subsequent cycles. Patients with CD20 expression of 20% or greater also received rituximab during the first four cycles. CNS prophylaxis was also administered with 12 doses of intrathecal chemotherapy with alternating methotrexate and cytarabine.
Patients who had a CR received maintenance with ponatinib and vincristine/prednisone monthly for 2 years, followed by ponatinib indefinitely.
Out of concern for vascular toxicity with long-term use of high-dose ponatinib, including the two deaths mentioned before, the protocol was amended after the first 37 patients were treated. The amended protocol reduced ponatinib to 30 mg starting at cycle 2, with further reduction to 15 mg once a CMR (absence of BCR-ABL on polymerase chain reaction) was achieved.
At a median follow-up of 44 months, the event-free survival rates – the primary endpoint – were 71% at 3 years and 68% at 5 years. The 3-year OS rate was 78%, and the 5-year OS rate was 74%.
All patients had complete remission and complete cytogenetic remission as assessed by conventional karyotyping. Additionally, 73 of 85 evaluable patients (86%) achieved a CMR at some point during therapy.
“We had previously reported that achievement of a complete molecular response by 3 months is associated with superior outcomes. Approximately three quarters of patients achieved this milestone,” Dr. Short said.
Grade 3 or greater adverse events of particular concern included transaminase elevations in 29% of patients, elevated bilirubin and pancreatitis in 15% each, and hypertension in 14%.
Four patients had grade 3 or greater venous thromboembolic or arterial events, including the two previously noted deaths from myocardial infarction, both of which occurred prior to the protocol amendment.
At the most recent follow-up, 11 patients had experienced relapse (no CNS-only relapses), and of this group, 5 died and 6 were still alive. Nineteen patients underwent hematopoietic stem cell transplant, and of this group, 13 were still alive and 6 died.
Causes of death in the nine patients who died while in CR included the two myocardial infarction deaths on study, three deaths from sepsis during consolidation, one from lung cancer, one from a head injury after a fall, one from myocardial infarction in a 79-year-old patient 4 years after stopping ponatinib (off study), and one from preexisting congestive heart failure in a 74-year-old patient.
In all, 47 patients were either in ongoing therapy or observation at last follow-up, including three patients who were transitioned to MRD-directed therapy including blinatumomab (Blincyto).
“As a next step, we are now evaluating lower-intensity regimens with ponatinib and blinatumomab in both the frontline and relapsed/refractory settings, with the goals of decreased chemotherapy-related toxicity, increased MRD-negativity rates, further decreased reliance on transplant, and improved long-term outcomes,” Dr. Short said.
The study was sponsored by MD Anderson with support from the National Cancer Institute. Dr. Short reported consulting for AstraZenca, honoraria from Amgen, and consulting and receiving research funding from Takeda Oncology.
SOURCE: Short NJ et al. ASH 2019, Abstract 283.
REPORTING FROM ASH 2019
CAR T cells produce complete responses in T-cell malignancies
ORLANDO – Anti-CD5 chimeric antigen receptor (CAR) T cells can produce complete responses (CRs) in patients with relapsed or refractory T-cell malignancies, according to findings from a phase 1 trial.
Three of 11 patients achieved a CR after CAR T-cell therapy, and one patient achieved a mixed response that deepened to a CR after transplant. Three responders, all of whom had T-cell lymphoma, were still alive and in CR at last follow-up.
There were no cases of severe cytokine release syndrome (CRS) or severe neurotoxicity, no serious infectious complications, and no nonhematologic grade 4 adverse events in this trial.
LaQuisa C. Hill, MD, of Baylor College of Medicine, Houston, presented these results at the annual meeting of the American Society of Hematology.
“While CD19 CAR T cells have revolutionized the treatment of relapsed/refractory B-cell malignancies, development of CAR T-cell platforms targeting T-cell-driven malignancies have been hindered by three main factors: CAR T-cell fratricide due to shared expression of target antigens leading to impaired expansion, ablation of normal T cells continuing to cause profound immunodeficiency, and the potential of transduced tumor cells providing a means of tumor escape,” Dr. Hill said.
Researchers have theorized that anti-CD5 CAR T cells can overcome these obstacles. In preclinical studies, anti-CD5 CAR T cells eliminated malignant blasts in vitro and in vivo and resulted in “limited and transient” fratricide (Blood. 2015 Aug 20;126[8]:983-92).
With this in mind, Dr. Hill and her colleagues tested CD5.28z CAR T cells in a phase 1 trial (NCT03081910). Eleven patients have been treated thus far – five with T-cell acute lymphoblastic leukemia (T-ALL), three with peripheral T-cell lymphoma (PTCL), two with angioimmunoblastic T-cell lymphoma (AITL), and one with Sézary syndrome.
The patients’ median age at baseline was 62 years (range, 21-71 years), and 63% were men. They had received a median of 5 prior therapies (range, 3-18). Two patients had relapsed after allogeneic hematopoietic stem cell transplant (HSCT), three had relapsed after autologous HSCT, and five were primary refractory.
Patients underwent lymphodepletion with fludarabine and cyclophosphamide, then received CAR T cells at doses of 1 x 107 or 5 x 107.
Response
Three lymphoma patients – two with AITL and one with PTCL – were still alive and in CR at last follow-up. The PTCL patient achieved a CR after CAR T-cell therapy and declined a subsequent HSCT. The patient has not received additional therapy and has retained the CR for 7 months.
One AITL patient achieved a CR and declined transplant as well. He relapsed after 7 months but received subsequent therapy and achieved another CR. The other AITL patient had a mixed response to CAR T-cell therapy but proceeded to allogeneic HSCT and achieved a CR that has lasted 9 months.
The remaining three lymphoma patients – two with PTCL and one with Sézary syndrome – progressed and died.
One T-ALL patient achieved a CR lasting 6 weeks, but the patient died while undergoing transplant workup. Two T-ALL patients did not respond to treatment and died. The remaining two patients progressed, and one of them died. The other patient who progressed is still alive and in CR after receiving subsequent therapy.
Factors associated with response
Dr. Hill said a shortened manufacturing process may be associated with enhanced response, as all responders received CAR T cells produced via a shorter manufacturing process. The shortened process involves freezing cells on day 4-5 post transduction, as opposed to day 7.
“While the numbers are too small to make any definitive conclusions, this seems to correlate with less terminal differentiation, which might improve potency,” Dr. Hill said. “However, additional analyses are ongoing.”
Dr. Hill also pointed out that CAR T-cell expansion was observed in all patients, with higher peak levels observed at the higher dose. In addition, CAR T-cell persistence was durable at both dose levels.
“We have been able to detect the CAR transgene at all follow-up time points, out to 9 months for some patients,” Dr. Hill said. “While limited persistence may play a role in nonresponders, it does not appear to be the only factor.”
Safety
“Surprisingly, no selective ablation of normal T cells has been observed,” Dr. Hill said. “As CAR T cells dwindled [after infusion], we were able to see recovery of normal T cells, all of which expressed normal levels of CD5. This was observed in all patients on study, except for one patient who had prolonged pancytopenia.”
Cytopenias were the most common grade 3/4 adverse events, including neutropenia (n = 8), anemia (n = 7), and thrombocytopenia (n = 5). Other grade 3/4 events included elevated aspartate aminotransferase (n = 2), hypoalbuminemia (n = 1), hyponatremia (n = 1), hypophosphatemia (n = 1), and elevated alanine aminotransferase (n = 1). There were no grade 5 adverse events.
Two patients developed grade 1 CRS, and two had grade 2 CRS. Both patients with grade 2 CRS were treated with tocilizumab, and their symptoms resolved.
One patient developed grade 2 immune effector cell-associated neurotoxicity syndrome, but this resolved with supportive care.
One patient had a central line–associated bloodstream infection (coagulase-negative staphylococci), and one had cytomegalovirus and BK virus reactivation. There were no fungal infections.
“We have demonstrated that CD5 CAR T cells can be manufactured from heavily pretreated patients with T-cell malignancies, and therapy is well tolerated,” Dr. Hill said. “We have seen strong and promising activity in T-cell lymphoma, which we hope to be able to translate to T-ALL as well.”
Dr. Hill said she and her colleagues hope to improve upon these results with a higher dose level of CD5 CAR T cells (1 x 108), which the team plans to start testing soon. The researchers may also investigate other target antigens, such as CD7, as well as the use of donor-derived CAR T cells for patients who have relapsed after allogeneic HSCT.
Dr. Hill said she has no relevant disclosures. Baylor College of Medicine is sponsoring this trial.
SOURCE: Hill L et al. ASH 2019. Abstract 199.
ORLANDO – Anti-CD5 chimeric antigen receptor (CAR) T cells can produce complete responses (CRs) in patients with relapsed or refractory T-cell malignancies, according to findings from a phase 1 trial.
Three of 11 patients achieved a CR after CAR T-cell therapy, and one patient achieved a mixed response that deepened to a CR after transplant. Three responders, all of whom had T-cell lymphoma, were still alive and in CR at last follow-up.
There were no cases of severe cytokine release syndrome (CRS) or severe neurotoxicity, no serious infectious complications, and no nonhematologic grade 4 adverse events in this trial.
LaQuisa C. Hill, MD, of Baylor College of Medicine, Houston, presented these results at the annual meeting of the American Society of Hematology.
“While CD19 CAR T cells have revolutionized the treatment of relapsed/refractory B-cell malignancies, development of CAR T-cell platforms targeting T-cell-driven malignancies have been hindered by three main factors: CAR T-cell fratricide due to shared expression of target antigens leading to impaired expansion, ablation of normal T cells continuing to cause profound immunodeficiency, and the potential of transduced tumor cells providing a means of tumor escape,” Dr. Hill said.
Researchers have theorized that anti-CD5 CAR T cells can overcome these obstacles. In preclinical studies, anti-CD5 CAR T cells eliminated malignant blasts in vitro and in vivo and resulted in “limited and transient” fratricide (Blood. 2015 Aug 20;126[8]:983-92).
With this in mind, Dr. Hill and her colleagues tested CD5.28z CAR T cells in a phase 1 trial (NCT03081910). Eleven patients have been treated thus far – five with T-cell acute lymphoblastic leukemia (T-ALL), three with peripheral T-cell lymphoma (PTCL), two with angioimmunoblastic T-cell lymphoma (AITL), and one with Sézary syndrome.
The patients’ median age at baseline was 62 years (range, 21-71 years), and 63% were men. They had received a median of 5 prior therapies (range, 3-18). Two patients had relapsed after allogeneic hematopoietic stem cell transplant (HSCT), three had relapsed after autologous HSCT, and five were primary refractory.
Patients underwent lymphodepletion with fludarabine and cyclophosphamide, then received CAR T cells at doses of 1 x 107 or 5 x 107.
Response
Three lymphoma patients – two with AITL and one with PTCL – were still alive and in CR at last follow-up. The PTCL patient achieved a CR after CAR T-cell therapy and declined a subsequent HSCT. The patient has not received additional therapy and has retained the CR for 7 months.
One AITL patient achieved a CR and declined transplant as well. He relapsed after 7 months but received subsequent therapy and achieved another CR. The other AITL patient had a mixed response to CAR T-cell therapy but proceeded to allogeneic HSCT and achieved a CR that has lasted 9 months.
The remaining three lymphoma patients – two with PTCL and one with Sézary syndrome – progressed and died.
One T-ALL patient achieved a CR lasting 6 weeks, but the patient died while undergoing transplant workup. Two T-ALL patients did not respond to treatment and died. The remaining two patients progressed, and one of them died. The other patient who progressed is still alive and in CR after receiving subsequent therapy.
Factors associated with response
Dr. Hill said a shortened manufacturing process may be associated with enhanced response, as all responders received CAR T cells produced via a shorter manufacturing process. The shortened process involves freezing cells on day 4-5 post transduction, as opposed to day 7.
“While the numbers are too small to make any definitive conclusions, this seems to correlate with less terminal differentiation, which might improve potency,” Dr. Hill said. “However, additional analyses are ongoing.”
Dr. Hill also pointed out that CAR T-cell expansion was observed in all patients, with higher peak levels observed at the higher dose. In addition, CAR T-cell persistence was durable at both dose levels.
“We have been able to detect the CAR transgene at all follow-up time points, out to 9 months for some patients,” Dr. Hill said. “While limited persistence may play a role in nonresponders, it does not appear to be the only factor.”
Safety
“Surprisingly, no selective ablation of normal T cells has been observed,” Dr. Hill said. “As CAR T cells dwindled [after infusion], we were able to see recovery of normal T cells, all of which expressed normal levels of CD5. This was observed in all patients on study, except for one patient who had prolonged pancytopenia.”
Cytopenias were the most common grade 3/4 adverse events, including neutropenia (n = 8), anemia (n = 7), and thrombocytopenia (n = 5). Other grade 3/4 events included elevated aspartate aminotransferase (n = 2), hypoalbuminemia (n = 1), hyponatremia (n = 1), hypophosphatemia (n = 1), and elevated alanine aminotransferase (n = 1). There were no grade 5 adverse events.
Two patients developed grade 1 CRS, and two had grade 2 CRS. Both patients with grade 2 CRS were treated with tocilizumab, and their symptoms resolved.
One patient developed grade 2 immune effector cell-associated neurotoxicity syndrome, but this resolved with supportive care.
One patient had a central line–associated bloodstream infection (coagulase-negative staphylococci), and one had cytomegalovirus and BK virus reactivation. There were no fungal infections.
“We have demonstrated that CD5 CAR T cells can be manufactured from heavily pretreated patients with T-cell malignancies, and therapy is well tolerated,” Dr. Hill said. “We have seen strong and promising activity in T-cell lymphoma, which we hope to be able to translate to T-ALL as well.”
Dr. Hill said she and her colleagues hope to improve upon these results with a higher dose level of CD5 CAR T cells (1 x 108), which the team plans to start testing soon. The researchers may also investigate other target antigens, such as CD7, as well as the use of donor-derived CAR T cells for patients who have relapsed after allogeneic HSCT.
Dr. Hill said she has no relevant disclosures. Baylor College of Medicine is sponsoring this trial.
SOURCE: Hill L et al. ASH 2019. Abstract 199.
ORLANDO – Anti-CD5 chimeric antigen receptor (CAR) T cells can produce complete responses (CRs) in patients with relapsed or refractory T-cell malignancies, according to findings from a phase 1 trial.
Three of 11 patients achieved a CR after CAR T-cell therapy, and one patient achieved a mixed response that deepened to a CR after transplant. Three responders, all of whom had T-cell lymphoma, were still alive and in CR at last follow-up.
There were no cases of severe cytokine release syndrome (CRS) or severe neurotoxicity, no serious infectious complications, and no nonhematologic grade 4 adverse events in this trial.
LaQuisa C. Hill, MD, of Baylor College of Medicine, Houston, presented these results at the annual meeting of the American Society of Hematology.
“While CD19 CAR T cells have revolutionized the treatment of relapsed/refractory B-cell malignancies, development of CAR T-cell platforms targeting T-cell-driven malignancies have been hindered by three main factors: CAR T-cell fratricide due to shared expression of target antigens leading to impaired expansion, ablation of normal T cells continuing to cause profound immunodeficiency, and the potential of transduced tumor cells providing a means of tumor escape,” Dr. Hill said.
Researchers have theorized that anti-CD5 CAR T cells can overcome these obstacles. In preclinical studies, anti-CD5 CAR T cells eliminated malignant blasts in vitro and in vivo and resulted in “limited and transient” fratricide (Blood. 2015 Aug 20;126[8]:983-92).
With this in mind, Dr. Hill and her colleagues tested CD5.28z CAR T cells in a phase 1 trial (NCT03081910). Eleven patients have been treated thus far – five with T-cell acute lymphoblastic leukemia (T-ALL), three with peripheral T-cell lymphoma (PTCL), two with angioimmunoblastic T-cell lymphoma (AITL), and one with Sézary syndrome.
The patients’ median age at baseline was 62 years (range, 21-71 years), and 63% were men. They had received a median of 5 prior therapies (range, 3-18). Two patients had relapsed after allogeneic hematopoietic stem cell transplant (HSCT), three had relapsed after autologous HSCT, and five were primary refractory.
Patients underwent lymphodepletion with fludarabine and cyclophosphamide, then received CAR T cells at doses of 1 x 107 or 5 x 107.
Response
Three lymphoma patients – two with AITL and one with PTCL – were still alive and in CR at last follow-up. The PTCL patient achieved a CR after CAR T-cell therapy and declined a subsequent HSCT. The patient has not received additional therapy and has retained the CR for 7 months.
One AITL patient achieved a CR and declined transplant as well. He relapsed after 7 months but received subsequent therapy and achieved another CR. The other AITL patient had a mixed response to CAR T-cell therapy but proceeded to allogeneic HSCT and achieved a CR that has lasted 9 months.
The remaining three lymphoma patients – two with PTCL and one with Sézary syndrome – progressed and died.
One T-ALL patient achieved a CR lasting 6 weeks, but the patient died while undergoing transplant workup. Two T-ALL patients did not respond to treatment and died. The remaining two patients progressed, and one of them died. The other patient who progressed is still alive and in CR after receiving subsequent therapy.
Factors associated with response
Dr. Hill said a shortened manufacturing process may be associated with enhanced response, as all responders received CAR T cells produced via a shorter manufacturing process. The shortened process involves freezing cells on day 4-5 post transduction, as opposed to day 7.
“While the numbers are too small to make any definitive conclusions, this seems to correlate with less terminal differentiation, which might improve potency,” Dr. Hill said. “However, additional analyses are ongoing.”
Dr. Hill also pointed out that CAR T-cell expansion was observed in all patients, with higher peak levels observed at the higher dose. In addition, CAR T-cell persistence was durable at both dose levels.
“We have been able to detect the CAR transgene at all follow-up time points, out to 9 months for some patients,” Dr. Hill said. “While limited persistence may play a role in nonresponders, it does not appear to be the only factor.”
Safety
“Surprisingly, no selective ablation of normal T cells has been observed,” Dr. Hill said. “As CAR T cells dwindled [after infusion], we were able to see recovery of normal T cells, all of which expressed normal levels of CD5. This was observed in all patients on study, except for one patient who had prolonged pancytopenia.”
Cytopenias were the most common grade 3/4 adverse events, including neutropenia (n = 8), anemia (n = 7), and thrombocytopenia (n = 5). Other grade 3/4 events included elevated aspartate aminotransferase (n = 2), hypoalbuminemia (n = 1), hyponatremia (n = 1), hypophosphatemia (n = 1), and elevated alanine aminotransferase (n = 1). There were no grade 5 adverse events.
Two patients developed grade 1 CRS, and two had grade 2 CRS. Both patients with grade 2 CRS were treated with tocilizumab, and their symptoms resolved.
One patient developed grade 2 immune effector cell-associated neurotoxicity syndrome, but this resolved with supportive care.
One patient had a central line–associated bloodstream infection (coagulase-negative staphylococci), and one had cytomegalovirus and BK virus reactivation. There were no fungal infections.
“We have demonstrated that CD5 CAR T cells can be manufactured from heavily pretreated patients with T-cell malignancies, and therapy is well tolerated,” Dr. Hill said. “We have seen strong and promising activity in T-cell lymphoma, which we hope to be able to translate to T-ALL as well.”
Dr. Hill said she and her colleagues hope to improve upon these results with a higher dose level of CD5 CAR T cells (1 x 108), which the team plans to start testing soon. The researchers may also investigate other target antigens, such as CD7, as well as the use of donor-derived CAR T cells for patients who have relapsed after allogeneic HSCT.
Dr. Hill said she has no relevant disclosures. Baylor College of Medicine is sponsoring this trial.
SOURCE: Hill L et al. ASH 2019. Abstract 199.
REPORTING FROM ASH 2019
CAR T-cell therapy advances in CLL
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
REPORTING FROM ASH 2019
Zanubrutinib achieved high response rate in del(17p) CLL cohort
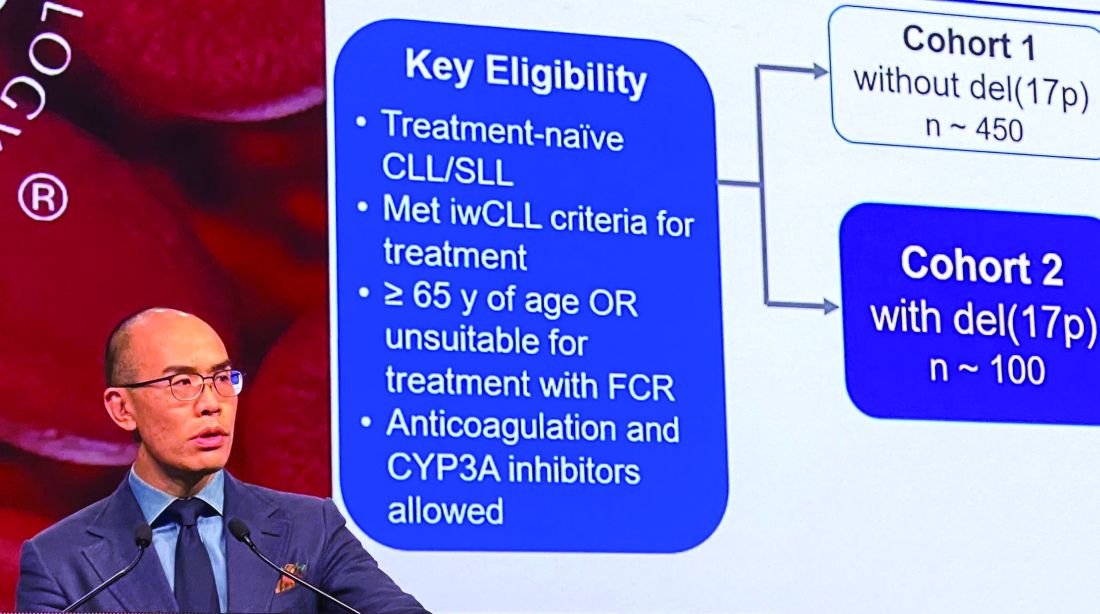
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
REPORTING FROM ASH 2019
Blinatumomab instead of chemo in young patients with relapsed ALL
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
Oral azacitidine: First maintenance therapy for AML
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.
Efficacy of postvenetoclax therapy may depend on prior agent exposure in CLL
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
REPORTING FROM ASH 2019
Kidney function in African American AML patients not linked to reduced survival compared with whites
ORLANDO – While African Americans with acute myeloid leukemia were more likely to have evidence of abnormal kidney function, the excess of this comorbidity didn’t affect overall survival, compared with whites, according to a study of more than 1,000 patients.
A total of 63% of African Americans with acute myeloid leukemia (AML) presented with a renal function abnormality that could have excluded them from a clinical trial, compared with 56% in the overall cohort; however, analysis of outcomes data suggested that renal function abnormalities were not associated with decreased survival in African Americans versus whites, said Abby Statler, PhD, MPH, of the Cleveland Clinic.
The findings may have implications for the design of clinical trials that might exclude patients on the basis of comorbidities that don’t actually affect survival, according to Dr. Statler.
“If we’re able to liberalize renal function eligibility criteria ... this may reduce racial disparities in clinical trial enrollment, which might be a major step in improving the diversity of cancer patient populations,” Dr. Statler said in a press conference at the annual meeting of the American Society of Hematology.
Overly restrictive criteria could be a significant barrier to clinical trial enrollment among minority patient populations, according to Dr. Statler.
Eligibility criteria are generally biased toward “fit” patient populations, which means they may discriminate against less-fit groups, such as African Americans who, compared with whites, have higher rates of comorbidities and report poorer overall health, according to Dr. Statler.
Laura Michaelis, MD, who chaired the press conference, said these findings suggest current clinical trial designs may be “too restrictive.”
“Once it’s published and validated, [these] data should definitely make us think twice about when you limit a patient’s enrollment in a trial,” Dr. Michaelis said in an interview.
Restrictive eligibility criteria may not only limit access to minority populations, but also may slow clinical trial accrual and completion, and make it harder to generalize clinical trial findings to the overall population, said Dr. Michaelis, associate professor of medicine in the division of hematology and oncology, Medical College of Wisconsin, Milwaukee.
The study by Dr. Statler and colleagues included 1,040 AML patients who received chemotherapy at Cleveland Clinic between 2003 and 2019. About 10% of the patients in the analysis were African American and 90% were white.
Median overall survival was not significantly different by race, at 13.7 months for African Americans and 14.9 months for whites (P = 0.89), according to results published in the study abstract.
Mild creatinine elevation did not appear to affect survival in this study, according to the investigator. Survival was not significantly different between patients with normal creatinine and those with creatinine up to 1.5 times the upper limit of normal. However, higher levels of creatinine were significantly associated with worse survival, Dr. Statler said.
Further analyses showed that these survival findings by creatinine level held up specifically in the African American subgroup as well, Dr. Statler said in the press conference.
Dr. Statler provided no disclosures related to the presentation. Study coauthors described disclosures related to Amgen, SimulStat, Bristol-Myers Squibb, Takeda, Pfizer, Novartis, Celgene Corporation, Abbvie, and Incyte, among others.
SOURCE: Statler A et al. ASH 2019, Abstract 381.
ORLANDO – While African Americans with acute myeloid leukemia were more likely to have evidence of abnormal kidney function, the excess of this comorbidity didn’t affect overall survival, compared with whites, according to a study of more than 1,000 patients.
A total of 63% of African Americans with acute myeloid leukemia (AML) presented with a renal function abnormality that could have excluded them from a clinical trial, compared with 56% in the overall cohort; however, analysis of outcomes data suggested that renal function abnormalities were not associated with decreased survival in African Americans versus whites, said Abby Statler, PhD, MPH, of the Cleveland Clinic.
The findings may have implications for the design of clinical trials that might exclude patients on the basis of comorbidities that don’t actually affect survival, according to Dr. Statler.
“If we’re able to liberalize renal function eligibility criteria ... this may reduce racial disparities in clinical trial enrollment, which might be a major step in improving the diversity of cancer patient populations,” Dr. Statler said in a press conference at the annual meeting of the American Society of Hematology.
Overly restrictive criteria could be a significant barrier to clinical trial enrollment among minority patient populations, according to Dr. Statler.
Eligibility criteria are generally biased toward “fit” patient populations, which means they may discriminate against less-fit groups, such as African Americans who, compared with whites, have higher rates of comorbidities and report poorer overall health, according to Dr. Statler.
Laura Michaelis, MD, who chaired the press conference, said these findings suggest current clinical trial designs may be “too restrictive.”
“Once it’s published and validated, [these] data should definitely make us think twice about when you limit a patient’s enrollment in a trial,” Dr. Michaelis said in an interview.
Restrictive eligibility criteria may not only limit access to minority populations, but also may slow clinical trial accrual and completion, and make it harder to generalize clinical trial findings to the overall population, said Dr. Michaelis, associate professor of medicine in the division of hematology and oncology, Medical College of Wisconsin, Milwaukee.
The study by Dr. Statler and colleagues included 1,040 AML patients who received chemotherapy at Cleveland Clinic between 2003 and 2019. About 10% of the patients in the analysis were African American and 90% were white.
Median overall survival was not significantly different by race, at 13.7 months for African Americans and 14.9 months for whites (P = 0.89), according to results published in the study abstract.
Mild creatinine elevation did not appear to affect survival in this study, according to the investigator. Survival was not significantly different between patients with normal creatinine and those with creatinine up to 1.5 times the upper limit of normal. However, higher levels of creatinine were significantly associated with worse survival, Dr. Statler said.
Further analyses showed that these survival findings by creatinine level held up specifically in the African American subgroup as well, Dr. Statler said in the press conference.
Dr. Statler provided no disclosures related to the presentation. Study coauthors described disclosures related to Amgen, SimulStat, Bristol-Myers Squibb, Takeda, Pfizer, Novartis, Celgene Corporation, Abbvie, and Incyte, among others.
SOURCE: Statler A et al. ASH 2019, Abstract 381.
ORLANDO – While African Americans with acute myeloid leukemia were more likely to have evidence of abnormal kidney function, the excess of this comorbidity didn’t affect overall survival, compared with whites, according to a study of more than 1,000 patients.
A total of 63% of African Americans with acute myeloid leukemia (AML) presented with a renal function abnormality that could have excluded them from a clinical trial, compared with 56% in the overall cohort; however, analysis of outcomes data suggested that renal function abnormalities were not associated with decreased survival in African Americans versus whites, said Abby Statler, PhD, MPH, of the Cleveland Clinic.
The findings may have implications for the design of clinical trials that might exclude patients on the basis of comorbidities that don’t actually affect survival, according to Dr. Statler.
“If we’re able to liberalize renal function eligibility criteria ... this may reduce racial disparities in clinical trial enrollment, which might be a major step in improving the diversity of cancer patient populations,” Dr. Statler said in a press conference at the annual meeting of the American Society of Hematology.
Overly restrictive criteria could be a significant barrier to clinical trial enrollment among minority patient populations, according to Dr. Statler.
Eligibility criteria are generally biased toward “fit” patient populations, which means they may discriminate against less-fit groups, such as African Americans who, compared with whites, have higher rates of comorbidities and report poorer overall health, according to Dr. Statler.
Laura Michaelis, MD, who chaired the press conference, said these findings suggest current clinical trial designs may be “too restrictive.”
“Once it’s published and validated, [these] data should definitely make us think twice about when you limit a patient’s enrollment in a trial,” Dr. Michaelis said in an interview.
Restrictive eligibility criteria may not only limit access to minority populations, but also may slow clinical trial accrual and completion, and make it harder to generalize clinical trial findings to the overall population, said Dr. Michaelis, associate professor of medicine in the division of hematology and oncology, Medical College of Wisconsin, Milwaukee.
The study by Dr. Statler and colleagues included 1,040 AML patients who received chemotherapy at Cleveland Clinic between 2003 and 2019. About 10% of the patients in the analysis were African American and 90% were white.
Median overall survival was not significantly different by race, at 13.7 months for African Americans and 14.9 months for whites (P = 0.89), according to results published in the study abstract.
Mild creatinine elevation did not appear to affect survival in this study, according to the investigator. Survival was not significantly different between patients with normal creatinine and those with creatinine up to 1.5 times the upper limit of normal. However, higher levels of creatinine were significantly associated with worse survival, Dr. Statler said.
Further analyses showed that these survival findings by creatinine level held up specifically in the African American subgroup as well, Dr. Statler said in the press conference.
Dr. Statler provided no disclosures related to the presentation. Study coauthors described disclosures related to Amgen, SimulStat, Bristol-Myers Squibb, Takeda, Pfizer, Novartis, Celgene Corporation, Abbvie, and Incyte, among others.
SOURCE: Statler A et al. ASH 2019, Abstract 381.
REPORTING FROM ASH 2019